The Growth Model of Forensically Important Lucilia sericata (Meigen) (Diptera: Calliphoridae) in South Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
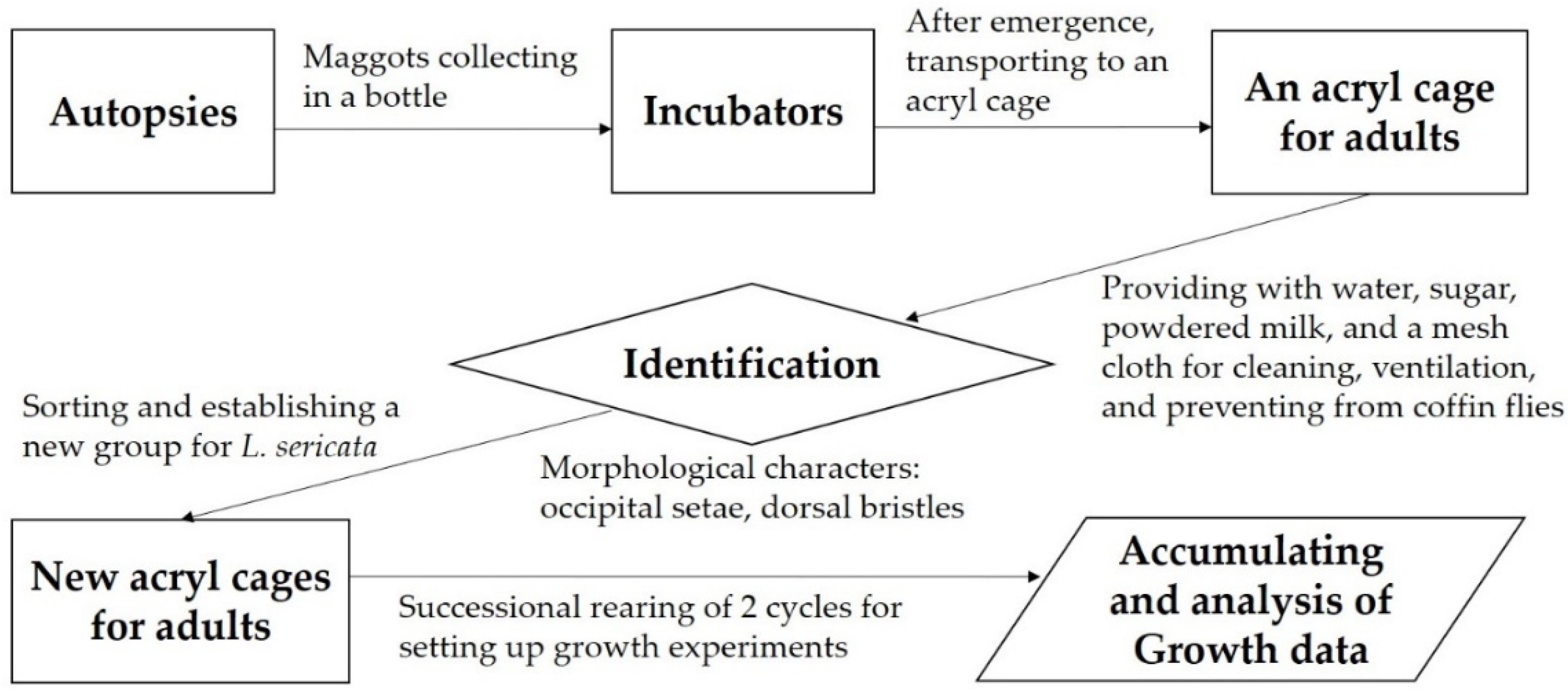
2.1. Identification and Rearing of Adult Flies
2.2. Sampling and Rearing of Maggots
2.3. Body Length, Larval Stages, and the Optimum Development Temperature
2.4. Data Fit and Statistical Analysis
3. Results
3.1. Body Length and Minimum Development Time
3.2. Base Temperature, Optimum Temperature, and Comparisons with Previous Studies
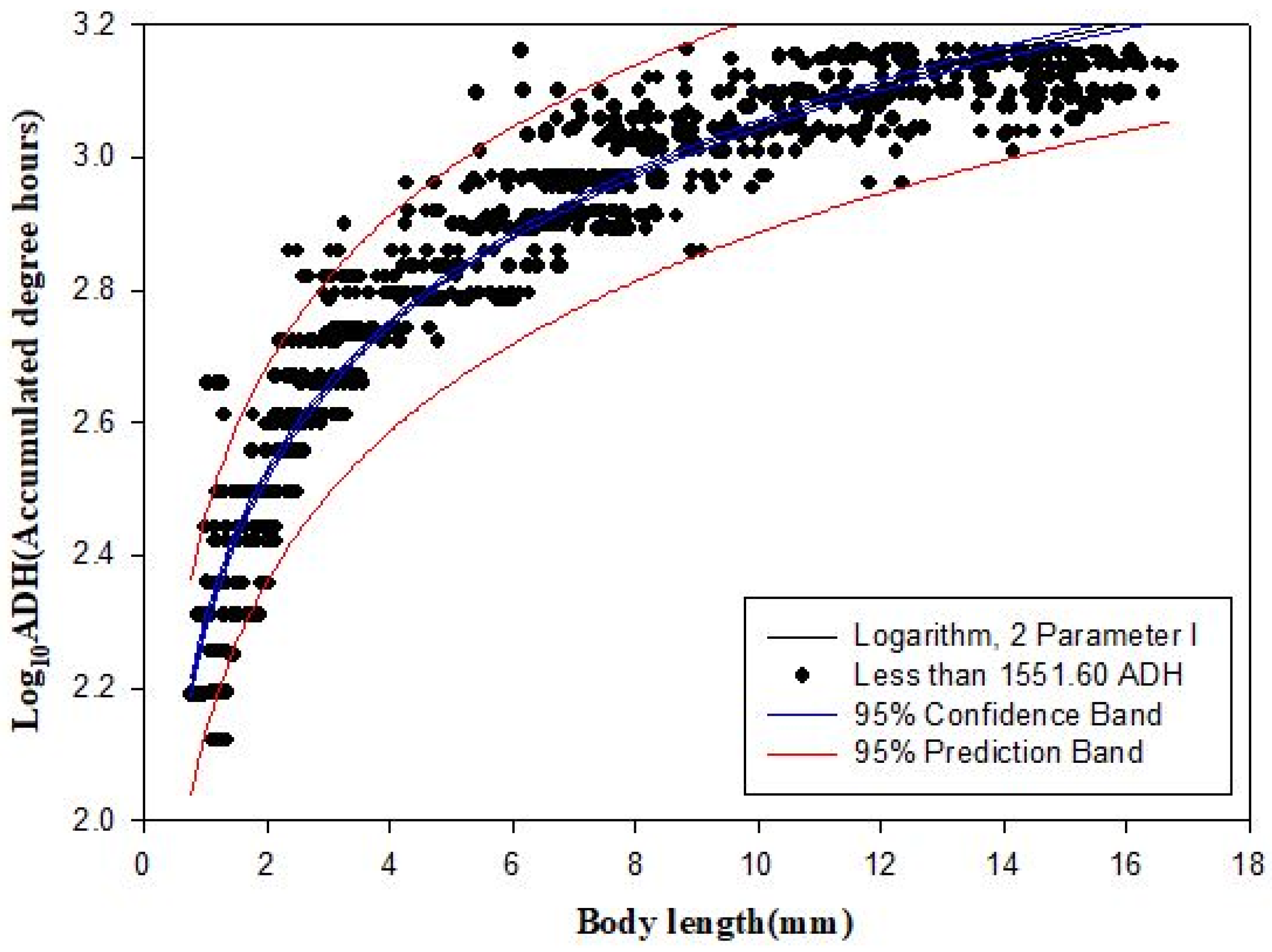
3.3. Minimum ADH Models and Scatter Plots
3.4. Linear and Nonlinear Regressions during Immature Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amendt, J.; Campobasso, C.P.; Goff, M.L.; Grassberger, M. Current Concepts in Forensic Entomology; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Byrd, H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Shin, S.E.; Jang, M.S.; Park, J.H.; Park, S.H. A forensic entomology case estimating the minimum postmortem interval using the distribution of fly pupae in fallow ground and maggots with freezing injury. Korean J. Leg. Med. 2015, 39, 17–21. [Google Scholar] [CrossRef][Green Version]
- Davidson, J. On the relationship between temperature and rate of development of insects at constant temperatures. J. Anim. Ecol. 1944, 13, 26–38. [Google Scholar] [CrossRef]
- Gennard, D.E. Forensic Entomology: An Introduction, 2nd ed.; John Wiley and Sons: Chichester, UK, 2012. [Google Scholar]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. Best practice in forensic entomology—Standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Foran, D.R. Generalized additive models and Lucilia sericata growth: Assessing confidence intervals and error rates in forensic entomology. J. Forensic Sci. 2008, 53, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G.V. A Manual of Forensic Entomology; British Museum (Natural History): London, UK, 1986. [Google Scholar]
- Benecke, M.; Josephi, E.; Zweihoff, R. Neglect of the elderly: Forensic entomology cases and considerations. Forensic Sci. Int. 2004, 146S, S195–S199. [Google Scholar] [CrossRef]
- Arnaldos, M.I.; García, M.D.; Romera, E.; Presa, J.J.; Luna, A. Estimation of postmortem interval in real cases based on experimentally obtained entomological evidence. Forensic Sci. Int. 2005, 149, 57–65. [Google Scholar] [CrossRef]
- Saigusa, K.; Takamiya, M.; Aoki, Y. Species identificaion of the forensically important flies in Iwate prefecture, Japan based on mitochondrial cytochrome oxidase gene subunit I (CO I) sequences. Legal Med. 2005, 7, 175–178. [Google Scholar] [CrossRef]
- Bugelli, V.; Forni, D.; Bassi, L.A.; Paolo, M.D.; Marra, D.; Lenzi, S.; Toni, C.; Giusiani, M.; Domenici, R.; Gherardi, M.; et al. Forensic entomology and the estimation of the minimum time since death in indoor cases. J. Forensic Sci. 2015, 60, 525–531. [Google Scholar] [CrossRef]
- Shin, S.E.; Lee, H.J.; Park, J.H.; Ko, K.S.; Kim, Y.H.; Kim, K.R.; Park, S.H. The first survey of forensically important entomofauna collected from medicolegal autopsies in South Korea. Biomed. Res. Int. 2015, 2015, 606728. [Google Scholar] [CrossRef]
- Choe, S.; Lee, D.; Park, H.; Jeon, H.K.; Kim, H.; Kang, J.H.; Jee, C.H.; Eom, K.S. Canine wound myiasis caused by Lucilia sericata (Diptera: Calliphoridae) in Korea. Korean J. Parasitol. 2016, 54, 667–671. [Google Scholar] [CrossRef]
- Kamal, A.S. Comparative study of thirteen species of sarcosaprophagous Calliphoridae and Sarcophagidae (Diptera) I. Bionomics. Ann. Entomol. Soc. Am. 1958, 51, 261–271. [Google Scholar] [CrossRef]
- Ash, N.; Greenberg, B. Developmental temperature responses of the sibling species Phaenicia sericata and Phaenicia pallescens. Ann. Entomol. Soc. Am. 1975, 68, 197–200. [Google Scholar] [CrossRef]
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J. Forensic Sci. 2000, 45, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, M.; Reiter, C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen- and isomorphen-diagram. Forensic Sci. Int. 2001, 120, 32–36. [Google Scholar] [CrossRef]
- Marchenko, M.I. Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Forensic Sci. Int. 2001, 120, 89–109. [Google Scholar] [CrossRef]
- Roe, A.; Higley, L.G. Development modeling of Lucilia sericata (Diptera: Calliphoridae). PeerJ 2015, 3, e803. [Google Scholar] [CrossRef]
- VanLaerhoven, S.L. Blind validation of postmortem interval estimates using developmental rates of blow flies. Forensic Sci. Int. 2008, 180, 76–80. [Google Scholar] [CrossRef]
- Núñez-Vázquez, C.; Tomberlin, J.K.; Cantú-Sifuentes, M.; García-Martínez, O. Laboratory development and field validation of Phormia regina (Diptera: Calliphoridae). J. Med. Entomol. 2013, 50, 252–260. [Google Scholar] [CrossRef]
- Kanō, R.; Shinonaga, S. Calliphoridae (Insecta: Diptera), Fauna Japonica; Biogeographical Society of Japan: Tokyo, Japan, 1968; p. 181. [Google Scholar]
- Tachibana, S.; Numata, H. Effects of temperature and photoperiod on the termination of larval diapause in Lucilia sericata (Diptera: Calliphoridae). Zoolog. Sci. 2004, 21, 197–202. [Google Scholar] [CrossRef]
- Rivers, D.B.; Thompson, C.; Brogan, R. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Entomol. Res. 2011, 101, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Benbow, M.E. Forensic Entomology: International Dimensions and Frontiers; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Adams, Z.J.; Hall, M.J. Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci. Int. 2003, 138, 50–61. [Google Scholar] [CrossRef]
- SAS Institute. PROC User’s Manual, Version 9.1; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Richards, C.S.; Rowlinson, C.C.; Cuttiford, L.; Grimsley, R.; Hall, M.J.R. Decomposed liver has a significantly adverse affect on the development rate of the blowfly Calliphora vicina. Int. J. Legal Med. 2013, 127, 259–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreau, G.; Michaud, J.P.; Schoenly, K.G. Experimental Design, Inferential Statistics, and Computer Modeling. In Forensic Entomology: International Dimensions and Frontiers; Tomberlin, J.K., Benbow, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 205–229. [Google Scholar]
- Johnson, A.P.; Wallman, J.F. Effect of massing on larval growth rate. Forensic Sci. Int. 2014, 241, 141–149. [Google Scholar] [CrossRef]
- Wells, J.D.; Lecheta, M.C.; Moura, M.O.; LaMotte, L.R. An evaluation of sampling methods used to produce insect growth models for postmortem interval estimation. Int. J. Legal Med. 2015, 129, 405–410. [Google Scholar] [CrossRef] [PubMed]
- LaMotte, L.R.; Roe, A.L.; Wells, J.D.; Higley, L.G. A statistical method to construct confidence sets on carrion insect age from development stage. J. Agric. Biol. Environ. Stat. 2017. [Google Scholar] [CrossRef]
- Goodbrod, J.R.; Goff, M.L. Effects of larval population density on rates of development and interactions between two species of Chrysomya (Diptera: Calliphoridae) in laboratory culture. J. Med. Entomol. 1990, 27, 338–343. [Google Scholar] [CrossRef]
- Charabidze, D.; Bourel, B.; Gosset, D. Larval-mass effect: Characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Sci. Int. 2011, 211, 61–66. [Google Scholar] [CrossRef]
- Kotzé, Z.; Villet, M.H.; Weldon, C.W. Effect of temperature on development of the blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Int. J. Legal Med. 2015, 129, 1155–1162. [Google Scholar] [CrossRef]
- Arnold, C.Y. The determination and significance of the base temperature in a linear heat unit system. Proc. Am. Soc. Hortic. Sci. 1959, 74, 430–445. [Google Scholar]
- Reibe, S.; Doetinchem, P.V.; Madea, B. A new simulation-based model for calculating post-mortem intervals using developmental data for Lucilia sericata (Dipt.: Calliphoridae). Parasitol. Res. 2010, 107, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, M.I. Development of Chrysomyia albiceps WD. (Diptera, Calliphoridae). Entomol. Rev. 1985, 64, 107–112. [Google Scholar]
- Greenberg, B.; Kunich, J.C. Entomology and the Law: Flies as Forensic Indicators; Cambridge University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Tarone, A.M.; Foran, D.R. Gene expression during blow fly development: Improving the precision of age estimates in forensic entomology. J. Forensic Sci. 2011, 56, S112–S122. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, S.E.; Park, S.H.; Jeong, S.J.; Park, S.H.; Moon, T.Y.; Lee, J.W. A study for estimating growth time of Calliphoridae flies using statistical models. J. Sci. Crim. Investig. 2019, 13, 89–94. [Google Scholar] [CrossRef]
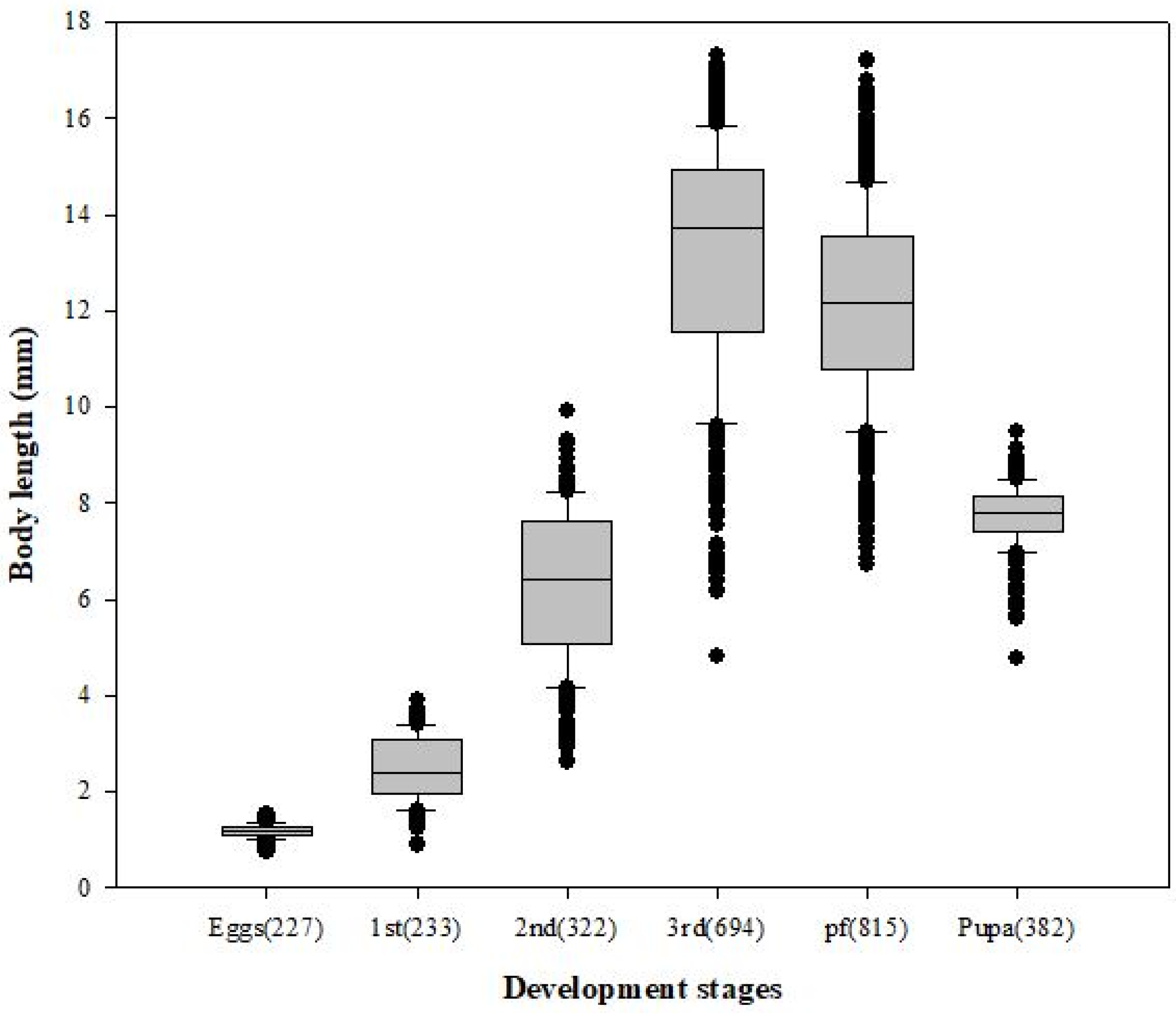
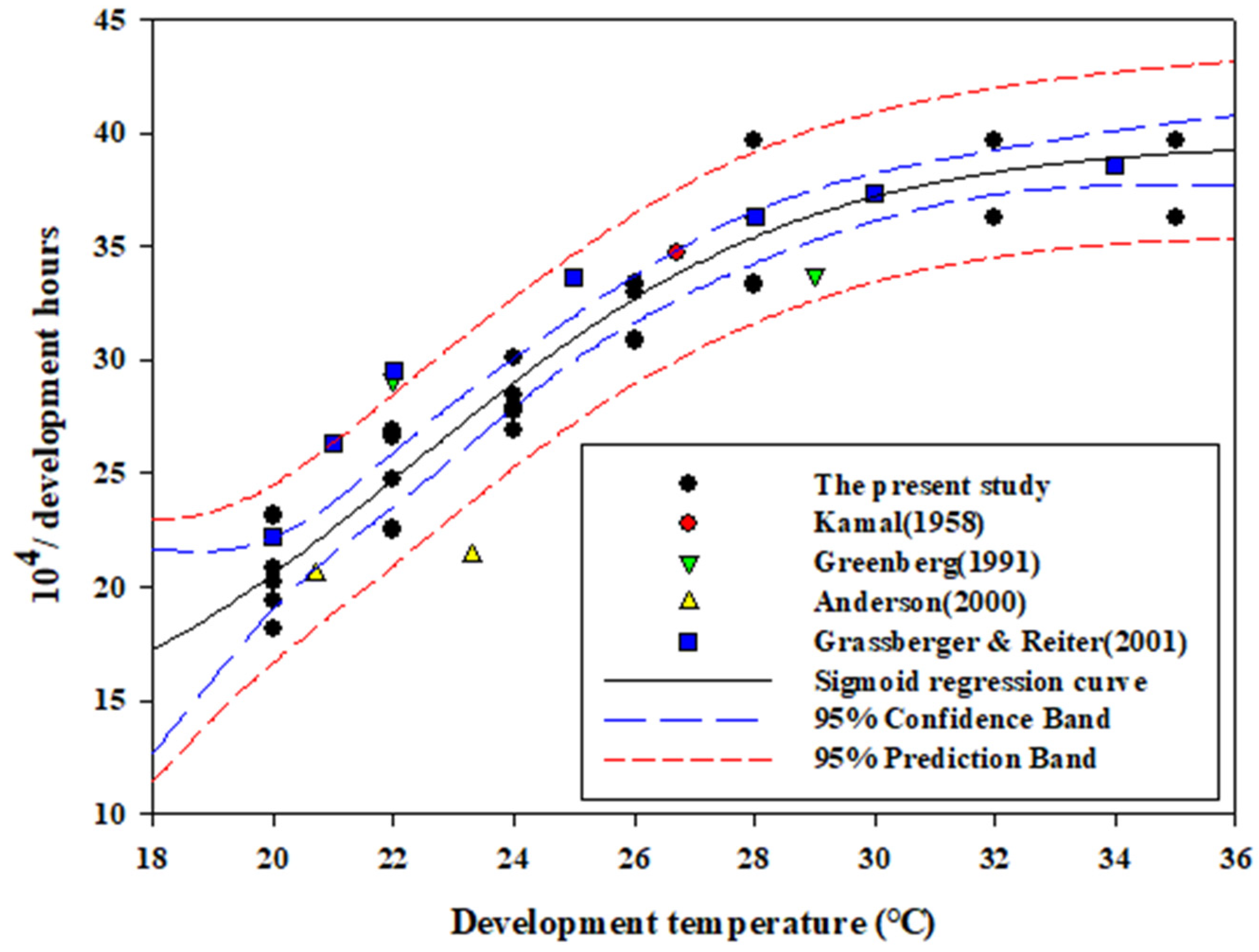

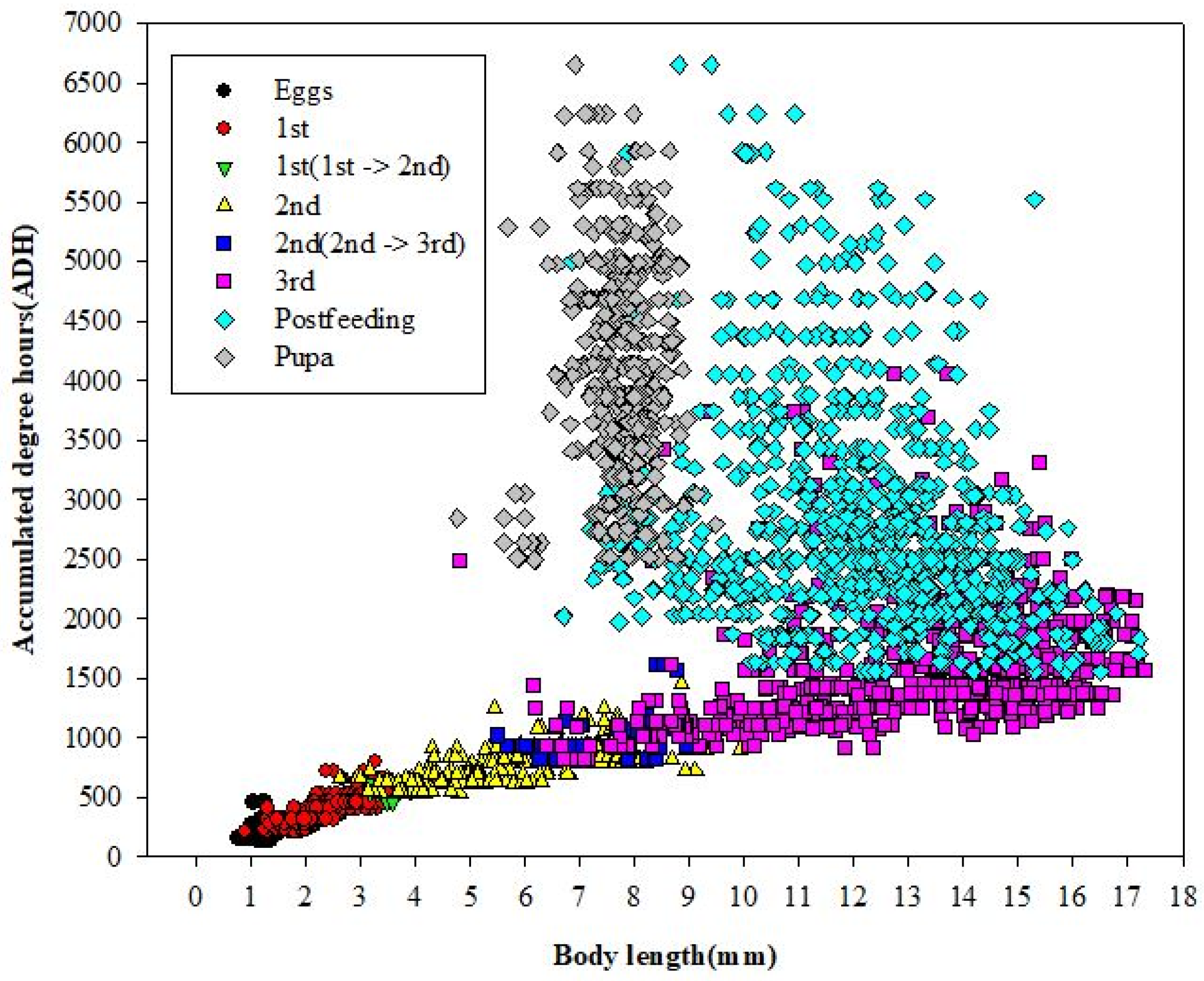
| Stage | Regression Equation | R2 | Base Temperature (°C) | p-Value |
|---|---|---|---|---|
| Egg | Y = 39.9305x − 479.1665 | 0.8701 | 12.0000 | 0.0207 * |
| First instar | Y = 25.4630x − 164.0213 | 0.5673 | 6.4416 | 0.1416 |
| Second instar | Y = 21.3656x − 147.9693 | 0.6338 | 6.9256 | 0.1071 |
| Third instar | Y = 12.5778x − 92.7091 | 0.6265 | 7.3709 | 0.1106 |
| Post-feeding | Y = 9.2416x − 65.3248 | 0.3773 | 7.0686 | 0.2703 |
| Pupa | Y = 4.4917x − 46.8452 | 0.9152 | 10.4293 | 0.0108 * |
| Egg to adult | Y = 1.9040x − 17.2758 | 0.9907 | 9.0734 | <0.0004 * |
| Model | Y | Regression Equation | R2adj | SE | MSE |
|---|---|---|---|---|---|
| Linear | ADH | Y = 238.508 + 83.076x | 0.872 | 142.800 | 204 × 102 |
| Quadratic | ADH | Y = 41.570 + 158.911x − 4.814x2 | 0.920 | 112.729 | 127 × 102 |
| Logarithm | ADH | Y = 38.392 + 466.960 Log10 (x) | 0.899 | 126.993 | 161 × 102 |
| Linear | Log10ADH | Log10 (Y) = 2.469 + 0.053x | 0.744 | 0.141 | 0.020 |
| Quadratic | Log10ADH | Log10 (Y) = 2.228 + 0.146x − 0.006x2 | 0.892 | 0.091 | 0.008 |
| Logarithm | Log10ADH | Log10 (Y) = 2.295+ 0.327 Log10 (x) | 0.911 | 0.083 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.E.; Park, J.H.; Jeong, S.J.; Park, S.H. The Growth Model of Forensically Important Lucilia sericata (Meigen) (Diptera: Calliphoridae) in South Korea. Insects 2021, 12, 323. https://doi.org/10.3390/insects12040323
Shin SE, Park JH, Jeong SJ, Park SH. The Growth Model of Forensically Important Lucilia sericata (Meigen) (Diptera: Calliphoridae) in South Korea. Insects. 2021; 12(4):323. https://doi.org/10.3390/insects12040323
Chicago/Turabian StyleShin, Sang Eon, Ji Hye Park, Su Jin Jeong, and Seong Hwan Park. 2021. "The Growth Model of Forensically Important Lucilia sericata (Meigen) (Diptera: Calliphoridae) in South Korea" Insects 12, no. 4: 323. https://doi.org/10.3390/insects12040323
APA StyleShin, S. E., Park, J. H., Jeong, S. J., & Park, S. H. (2021). The Growth Model of Forensically Important Lucilia sericata (Meigen) (Diptera: Calliphoridae) in South Korea. Insects, 12(4), 323. https://doi.org/10.3390/insects12040323





