Determining the Sterilization Doses under Hypoxia for the Novel Black Pupae Genetic Sexing Strain of Anastrepha fraterculus (Diptera, Tephritidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Anastrepha fraterculus Colonies
2.2. Irradiation Procedures
2.3. Evaluation of Sterilization under Hypoxia
2.4. Data Analysis
3. Results
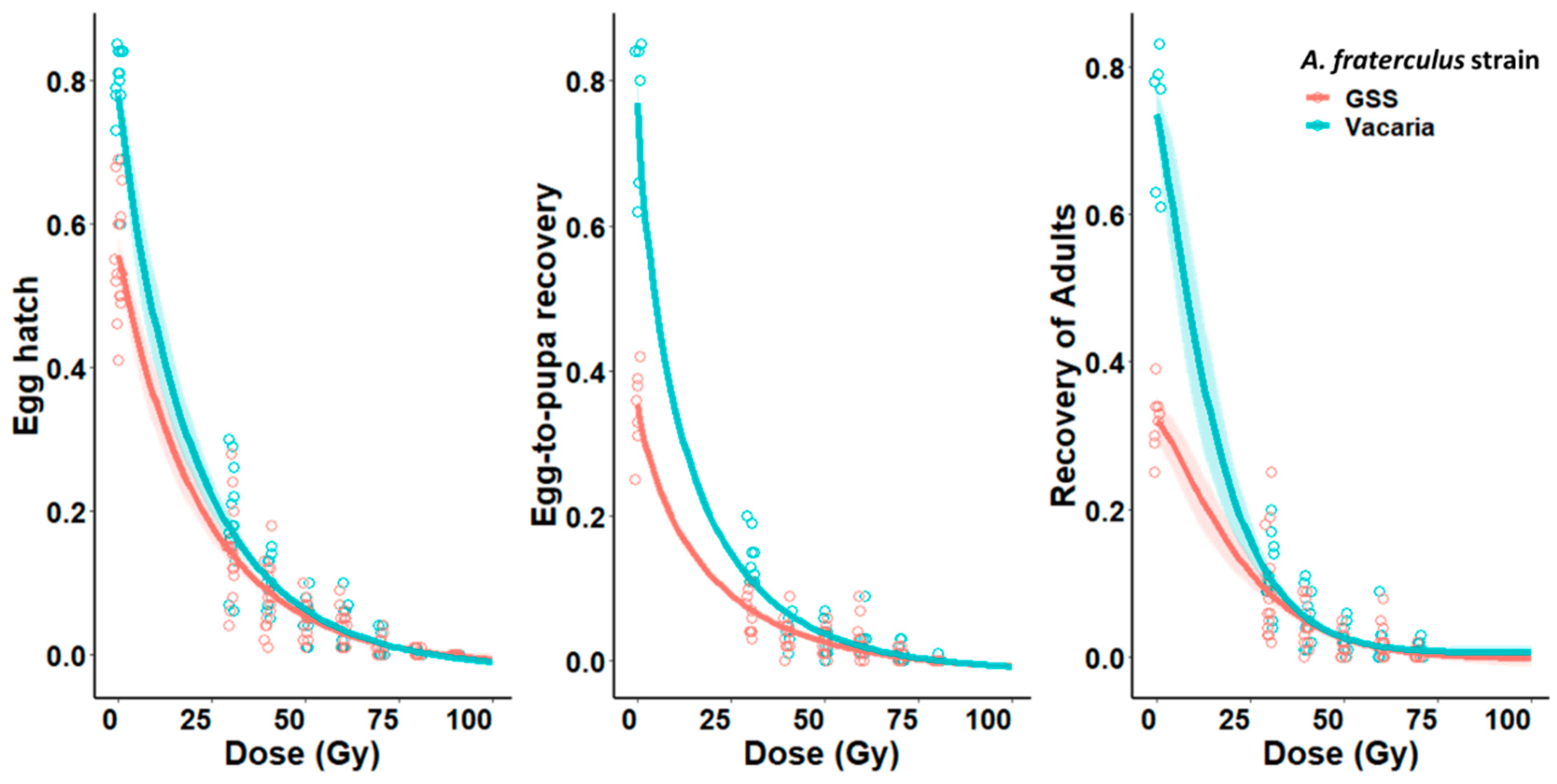
3.1. Irradiation of Males under Hypoxia
3.2. Irradiation under Hypoxia of Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raga, A.; Prestes, D.; Souza Filho, M.; Sato, M.; Siloto, R.; Guimarães, J.; Zucchi, R. Fruit fly (Diptera: Tephritoidea) infestation in citrus in the State of São Paulo, Brazil. Neotrop. Entomol. 2004, 33, 85–89. [Google Scholar] [CrossRef][Green Version]
- Prezotto, L.; Perondini, A.; Hernández-Ortiz, V.; Frías, D.; Selivon, D. What Can Integrated Analysis of Morphological and Genetic Data Still Reveal about the Anastrepha fraterculus (Diptera: Tephritidae) Cryptic Species Complex? Insects 2019, 10, 408. [Google Scholar] [CrossRef]
- Hernández-Ortiz, V.; Barradas-Juanz, N.; Díaz-Castelazo, C. A Review of the Natural Host Plants of the Anastrepha fraterculus Complex in the Americas. In Area-Wide Management of Fruit Fly Pests; Pérez-Staples, D., Díaz-Fleischer, F., Montoya, P., Vera, M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 89–122. [Google Scholar] [CrossRef]
- Fundecitrus. Fundo de Defesa da Citricultura. Estimativa da safra de laranja 2020/2021 do Cinturão Citrícola de São Paulo e Triângulo/Sudoeste Mineiro: Sumário Executivo 2020/21. Available online: Fundecitrus.com.br (accessed on 20 November 2020).
- Cladera, J.; Vilardi, J.; Juri, M.; Paulin, L.; Giardini, M.; Gómez Cendra, P.; Segura, D.; Lanzavecchia, S. Genetics and biology of Anastrepha fraterculus: Research supporting the use of the sterile insect technique (SIT) to control this pest in Argentina. BMC Genet. 2014, 15, S12. [Google Scholar] [CrossRef]
- Vera, T.; Abraham, S.; Oviedo, A.; Willink, E. Demographic and quality control parameters of Anastrepha fraterculus (Diptera: Tephritidae) maintained under artificial rearing. Fla. Entomol. 2007, 90, 53–57. [Google Scholar] [CrossRef]
- Walder, J.; Morelli, R.; Costa, K.; Faggioni, K.; Sanches, P.; Paranhos, B.; Bento, J.; Costa, M. Large scale artificial rearing of Anastrepha sp.1 aff. fraterculus (Diptera: Tephritidae) in Brazil. Sci. Agric. 2014, 71, 281–286. [Google Scholar] [CrossRef]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 355–398. [Google Scholar] [CrossRef]
- Robinson, A. Mutations and their use in insect control. Mutat. Res. Rev. Mutat. 2002, 511, 113–132. [Google Scholar] [CrossRef]
- Parker, A.G.; Vreysen, M.J.B.; Bouyer, J.; Calkins, C.O. Sterile Insect Quality Control/Assurance. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 399–440. [Google Scholar]
- Parker, A.; Mehta, K. Sterile insect technique: A model for dose optimization for improved sterile insect quality. Fla. Entomol. 2007, 90, 88–95. [Google Scholar] [CrossRef]
- Arthur, V.; Machi, A.; Mastrangelo, T. Ionizing Radiations in Entomology. In Evolution of Ionizing Radiation Research; Nenoi, M., Ed.; InTechOpen: London, UK, 2015; pp. 213–234. [Google Scholar] [CrossRef]
- López-Martínez, G.; Hahn, D. Early Life Hormetic Treatments Decrease Irradiation-Induced Oxidative Damage, Increase Longevity, and Enhance Sexual Performance during Old Age in the Caribbean Fruit Fly. PLoS ONE 2014, 9, e88128. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Ortiz, U.; Pérez-Staples, D.; Liedo, P.; Toledo, J. Sexual Competitiveness, Field Survival, and Dispersal of Anastrepha obliqua (Diptera: Tephritidae) Fruit Flies Irradiated at Different Doses. J. Econ. Entomol. 2018, 111, 761–769. [Google Scholar] [CrossRef]
- Walder, J.; Calkins, C. Effects of gamma radiation on the sterility and behavioral quality of the caribbean fruit fly, Anastrepha suspensa (Loew) (Diptera:Tephritidae). Sci. Agrícola 1993, 50, 157–165. [Google Scholar] [CrossRef]
- Allinghi, A.; Gramajo, C.; Willink, E.; Vilardi, J. Induction of sterility in Anastrepha fraterculus (Diptera: Tephritidae) by gamma radiation. Fla. Entomol. 2007, 90, 96–102. [Google Scholar] [CrossRef]
- Rull, J.; Birke, A.; Ortega, R.; Montoya, P.; López, L. Quantity and safety vs. quality and performance: Conflicting interests during mass rearing and transport affect the efficiency of sterile insect technique programs. Entomol. Exp. Appl. 2011, 142, 78–86. [Google Scholar] [CrossRef]
- Rull, J.; Encarnación, N.; Birke, A. Mass Rearing History and Irradiation Affect Mating Performance of the Male Fruit Fly, Anastrepha obliqua. J. Insect Sci. 2012, 12, 1–17. [Google Scholar] [CrossRef]
- Rull, J.; Arredondo, J.; Diaz-Fleischer, F. Improved mating performance of male Anastrepha ludens (Diptera: Tephritidae) irradiated at low doses for release in sterile insect technique programmes. Int. J. Trop. Insect Sci. 2014, 34, S28–S34. [Google Scholar] [CrossRef]
- Hooper, G.; Katiyar, K. Competitiveness of Gamma-Sterilized Males of the Mediterranean Fruit Fly1. J. Econ. Entomol. 1971, 64, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- FAO/IAEA (Food and Agriculture Organization–International Atomic Energy). Guideline for Packing, Shipping, Holding and Release of Sterile Flies in Area-Wide Fruit Fly Control Programmes, 2nd ed.; Zavala-López, J.L., Enkerlin, W.R., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; p. 140. [Google Scholar]
- Cancino, J.; López-Arriaga, F. Effect of hypoxia and its repercussions in packing pupae of the parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) for shipment. Biocontrol. Sci. Technol. 2016, 26, 665–677. [Google Scholar] [CrossRef]
- Dowell, R.; Worley, J.; Gomes, P. Supply, Emergence, and Release of Sterile Insects. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 441–484. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Kovaleski, A.; Botteon, V.; Scopel, W.; Costa, M. Optimization of the sterilizing doses and overflooding ratios for the South American fruit fly. PLoS ONE 2018, 13, e0201026. [Google Scholar] [CrossRef]
- Dias, V.; Hallman, G.; Martínez-Barrera, O.; Hurtado, N.; Cardoso, A.; Parker, A.; Caravantes, L.; Rivera, C.; Araújo, A.; Maxwell, F.; et al. Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies. Insects 2020, 11, 371. [Google Scholar] [CrossRef]
- Rendón, P.; McInnis, D.; Lance, D.; Stewart, J. Medfly (Diptera:Tephritidae) Genetic Sexing: Large-Scale Field Comparison of Males-Only and Bisexual Sterile Fly Releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef]
- Orozco, D.; Hernández, M.; Meza, J.; Quintero, J. Do sterile females affect the sexual performance of sterile males of Anastrepha ludens (Diptera: Tephritidae)? J. Appl. Entomol. 2012, 137, 321–326. [Google Scholar] [CrossRef]
- Zepeda-Cisneros, C.; Meza Hernández, J.; García-Martínez, V.; Ibañez-Palacios, J.; Zacharopoulou, A.; Franz, G. Development, genetic and cytogenetic analyses of genetic sexing strains of the Mexican fruit fly, Anastrepha ludens Loew (Diptera: Tephritidae). BMC Genet. 2014, 15, S1. [Google Scholar] [CrossRef] [PubMed]
- Meza, J.; Bourtzis, K.; Zacharopoulou, A.; Gariou-Papalexiou, A.; Cáceres, C. Development and characterization of a pupal-colour based genetic sexing strain of Anastrepha fraterculus sp. 1 (Diptera: Tephritidae). BMC Genet. 2020, 21. [Google Scholar] [CrossRef]
- Mitchell, S.; Tanaka, N.; Steiner, L.F. Methods of Mass Culturing Melon Flies and Oriental and Mediterranean Fruit Flies; U.S. Dept. of Agriculture, Agricultural Research Service; ARS: Hilo, HI, USA, 1965; pp. 33–104.
- International Atomic Energy Agency. Dosimetry System for SIT: Standard Operating Procedure for Gafchromic Þlm. International Atomic Energy Agency: Vienna, Austria. Available online: http://www.naweb.iaea.org/nafa/ipc/public/ipc-gafchromic-dosimetry-sit.html (accessed on 10 December 2020).
- Food and Agriculture Organization of the United Nations; International Atomic Energy Agency; United States Department of Agriculture. Product Quality Control for Sterile Mass-Reared and Released Tephritid Fruit Flies; Version 7.0; IAEA: Vienna, Austria, 2019; p. 148.
- Ritz, C.; Baty, F.; Streibig, J.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2—Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 10 December 2020).
- Giraud-Billoud, M.; Rivera-Ingraham, G.; Moreira, D.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernández, J.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty years of the ‘Preparation for Oxidative Stress’ (POS) theory: Ecophysiological advantages and molecular strategies. Comp. Biochem. Phys. A 2019, 234, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Greenlee, K.; Verberk, W. Functional Hypoxia in Insects: Definition, Assessment, and Consequences for Physiology, Ecology, and Evolution. Annu. Rev. Entomol. 2018, 63, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; López-Martínez, G. A dose of experimental hormesis: When mild stress protects and improves animal performance. Comp. Biochem. Phys. A 2020, 242, 110658. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, G.; Hahn, D. Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. J. Exp. Bio. 2012, 215, 2150–2161. [Google Scholar] [CrossRef]
- López-Martínez, G.; Carpenter, J.; Hight, S.; Hahn, D. Low-Oxygen Atmospheric Treatment Improves the Performance of Irradiation-Sterilized Male Cactus Moths Used in SIT. J. Econ. Entomol. 2014, 107, 185–197. [Google Scholar] [CrossRef]
- Ohinata, K.; Ashraf, M.; Harris, E. Mediterranean Fruit Flies: Sterility and Sexual Competitiveness in the Laboratory After Treatment with Gamma Irradiation in Air, Carbon Dioxide, Helium, Nitrogen or Partial Vacuum1. J. Econ. Entomol. 1977, 70, 165–168. [Google Scholar] [CrossRef]
- Fisher, K. Irradiation Effects in Air and in Nitrogen on Mediterranean Fruit Fly (Diptera: Tephritidae) Pupae in Western Australia. J. Econ. Entomol. 1997, 90, 1609–1614. [Google Scholar] [CrossRef]
- Caceres, C. Mass rearing of temperature sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata). Genetica 2002, 116, 107–116. [Google Scholar] [CrossRef]
- Orozco-Dávila, D.; Adriano-Anaya, M.; Quintero-Fong, L.; Salvador-Figueroa, M. Sterility and Sexual Competitiveness of Tapachula-7 Anastrepha ludens Males Irradiated at Different Doses. PLoS ONE 2015, 10, e0135759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robinson, A. Influence of Anoxia during Gamma Irradiation on the Fertility and Competitiveness of the Adult Male Codling Moth, Laspeyresia pomonella (L.). Radiat. Res. 1975, 61, 526. [Google Scholar] [CrossRef] [PubMed]
- Langley, P.; Curtis, C.; Brady, J. The viability, fertility and behaviour of tsetse flies (Glossina morsitans) sterilized by irradiation under various conditions. Entomol. Exp. Appl. 1974, 17, 97–111. [Google Scholar] [CrossRef]
- Sassù, F.; Nikolouli, K.; Pereira, R.; Vreysen, M.; Stauffer, C.; Cáceres, C. Irradiation dose response under hypoxia for the application of the sterile insect technique in Drosophila suzukii. PLoS ONE 2019, 14, e0226582. [Google Scholar] [CrossRef]
- Balock, J.; Burditt, A.; Christenson, L. Effects of Gamma Radiation on Various Stages of Three Fruit Fly Species. J. Econ. Entomol. 1963, 56, 42–46. [Google Scholar] [CrossRef]
- Williamson, D.; Mitchell, S.; Seo, S. Gamma Irradiation of the Mediterranean Fruit Fly (Diptera: Tephritidae): Effects of Puparial Age under Induced Hypoxia on Female Sterility. Ann. Entomol. Soc. Am. 1985, 78, 101–106. [Google Scholar] [CrossRef]
- Nestel, D.; Nemny-Lavy, E.; Mohammad Islam, S.; Wornoayporn, V.; Cáceres, C. Effects of pre-irradiation conditioning of Medfly pupae (Diptera: Tephritidae): Hypoxia and quality of sterile males. Fla. Entomol. 2007, 90, 80–87. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Parker, A.; Jessup, A.; Pereira, R.; Orozco-Dávila, D.; Islam, A.; Dammalage, T.; Walder, J. A New Generation of X Ray Irradiators for Insect Sterilization. J. Econ. Entomol. 2010, 103, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Santos, E.; Rendón, P.; Ruiz-Montoya, L.; Toledo, J.; Liedo, P. Effect of Irradiation Doses on Sterility and Biological Security in a Genetically Modified Strain of the Mediterranean Fruit Fly (Diptera: Tephritidae). J. Econ. Entomol. 2017, 110, 1483–1494. [Google Scholar] [CrossRef]
- Sayed, R.; Badr, F. Biochemical patterns of protein in gamma irradiated males of Ceratitis capitata (Wied.). Int. J. Trop. Insect Sci. 2019, 39, 295–300. [Google Scholar] [CrossRef]
- Moreno, D.; Sanchez, M.; Robacker, D.; Worley, J. Mating Competitiveness of Irradiated Mexican Fruit Fly (Diptera: Tephritidae). J. Econ. Entomol. 1991, 84, 1227–1234. [Google Scholar] [CrossRef]
- Arredondo, J.; Tejeda, M.; Ruiz, L.; Meza, J.; Pérez-Staples, D. Timing of Irradiation and Male Mating History Effects on Female Remating in Anastrepha ludens (Diptera: Tephritidae). Fla. Entomol. 2017, 100, 566–570. [Google Scholar] [CrossRef][Green Version]
- Toledo, J.; Rull, J.; Oropeza, A.; Hernández, E.; Liedo, P. Irradiation of Anastrepha obliqua (Diptera: Tephritidae) Revisited: Optimizing Sterility Induction. J. Econ. Entomol. 2004, 97, 383–389. [Google Scholar] [CrossRef]
- Rull, J.; Diaz-Fleischer, F.; Arredondo, J. Irradiation of Anastrepha ludens (Diptera: Tephritidae) Revisited: Optimizing Sterility Induction. J. Econ. Entomol. 2007, 100, 1153–1159. [Google Scholar] [CrossRef]
- Costa, K.; Costa, M.; Botteon, V.; Faggioni, K.; Costa, N.; Mastrangelo, T. Quality control and characterization of the testicles and ovaries of irradiated Anastrepha obliqua from Brazil. Sci. Agr. 2020, 77, 1–7. [Google Scholar] [CrossRef]
- Toledo-Arreola, J. Dosis Óptima de Irradiación a Pupas de Anastrepha serpentina (Wiedemann) (Diptera: Tephritidae) Para la Obtención de Adultos Estériles Sexualmente Competitivos. Master’s Thesis, Instituto Tecnológico y de Estudios Superiores de Monterrey, Tecnológico, Mexico, 1992. [Google Scholar]
- Landeta-Escamilla, A.; Hernández, E.; Arredondo, J.; Díaz-Fleischer, F.; Pérez-Staples, D. Male irradiation affects female remating behavior in Anastrepha serpentina (Diptera: Tephritidae). J. Insect Physiol. 2016, 85, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, J.; Ruiz, L.; Montoya, P.; Díaz-Fleischer, F. Packing and Postirradiation Handling of the Anastrepha ludens (Diptera: Tephritidae) Tapachula-7 Genetic Sexing StraIn Combined Effects of Hypoxia, Pupal Size, and Temperature on Adult Quality. J. Econ. Entomol. 2018, 111, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Benelli, M.; Mainali, B.; Taylor, P.; Rempoulakis, P. Reduced quality of sterile Queensland fruit fly following post-production stress from hypoxia, irradiation and vibration. J. Pest Sci. 2020, 94, 473–485. [Google Scholar] [CrossRef]
| Dose (Gy) | Biological Parameters | |||||
|---|---|---|---|---|---|---|
| Egg Hatch (%) | Egg-to-Pupa Recovery (%) | Recovery of Adults (%) | ||||
| VAC | GSS | VAC | GSS | VAC | GSS | |
| 0 | 80.3 ± 2.8 | 51.5 ± 1.2 | 76.6 ± 4.1 | 35.5 ± 1.9 | 73.5 ± 3.8 | 32.1 ± 1.5 |
| 30 | 18.03 ± 2.1 | 13.9 ± 1.6 | 12.1 ± 1.5 | 9.7 ± 1.8 | 10.9 ± 1.3 | 9.2 ± 1.7 |
| 40 | 9.3 ± 0.8 | 7.9 ± 1.2 | 5.2 ± 0.9 | 4.4 ± 0.8 | 4.8 ± 0.8 | 4.0 ± 0.7 |
| 50 | 5.2 ± 0.7 | 5.2 ± 0.7 | 2.6 ± 0.5 | 2.4 ± 0.5 | 2.3 ± 0.4 | 2.0 ± 0.4 |
| 60 | 3.9 ± 0.7 | 3.9 ± 0.6 | 2.03 ± 0.5 | 2.4 ± 0.7 | 1.7 ± 0.6 | 1.9 ± 0.6 |
| 70 | 1.7 ± 0.4 | 1.1 ± 0.3 | 0.73 ± 0.3 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.2 ± 0.15 |
| 80 | 0.13 ± 0.1 | 0.17 ± 0.06 | 0.07 ± 0.05 | 0 | 0.03 ± 0.03 | 0 |
| 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Weibull model | y = (0.78)exp(exp(0.92(log(x) − log(20.6)))) | y = (0.55)exp(exp(0.96(log(x) − log(23.1)))) | y = (0.77)exp(exp(0.77(log(x) − log(13.9)))) | y = (0.35)exp(exp(0.82(log(x) − log(19.1)))) | y = (0.74)exp(exp(1.18(log(x) − log(17.1)))) | y = (0.32) exp(exp (1.26 (log(x) − log(24.7)))) |
| Parameter/Strain | D50 (95% CI) † | D90 (95% CI) | D99 (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Egg hatch | VAC | 8.1 (5.7; 10.6) | t = 8.45 p < 10−3 | 41.1 (37.02; 45.1) | t = 1.4 ns § p = 0.15 | 74.05 (57.9; 90.2) | t = 0.0043 ns p = 0.99 |
| GSS | 2.2 (0.9; 3.4) | 37.4 (33.9; 40.8) | 74.1 (58.9; 89.3) | ||||
| Egg-to-Pupa Recovery | VAC | 4.5 (4.3; 4.8) | --- | 32.3 (32.2; 32.5) | t = 57.8 p < 10−3 | 68.5 (66.8; 70.2) | t = 3.7 p = 0.0002 |
| GSS | NE ‡ | 23.1 (22.8; 23.4) | 64.6 (63.2; 65.9) | ||||
| Recovery of Adults | VAC | 7.7 (4.3; 11.1) | --- | 31.3 (29.3; 33.3) | t = 2.1 p = 0.04 | 68.6 (51.1; 86.1) | t = 0.46 ns p = 0.64 |
| GSS | NE | 27.6 (24.5; 30.7) | 62.9 (45.4; 80.5) | ||||
| Dose (Gy) | Biological Parameters | |||
|---|---|---|---|---|
| Adult Emergence (%) | Sex Ratio (♀/♂ + ♀) | |||
| VAC | GSS | VAC | GSS | |
| 0 | 96.1 ± 1.1 | 90.8 ± 1.5 | 0.4 ± 0.01 (n = 1323) | 0.44 ± 0.02 (n = 770) |
| 30 | 92.04 ± 2.1 | 92.6 ± 2.4 | 0.4 ± 0.03 (n = 329) | 0.46 ± 0.04 (n = 275) |
| 40 | 93.5 ± 1.5 | 83.9 ± 7.0 | 0.4 ± 0.1 (n = 143) | 0.5 ± 0.05 (n = 120) |
| 50 | 83.9 ± 6.6 | 69.9 ± 9.7 | 0.39 ± 0.1 (n = 68) | 0.28 ± 0.07 (n = 60) |
| 60 | 61.8 ± 9.7 | 66.1 ± 9.5 | 0.38 ± 0.1 (n = 50) | 0.38 ± 0.1 (n = 58) |
| 70 | 40.6 ± 11.9 | 17.8 ± 9.7 | 0.19 ± 0.1 (n = 18) | 0.02 ± 0.01 (n = 7) |
| 80 | 6.7 ± 6.6 | 0 | 0.07 ± 0.06 (n = 1) | NE |
| 90 | 0 | 0 | NE † | NE |
| 100 | 0 | 0 | NE | NE |
| Kruskal-Wallis Analysis of Variance | χ2 = 77.4 p < 10−3 | χ2 = 83.6 p < 10−3 | χ2 = 57.9 p < 10−3 | χ2 = 86.5 p < 10−3 |
| Strain | Dose (Gy) | Biological Parameters | ||||
|---|---|---|---|---|---|---|
| Egg Hatch (%) | Egg-to-Pupa Recovery (%) | Recovery of Adults (%) | Adult Emergence (%) | Sex Ratio (♀/♂ + ♀) | ||
| VAC | 0 | 78.3 ± 1.6 | 76.6 ± 2.6 | 73.5 ± 3.3 | 96.1 ± 0.7 | 0.4 ± 0.01 (n = 1324) |
| 30 | 27.2 ± 3.9 | 18.9 ± 2.4 | 18.0 ± 2.2 | 95.6 ± 1.5 | 0.5 ± 0.03 (n = 540) | |
| 40 | 10.2 ± 5.1 | 0.1 ± 0.05 | 0.1 ± 0.05 | 6.7 ± 6.6 | 0.07 ± 0.06 (n = 3) | |
| 50 | 0 | 0 | 0 | 0 | NE † | |
| 60 | 0 | 0 | 0 | 0 | NE | |
| Kruskal–Wallis Analysis of Variance | χ2 = 63.6 p < 10−3 | χ2 = 54.2 p < 10−3 | χ2 = 59.5 p < 10−3 | χ2 = 55.9 p < 10−3 | χ2 = 55.8 p < 10−3 | |
| GSS | 0 | 74.7 ± 1.9 | 73.1 ± 2.5 | 69.3 ± 2.8 | 94.7 ± 1.4 | 0.39 ± 0.01 (n = 1662) |
| 30 | 16.9 ± 2.7 | 14.3 ± 2.7 | 13.6 ± 2.6 | 80.1 ± 8.7 | 0.42 ± 0.06 (n = 407) | |
| 40 | 26.1 ± 10.3 | 0.2 ± 0.11 | 0.2 ± 0.1 | 6.7 ± 6.6 | 0.02 ± 0.01 (n = 6) | |
| 50 | 0 | 0 | 0 | 0 | NE | |
| 60 | 0 | 0 | 0 | 0 | NE | |
| Kruskal–Wallis Analysis of Variance | χ2 = 54.3 p < 10−3 | χ2 = 55.3 p < 10−3 | χ2 = 54.3 p < 10−3 | χ2 = 50.5 p < 10−3 | χ2 = 50.7 p < 10−3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giustina, P.D.; Mastrangelo, T.; Ahmad, S.; Mascarin, G.; Caceres, C. Determining the Sterilization Doses under Hypoxia for the Novel Black Pupae Genetic Sexing Strain of Anastrepha fraterculus (Diptera, Tephritidae). Insects 2021, 12, 308. https://doi.org/10.3390/insects12040308
Giustina PD, Mastrangelo T, Ahmad S, Mascarin G, Caceres C. Determining the Sterilization Doses under Hypoxia for the Novel Black Pupae Genetic Sexing Strain of Anastrepha fraterculus (Diptera, Tephritidae). Insects. 2021; 12(4):308. https://doi.org/10.3390/insects12040308
Chicago/Turabian StyleGiustina, Paloma Della, Thiago Mastrangelo, Sohel Ahmad, Gabriel Mascarin, and Carlos Caceres. 2021. "Determining the Sterilization Doses under Hypoxia for the Novel Black Pupae Genetic Sexing Strain of Anastrepha fraterculus (Diptera, Tephritidae)" Insects 12, no. 4: 308. https://doi.org/10.3390/insects12040308
APA StyleGiustina, P. D., Mastrangelo, T., Ahmad, S., Mascarin, G., & Caceres, C. (2021). Determining the Sterilization Doses under Hypoxia for the Novel Black Pupae Genetic Sexing Strain of Anastrepha fraterculus (Diptera, Tephritidae). Insects, 12(4), 308. https://doi.org/10.3390/insects12040308






