Remarkable Population Resilience in a North African Endemic Damselfly in the Face of Rapid Agricultural Transformation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Demographic Parameter
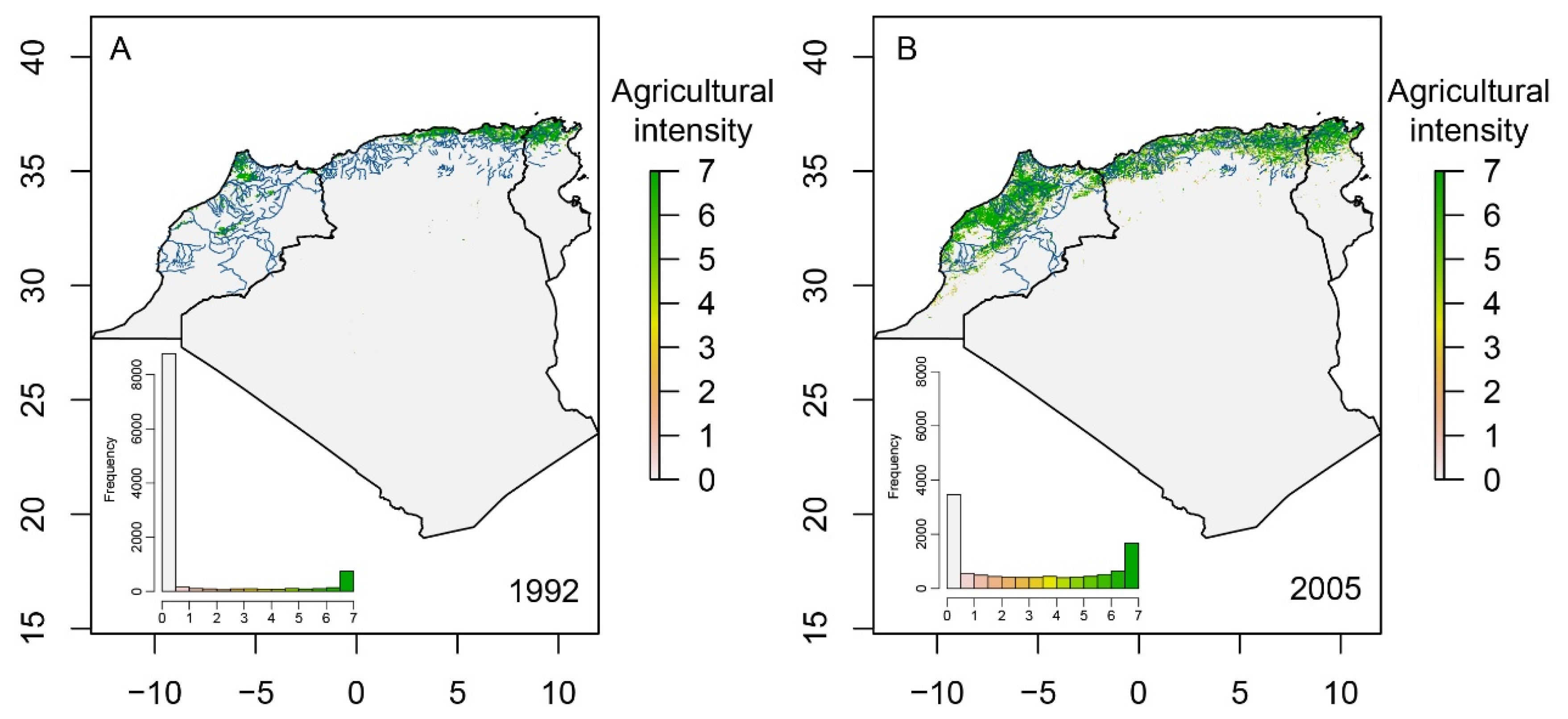
2.3. Agricultural Intensity Data
2.4. Geographic Range Estimation
3. Results
3.1. Recapture and Survival Probability
3.2. Occupancy of Terrestrial Habitat
3.3. Agricultural Extent and Occupancy
3.4. Distribution Modelling
4. Discussion
4.1. Demographic Parameters
4.2. Population Size
4.3. Terrestrial Habitat Occupancy
4.4. Geographic Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zabel, F.; Delzeit, R.; Schneider, J.M.; Seppelt, R.; Mauser, W.; Václavík, T. Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity. Nat. Commun. 2019, 10, 2844. [Google Scholar] [CrossRef]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Daily, G.C.; Ehrlich, P.R.; Fay, J.P. Countryside biogeography of moths in a fragmented landscape: Biodiversity in native and agricultural habitats. Conserv. Biol. 2001, 15, 378–388. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Friberg, N. Impacts and indicators of change in lotic ecosystems. Wiley Interdiscip. Rev. Water 2014, 1, 513–531. [Google Scholar] [CrossRef]
- Joffe, G. The impending water crisis in the MENA region. Int. Spect. 2016, 51, 55–66. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Tropp, H.; Jagerskog, A. Water Scarcity Challenges in the Middle East and North Africa (MENA); Human Development Report; Stockholm International Water Institute: Stockholm, Sweden, 2006; Available online: http://hdr.undp.org/hdr2006/pdfs/background-docs/Thematic_Papers/SIWI.pdf (accessed on 2 March 2021).
- Nin-Pratt, A.; El-Enbaby, H.; Figueroa, J.L.; ElDidi, H.; Breisinger, C. Agriculture and Economic Transformation in the Middle East and North Africa: A Review of the Past with Lessons for the Future; International Food Policy Research Institute: Washington, DC, USA, 2017. [Google Scholar]
- Rolls, R.J.; Leigh, C.; Sheldon, F. Mechanistic effects of low-flow hydrology on riverine ecosystems: Ecological principles and consequences of alteration. Freshw. Sci. 2012, 31, 1163–1186. [Google Scholar] [CrossRef]
- Vilenica, M.; Kerovec, M.; Pozojević, I.; Mihaljević, Z. Odonata assemblages in anthropogenically impacted lotic habitats. J. Limnol. 2021, 80. [Google Scholar] [CrossRef]
- Malaj, E.; Peter, C.; Grote, M.; Kühne, R.; Mondy, C.P.; Usseglio-Polatera, P.; Brack, W.; Schäfer, R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. USA 2014, 111, 9549–9554. [Google Scholar] [CrossRef]
- Karrouch, L.; Chahlaoui, A.; Essahale, A. Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Boufekrane River, Meknes, Morocco. J. Geosci. Environ. Prot. 2017, 5, 77812. [Google Scholar] [CrossRef][Green Version]
- Saal, I.; Bouchelouche, D.; Hamache, C.; Arab, A. Evaluation of the surface water quality in the Kebir-Rhumel catchment area (northeast Algeria) using biotic indices and physico-chemical analyses. Environ. Sci. Pollut. Res. 2020, 1–15. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Kaunisto, K.M.; Roslin, T.; Sääksjärvi, I.E.; Vesterinen, E.J. Pellets of proof: First glimpse of the dietary composition of adult odonates as revealed by metabarcoding of feces. Ecol. Evol. 2017, 7, 8588–8598. [Google Scholar] [CrossRef]
- Kaunisto, K.M.; Roslin, T.; Forbes, M.R.; Morrill, A.; Sääksjärvi, I.E.; Puisto, A.I.; Lilley, T.M.; Vesterinen, E.J. Threats from the air: Damselfly predation on diverse prey taxa. J. Anim. Ecol. 2020, 89, 1365–1374. [Google Scholar] [CrossRef]
- Walser, C.; Bart, H. Influence of agriculture on in-stream habitat and fish community structure in Piedmont watersheds of the Chattahoochee River System. Ecol. Freshw. Fish 1999, 8, 237–246. [Google Scholar] [CrossRef]
- Kadoya, T.; Suda, S.I.; Washitani, I. Dragonfly crisis in Japan: A likely consequence of recent agricultural habitat degradation. Biol. Conserv. 2009, 142, 1899–1905. [Google Scholar] [CrossRef]
- Khelifa, R.; Deacon, C.; Mahdjoub, H.; Simaika, J.P.; Suhling, F.; Samways, M.J. Dragonfly conservation in the increasingly stressed African Mediterranean-type ecosystems. In revision.
- Samraoui, B.; Boudot, J.P.; Ferreira, S.; Riservato, E.; Jović, M.; Kalkman, V.J.; Schneider, W. The status and distribution of dragonflies. In The Status and Distribution of Freshwater Biodiversity in Northern Africa; García, N., Cuttelod, A., Abdul Malak, D., Eds.; IUCN: Gland, Switzerland; Cambridge, UK; Malaga, Spain, 2010; pp. 51–69. [Google Scholar]
- Khelifa, R.; Zebsa, R.; Amari, H.; Mellal, M.K.; Mahdjoub, H.; Kahalerras, A. A hotspot for threatened Mediterranean odonates in the Seybouse River (Northeast Algeria): Are IUCN population sizes drastically underestimated? Int. J. Odonatol. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Khelifa, R. Sensitivity of biodiversity indices to life history stage, habitat type and landscape in Odonata community. Biol. Conserv. 2019, 237, 63–69. [Google Scholar] [CrossRef]
- Khelifa, R.; Guebailia, A.; Mahdjoub, H.; Aouaouche, M.S.; Houhamdi, M. Aspects of life history of Platycnemis subdilatata (Zygoptera: Platycnemididae) in Northeast Algeria. Int. J. Odonatol. 2015, 18, 317–327. [Google Scholar] [CrossRef]
- Khelifa, R.; Mahdjoub, H.; Aouaouche, M.S.; Houhamdi, M. Reproductive behaviour of a North African endemic damselfly, Platycnemis subdilatata (Odonata: Platycnemididae) and probable senescence effects. Int. J. Odonatol. 2016, 19, 157–167. [Google Scholar] [CrossRef]
- Dijkstra, K.D.; Schröter, A. Field Guide to the Dragonflies of Britain and Europe; Bloomsbury Publishing: London, UK, 2020. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lebreton, J.D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef]
- Laake, J. RMark: An R Interface for Analysis of Capture–Recapture Data with MARK; AFSC Processed Rep 2013-01; Alaska Fisheries Science Center, NOAA, National Marine Fisheries Service: Seattle, WA, USA, 2013.
- Choquet, R.; Lebreton, J.D.; Gimenez, O.; Reboulet, A.M.; Pradel, R. U-CARE: Utilities for performing goodness of fit tests and manipulating capture–recapture data. Ecography 2009, 32, 1071–1074. [Google Scholar] [CrossRef]
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Cook, L.; Brower, L.; Croze, H. The accuracy of a population estimation from multiple recapture data. J. Anim. Ecol. 1967, 36, 57–60. [Google Scholar] [CrossRef]
- Schwarz, C.J.; Arnason, A.N. A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 1996, 52, 860–873. [Google Scholar] [CrossRef]
- Crosbie, S.; Manly, B. Parsimonious modelling of capture-mark-recapture studies. Biometrics 1985, 41, 385–398. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Hansen, M.C.; DeFries, R.S.; Townshend, J.R.; Sohlberg, R. Global land cover classification at 1 km spatial resolution using a classification tree approach. Int. J. Remote Sens. 2000, 21, 1331–1364. [Google Scholar] [CrossRef]
- European Space Agency. GlobCover Land Cover V2.3; ESA GlobCover Project, Led by MEDIAS; European Space Agency: Paris, France, 2011; Available online: http://due.esrin.esa.int/page_globcover.php (accessed on 1 March 2021).
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Naimi, B.; Hamm, N.A.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Garcia, R.A.; Burgess, N.D.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Exploring consensus in 21st century projections of climatically suitable areas for African vertebrates. Glob. Chang. Biol. 2012, 18, 1253–1269. [Google Scholar] [CrossRef]
- Murray, K.; Conner, M.M. Methods to quantify variable importance: Implications for the analysis of noisy ecological data. Ecology 2009, 90, 348–355. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef]
- Khelifa, R.; Mahdjoub, H.; Baaloudj, A.; Cannings, R.A.; Samways, M.J. Effects of both climate change and human water demand on a highly threatened damselfly. Sci. Rep. 2021, 11, 7725. [Google Scholar] [CrossRef]
- Cordero-Rivera, A.; Stoks, R. Mark-recapture studies and demography. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 2008; pp. 7–20. [Google Scholar]
- Khelifa, R.; Zebsa, R.; Amari, H.; Mellal, M.K.; Mahdjoub, H. Field estimates of fitness costs of the pace-of-life in an endangered damselfly. J. Evol. Biol. 2019, 32, 943–954. [Google Scholar] [CrossRef]
- Khelifa, R.; Mellal, M.K. Host-plant-based restoration as a potential tool to improve conservation status of odonate specialists. Insect Conserv. Divers. 2017, 10, 151–160. [Google Scholar] [CrossRef]
- Khelifa, R.; Mellal, M.K.; Zouaimia, A.; Amari, H.; Zebsa, R.; Bensouilah, S.; Laouar, A.; Houhamdi, M. On the restoration of the last relict population of a dragonfly Urothemis edwardsii Selys (Libellulidae: Odonata) in the Mediterranean. J. Insect Conserv. 2016, 20, 797–805. [Google Scholar] [CrossRef]
- Zebsa, R.; Khelifa, R.; Kahalerras, A. Adult movement pattern and habitat preferences of the Maghribian endemic Gomphus lucasii (Odonata: Gomphidae). J. Insect Sci. 2015, 15, 151. [Google Scholar] [CrossRef]
- Liker, A.; Szekely, T. Mortality costs of sexual selection and parental care in natural populations of birds. Evolution 2005, 59, 890–897. [Google Scholar] [CrossRef]
- Cordero, A.; Perez, F.E.; Andres, J. The effect of handling damage, mobility, body size, and fluctuating asymmetry on lifetime mating success of Ischnura graellsii (Rambur) (Zygoptera: Coenagrionidae). Odonatologica 2002, 31, 117–128. [Google Scholar]
- Sherratt, T.; Laird, R.; Hassall, C.; Lowe, C.; Harvey, I.; Watts, P.; Cordero-Rivera, A.; Thompson, D. Empirical evidence of senescence in adult damselflies (Odonata: Zygoptera). J. Anim. Ecol. 2010, 79, 1034–1044. [Google Scholar] [CrossRef]
- Conrad, K.; Willson, K.; Harvey, I.; Thomas, C.; Sherratt, T. Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 1999, 22, 524–531. [Google Scholar] [CrossRef]
- Tscharntke, T.; Steffan-Dewenter, I.; Kruess, A.; Thies, C. Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol. Appl. 2002, 12, 354–363. [Google Scholar]
- Marini, L.; Fontana, P.; Klimek, S.; Battisti, A.; Gaston, K.J. Impact of farm size and topography on plant and insect diversity of managed grasslands in the Alps. Biol. Conserv. 2009, 142, 394–403. [Google Scholar] [CrossRef]
- Bried, J.T.; Ervin, G.N. Abundance patterns of dragonflies along a wetland buffer. Wetlands 2006, 26, 878–883. [Google Scholar] [CrossRef]
- Korbaa, M.; Ferreras-Romero, M.; Ruiz-García, A.; Boumaiza, M. TSOI—A new index based on Odonata populations to assess the conservation relevance of watercourses in Tunisia. Odonatologica 2018, 47, 43–72. [Google Scholar]
- El Haissoufi, M.; De Knijf, G.; van’t Bosch, J.; Bennas, N.; Millán Sánchez, A. Contribution to the knowledge of the Moroccan Odonata, with first records of Orthetrum sabina, and an overview of first and last dates for all species. Odonatologica 2015, 44, 225–254. [Google Scholar]
- Dodds, W.K. Eutrophication and trophic state in rivers and streams. Limnol. Oceanogr. 2006, 51, 671–680. [Google Scholar] [CrossRef]
- Reece, B.A.; Mcintyre, N.E. Community assemblage patterns of odonates inhabiting a wetland complex influenced by anthropogenic disturbance. Insect Conserv. Divers. 2009, 2, 73–80. [Google Scholar] [CrossRef]
- Khelifa, R. Partial bivoltinism and emergence patterns in the North African endemic damselfly Calopteryx exul: Conservation implications. Afr. J. Ecol 2017, 55, 145–151. [Google Scholar] [CrossRef]
- Guettaf, M.; Maoui, A.; Ihdene, Z. Assessment of water quality: A case study of the Seybouse River (North East of Algeria). Appl. Water Sci. 2017, 7, 295–307. [Google Scholar] [CrossRef]
- Tippler, C.; Wright, I.A.; Davies, P.J.; Evans, C.R. Are Odonata nymph adversely affected by impaired water quality in urban streams. Austral Ecol. 2018, 43, 890–902. [Google Scholar] [CrossRef]
- Stoks, R.; Johansson, F.; De Block, M. Life-history plasticity under time stress in damselfly larvae. In Dragonflies and Damselflies. Model Organisms for Ecological and Evolutionary Research; Oxford University Press: Oxford, UK, 2008; pp. 39–51. [Google Scholar]
- Janni, O.; Viganò, M.; Corso, A. First records of Diplacodes lefebvrii (Rambur, 1842) for Sicily and additional record of Trithemis kirbyi Selys, 1891 (Odonata Libellulidae). Biodivers. J. 2020, 11, 65–68. [Google Scholar] [CrossRef]
- Kalfayan, M.; Krieg-Jacquier, R. First records of Trithemis arteriosa and Brachytron pratense on the island of Samos, Greece (Odonata: Anisoptera). Not. Odonatol. 2018, 9, 31–36. [Google Scholar]
- Lind, L.; Hasselquist, E.M.; Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J. Environ. Manag. 2019, 249, 109391. [Google Scholar] [CrossRef] [PubMed]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef] [PubMed]





| Estimate | Std. Error | z | p | |
|---|---|---|---|---|
| (Intercept) | −0.105 | 0.214 | −0.491 | 0.6233 |
| Age[M] | 0.967 | 0.263 | 3.679 | 0.0002 |
| Age[T] | −1.686 | 0.505 | −3.340 | 0.0008 |
| Sex[Male] | 0.201 | 0.292 | 0.687 | 0.4920 |
| Land[Grassland] | 1.930 | 0.246 | 7.856 | <0.0001 |
| Age[M] × Sex[Male] | −0.173 | 0.362 | −0.478 | 0.6329 |
| Age[T] × Sex[Male] | −1.810 | 1.142 | −1.586 | 0.1128 |
| Age[M] × Land[Grassland] | −1.162 | 0.314 | −3.695 | 0.0002 |
| Age[T] × Land[Grassland] | 0.431 | 0.545 | 0.791 | 0.4292 |
| Sex[Male] × Land[Grassland] | −0.056 | 0.337 | −0.166 | 0.8683 |
| Age[M] × Sex[Male] × Land[Grassland] | −0.339 | 0.439 | −0.772 | 0.4402 |
| Age[T] × Sex[Male] × Land[Grassland] | 1.524 | 1.179 | 1.292 | 0.1962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khelifa, R.; Mahdjoub, H.; Baaloudj, A.; Cannings, R.A.; Samways, M.J. Remarkable Population Resilience in a North African Endemic Damselfly in the Face of Rapid Agricultural Transformation. Insects 2021, 12, 353. https://doi.org/10.3390/insects12040353
Khelifa R, Mahdjoub H, Baaloudj A, Cannings RA, Samways MJ. Remarkable Population Resilience in a North African Endemic Damselfly in the Face of Rapid Agricultural Transformation. Insects. 2021; 12(4):353. https://doi.org/10.3390/insects12040353
Chicago/Turabian StyleKhelifa, Rassim, Hayat Mahdjoub, Affef Baaloudj, Robert A. Cannings, and Michael J. Samways. 2021. "Remarkable Population Resilience in a North African Endemic Damselfly in the Face of Rapid Agricultural Transformation" Insects 12, no. 4: 353. https://doi.org/10.3390/insects12040353
APA StyleKhelifa, R., Mahdjoub, H., Baaloudj, A., Cannings, R. A., & Samways, M. J. (2021). Remarkable Population Resilience in a North African Endemic Damselfly in the Face of Rapid Agricultural Transformation. Insects, 12(4), 353. https://doi.org/10.3390/insects12040353






