Ultrastructure of the Olfactory Sensilla across the Antennae and Maxillary Palps of Bactrocera dorsalis (Diptera: Tephritidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Field Emission Scanning Electron Microscope (FESEM)
2.3. Transmission Electron Microscope (TEM)
2.4. Statistical Analyses
3. Results
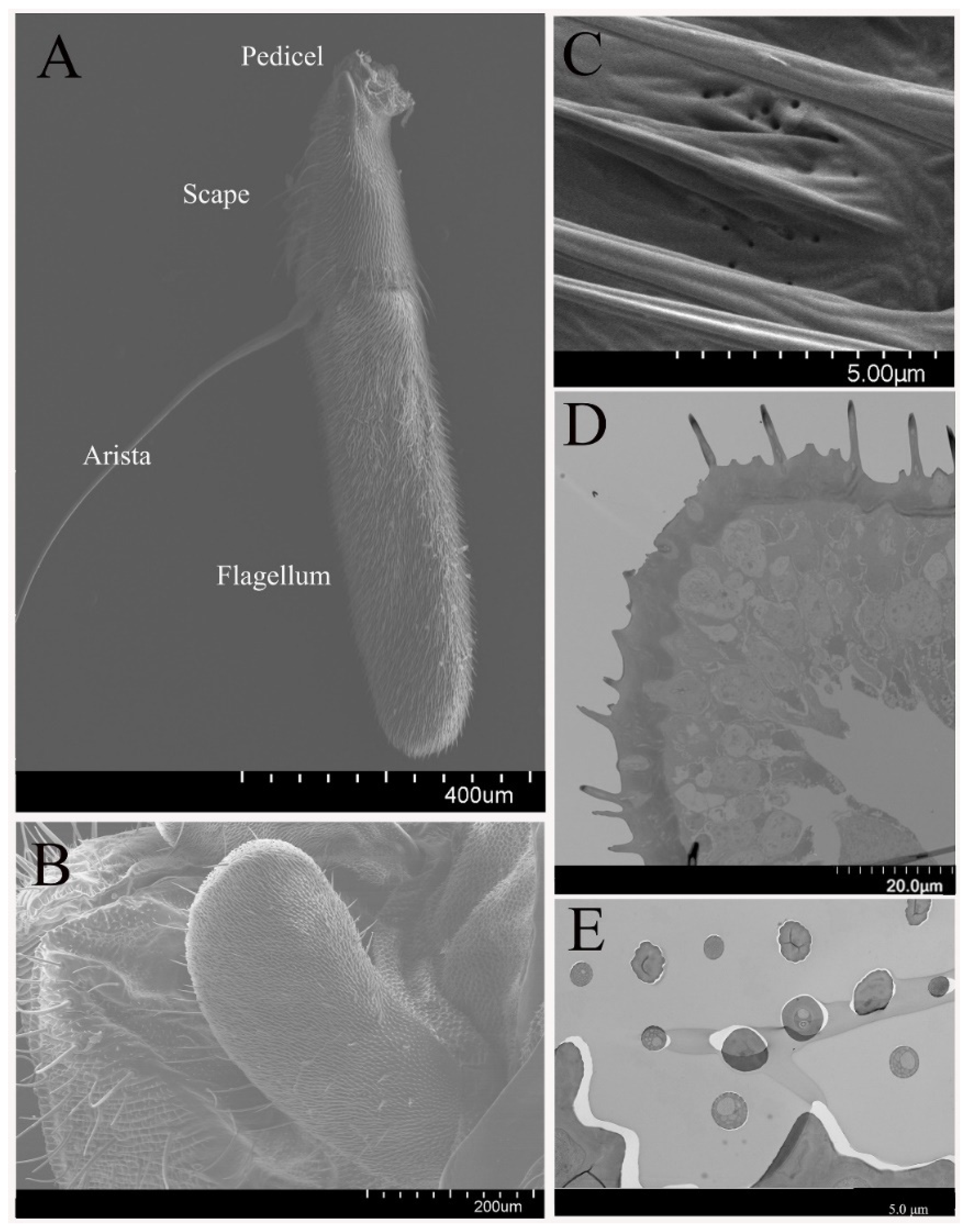
3.1. General Morphology of Antenna and Maxillary Palps
3.2. Sensilla on the Antenna and Maxillary Palp
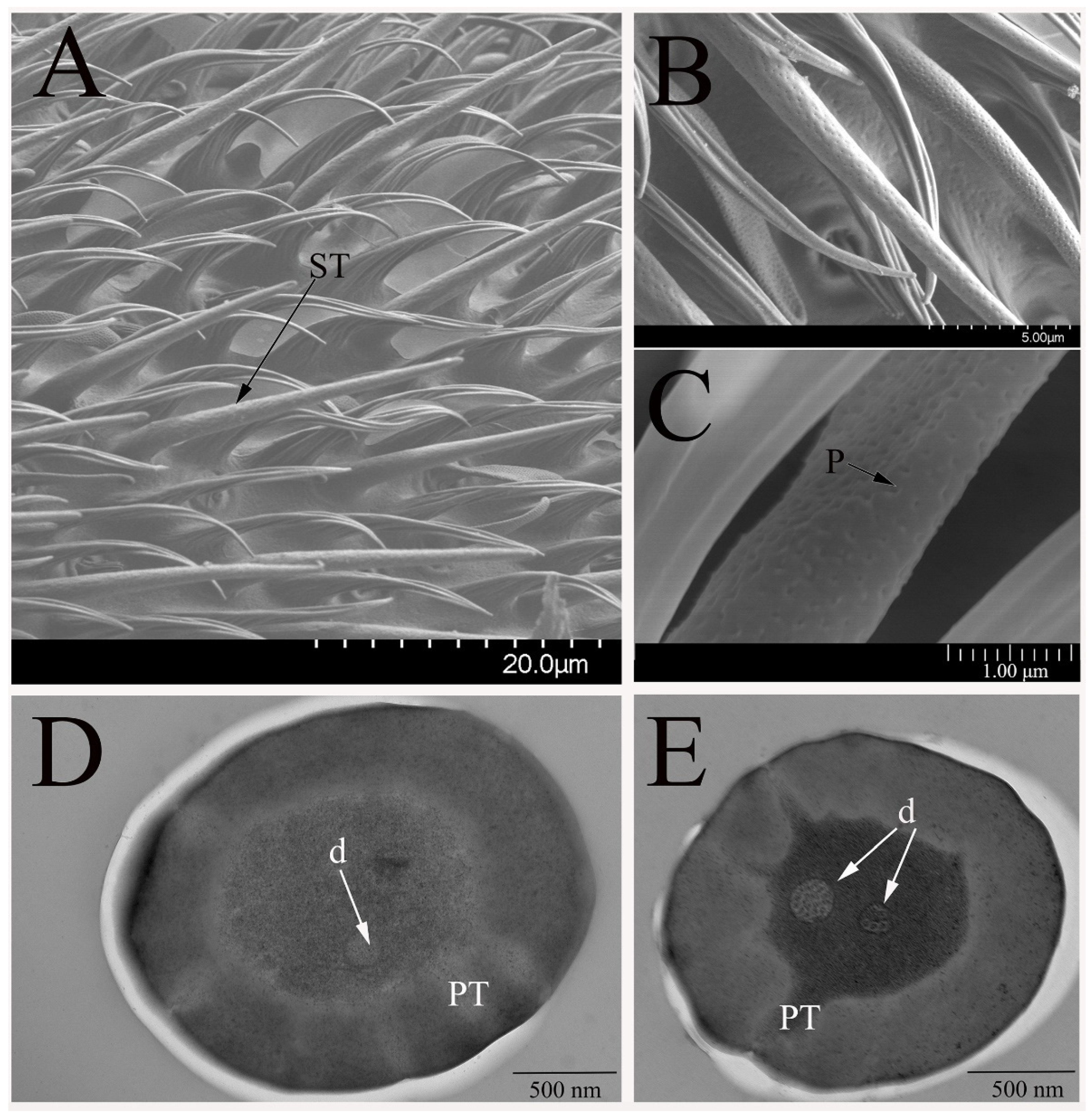
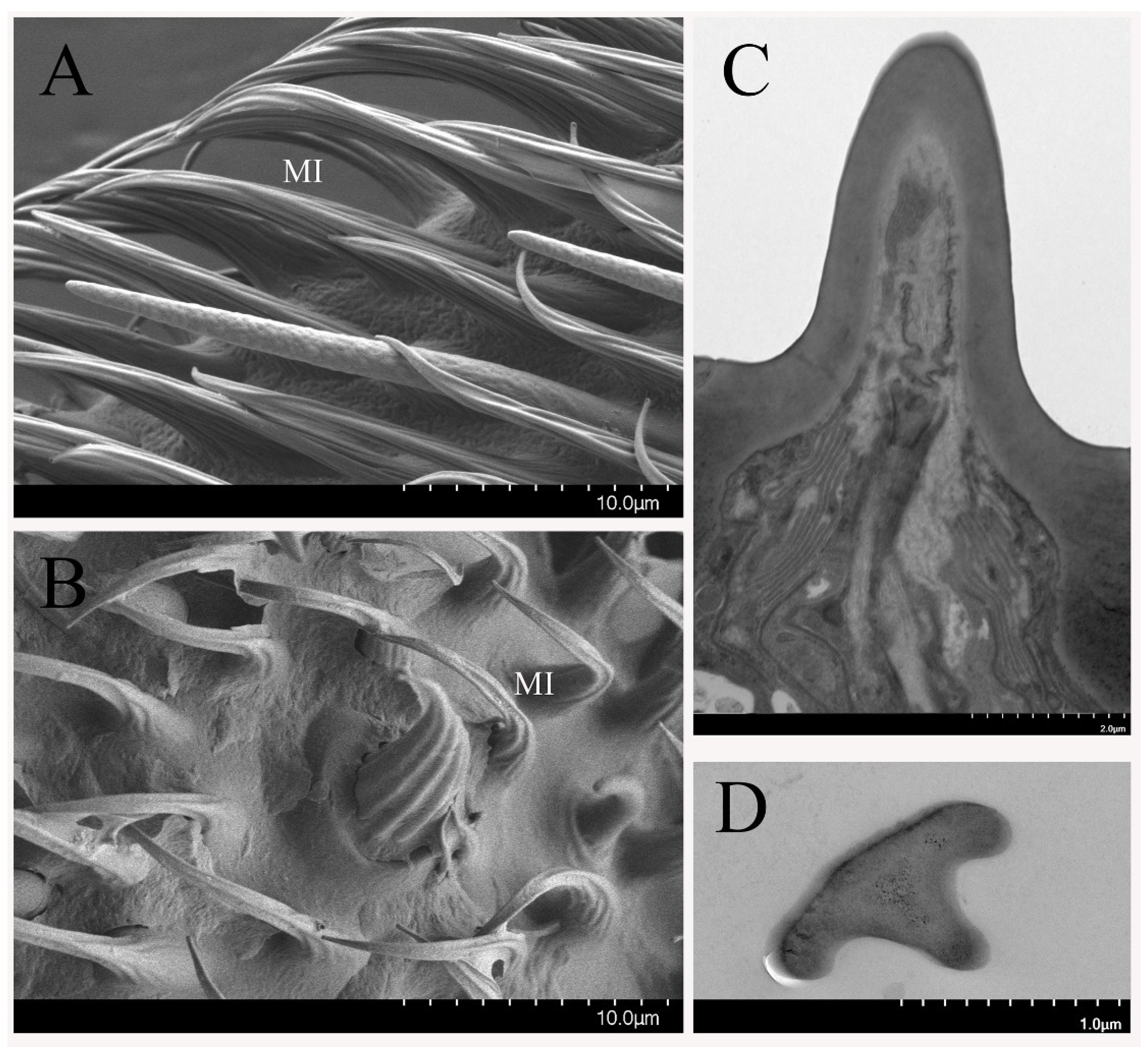
3.2.1. Sensilla Trichodea
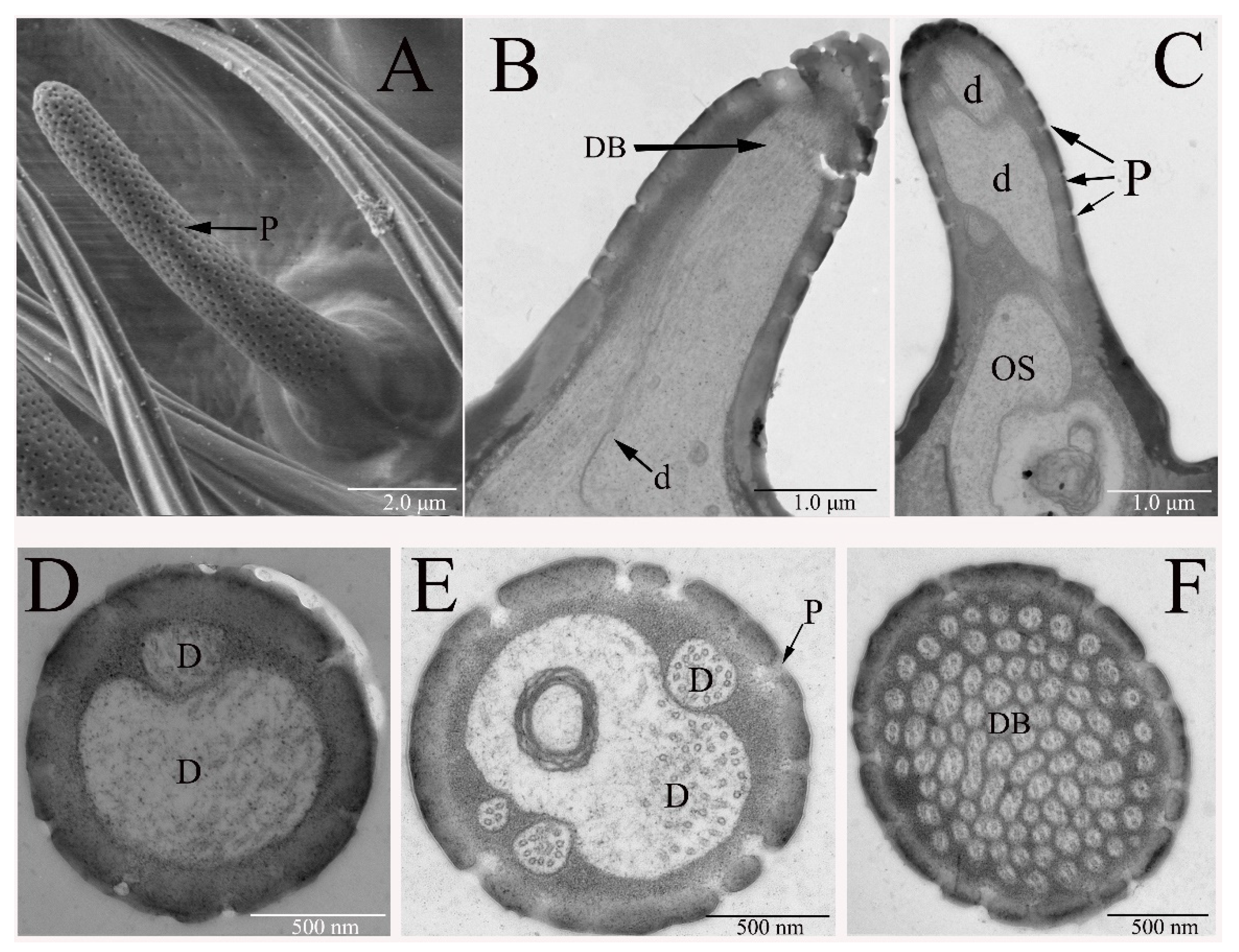
3.2.2. Sensilla Basiconica
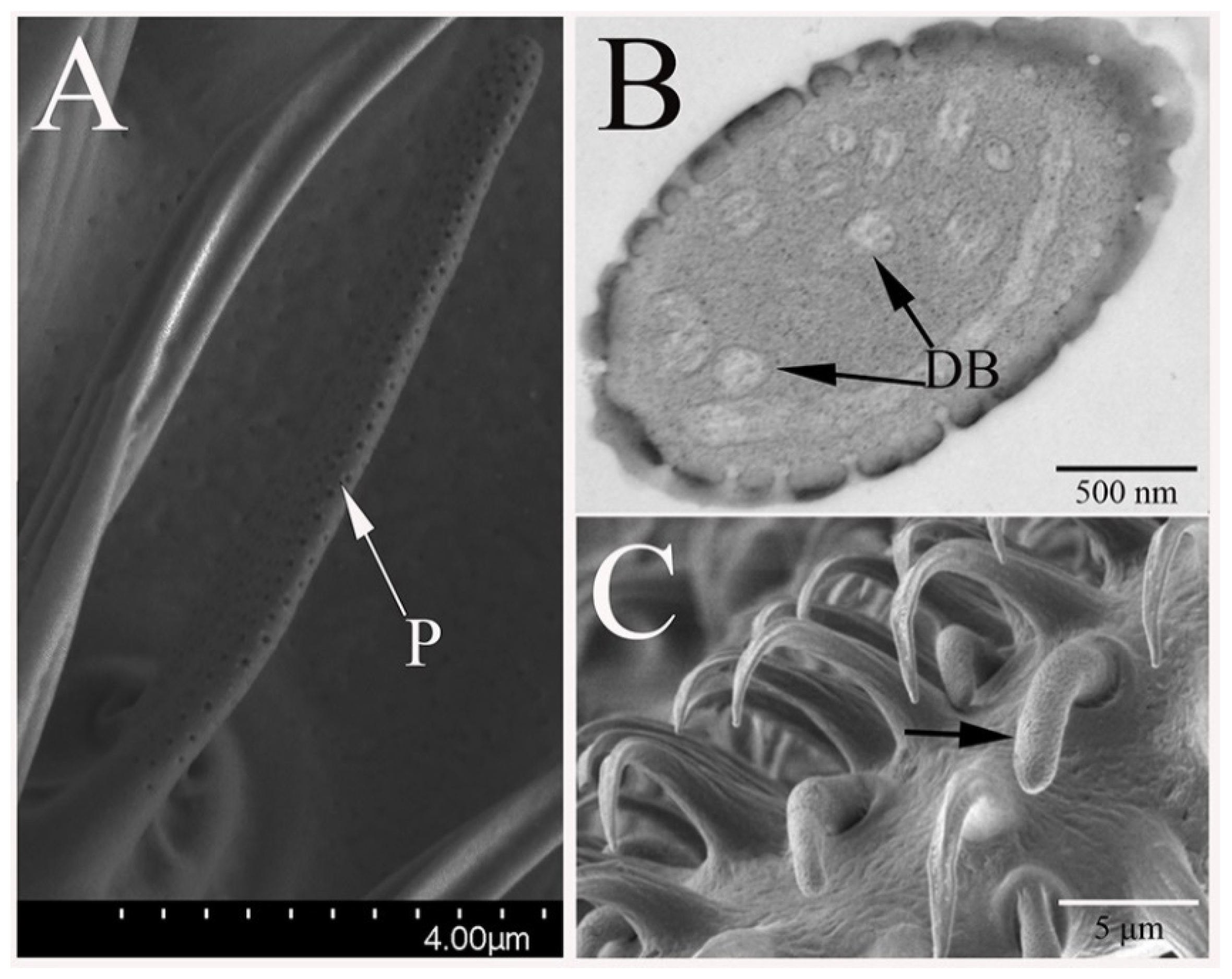
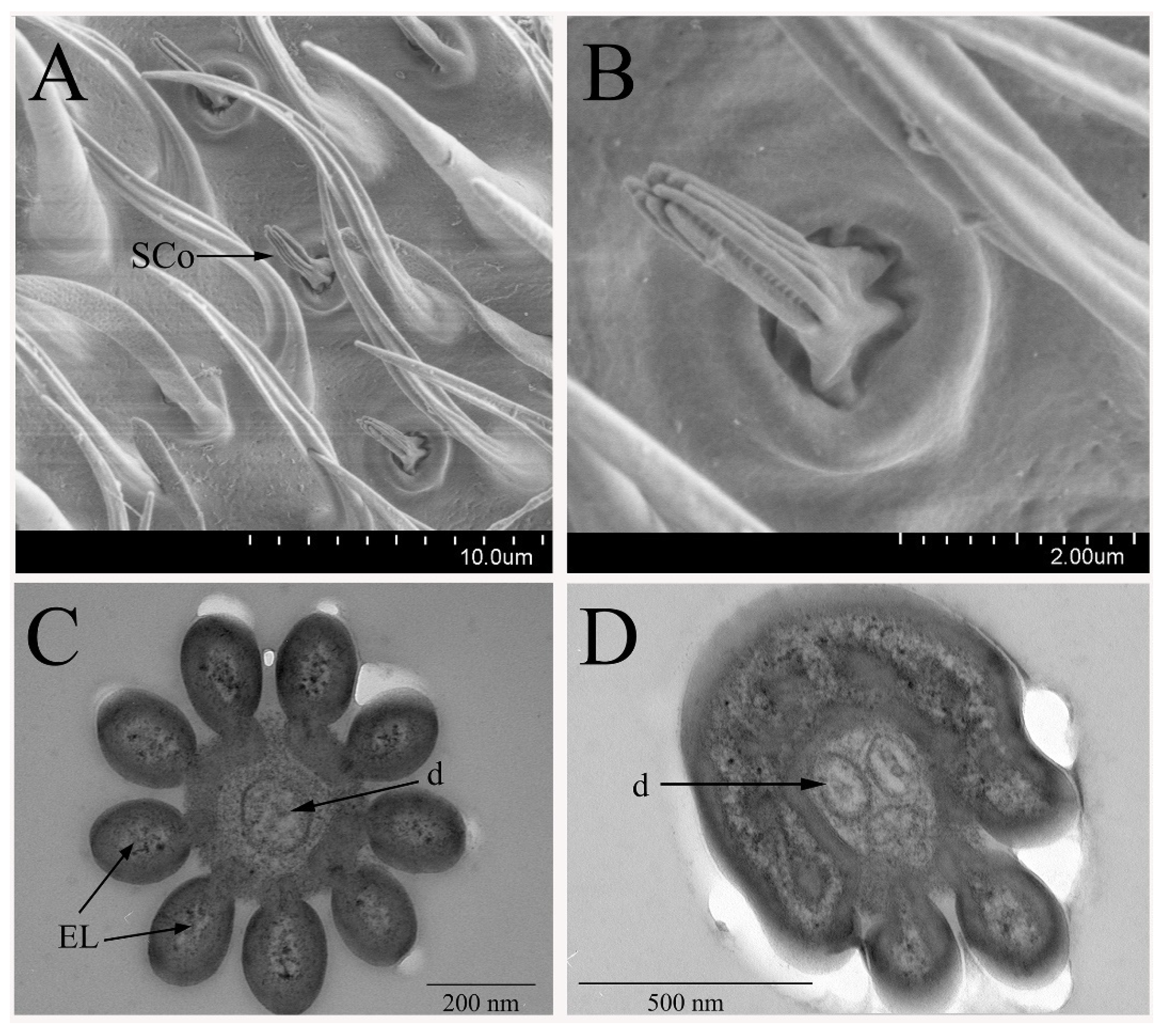
3.2.3. Sensilla Coeloconica
3.2.4. Non-Olfactory Sensilla
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Zhang, D.J.; Xu, Y.J.; Wang, L.; Cheng, D.F.; Qi, Y.X.; Zeng, L.; Lu, Y.Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Inter. Agric. 2019, 18, 771–787. [Google Scholar] [CrossRef]
- Wei, D.; Dou, W.; Jiang, M.X.; Wang, J.J. Oriental fruit fly Bactrocera dorsalis (Hendel). In Biological Invasions and Its Management in China; Wang, F.H., Jiang, M.X., Zhang, A.B., Eds.; Springer: Cham, Switzerland, 2017; pp. 267–283. [Google Scholar] [CrossRef]
- Onagbola, E.O.; Meyer, W.L.; Boina, D.R.; Stelinski, L.L. Morphological characterization of the antennal sensilla of the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), with reference to their probable functions. Micron 2008, 39, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Li, R.T.; Huang, L.Q.; Dong, J.F.; Wang, C.Z. A moth odorant receptor highly expressed in the ovipositor is involved in detecting host-plant volatiles. eLife 2020, 9, e53706. [Google Scholar] [CrossRef]
- Visser, H. Host odor perception in pytophagous isects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Li, Y.P.; Du, X.; Liu, F.F.; Li, Y.; Liu, T.X. Ultrastructure of the sensilla on antennae and mouthparts of larval and adult Plutella xylostella (Lepidoptera: Plutellidae). J. Inter. Agric. 2018, 17, 1409–1420. [Google Scholar] [CrossRef]
- Seada, M.; Hamza, A. Differential morphology of the sensory sensilla of antennae, palpi, foretarsi and ovipositor of adult Tribolium castaneum (Herbst) (Coleoptera:Tenebrionidae). Ann. Agric. Sci. 2018, 63, 1–8. [Google Scholar] [CrossRef]
- Pezzi, M.; Scapoli, C.; Mamolini, E.; Leis, M.; Bonacci, T.; Whitmore, D.; Krčmar, S.; Furini, M.; Giannerini, S.; Chicca, M.; et al. Ultrastructural characterization of sensilla and microtrichia on the antenna of female Haematopota pandazisi (Diptera: Tabanidae). Parasitol. Res. 2018, 117, 959–970. [Google Scholar] [CrossRef]
- Zheng, W.W.; Peng, W.; Zhu, C.P.; Zhang, Q.; Saccone, G.; Zhang, H.Y. Identification and expression profile analysis of odorant binding proteins in the oriental fruit fly Bactrocera dorsalis. Int. J. Mol. Sci. 2013, 14, 14936–14949. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, H.M.; Wang, P.D.; Hu, M.Y.; Zhong, G.H. Bdor\Orco is important for oviposition-deterring behavior induced by both the volatile and non-volatile repellents in Bactrocera dorsalis (Diptera: Tephritidae). J. Insect Physiol. 2014, 65, 51–56. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Zhang, H.; Wang, Z.B.; Bin, S.Y.; He, H.L.; Lin, J.T. Discovery of chemosensory genes in the oriental fruit Fly, Bactrocera dorsalis. PLoS ONE 2015, 10, e0129794. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Xu, L.; Zhang, Y.X.; Wei, D.; Wang, J.J.; Jiang, H.B. Genome-wide identification and expression profiling of odorant-binding proteins in the oriental fruit fly, Bactrocera dorsalis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100605. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, X.F.; Xu, L.; Keesey, I.; Lei, Z.R.; Smagghe, G.; Wang, J.J. An antennae-specific odorant-binding protein is involved in Bactrocera dorsalis olfaction. Front. Ecol. Evol. 2020, 8, 63. [Google Scholar] [CrossRef]
- Wen-Yung, L.; Jui-Chun, C.; Tai-Lang, L.; Yu-Bing, H. Ultrastructure of the antennal sensilla of the oriental fruit-fly Bactrocera (=Dacus) dorsalis (Hendel) (Diptera: Tephritidae). Zool. Stud. 1995, 34, 21–28. [Google Scholar]
- Hu, F.; Zhang, G.N.; Jia, F.X.; Dou, W.; Wang, J.J. Morphological characterization and distribution of antennal sensilla of six fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2010, 103, 661–670. [Google Scholar] [CrossRef]
- Castrejon-Gomez, V.R.; Rojas, J.C. Antennal sensilla of Anastrepha serpentina (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2009, 102, 310–316. [Google Scholar] [CrossRef]
- Degwi, M.S.E.; Zahran, N.F. Morphological effect of gamma radiation on the antennal sensory structure of peach fruit fly, Bactrocera zonata, using electron microscopy. Entomol. News 2019, 128, 177–187. [Google Scholar] [CrossRef]
- Zhang, G.N.; Hull-Sanders, H.; Hu, F.; Dou, W.; Niu, J.Z.; Wang, J.J. Morphological characterization and distribution of sensilla on maxillary palpi of six Bactrocera fruit flies (Diptera: Tephritidae). FLA Entomol. 2011, 97, 379–388. [Google Scholar] [CrossRef]
- Oh, H.W.; Jeong, S.A.; Kim, J.; Park, K.C. Morphological and functional heterogeneity in olfactory perception between antennae and maxillary palps in the pumpkin fruit fly, Bactrocera depressa. Arch. Insect Biochem. Physiol. 2019, 101, e21560. [Google Scholar] [CrossRef]
- Sukontason, K.; Sukontason, K.L.; Piangjai, S.; Boonchu, N.; Chaiwong, T.; Ngern-Klun, R.; Sripakdee, D.; Vogtsberger, R.C.; Olson, J.K. Antennal sensilla of some forensically important flies in families Calliphoridae, Sarcophagidae and Muscidae. Micron 2004, 35, 671–679. [Google Scholar] [CrossRef]
- Sukontason, K.; Methanitikorn, R.; Chaiwong, T.; Kurahashi, H.; Vogtsberger, R.C.; Sukontason, K.L. Sensilla of the antenna and palp of Hydrotaea chalcogaster (Diptera: Muscidae). Micron 2007, 38, 218–223. [Google Scholar] [CrossRef]
- Arzuffi, R.; Robledo, N.; Valdez, J. Antennal sensilla of Toxotrypana curvicauda (Diptera: Tephritidae). FLA Entomol. 2008, 91, 669–673. [Google Scholar]
- Sun, L.; Wang, Q.; Zhang, Y.; Tu, X.; Yan, Y.; Wang, Q.; Dong, K.; Zhang, Y.; Xiao, Q. The sensilla trichodea-biased EoblPBP1 binds sex pheromones and green leaf volatiles in Ectropis obliqua Prout, a geometrid moth pest that uses type-II sex pheromones. J. Insect Physiol. 2019, 116, 17–24. [Google Scholar] [CrossRef]
- Chen, L.; Fadamiro, H.Y. Antennal sensilla of the decapitating phorid fly, Pseudacteon tricuspis (Diptera: Phoridae). Micron 2008, 39, 517–525. [Google Scholar] [CrossRef]
- Wang, S.N.; Shan, S.; Liu, J.T.; Li, R.J.; Lu, Z.Y.; Dhiloo, K.H.; Khashaveh, A.; Zhang, Y.J. Characterization of antennal chemosensilla and associated odorant binding as well as chemosensory proteins in the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidae). Sci. Rep. 2018, 8, 7649. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Q.; Yang, S.; Wang, Q.; Zhang, Z.; Khashaveh, A.; Zhang, Y.J.; Guo, Y.Y. Functional analysis of female-biased odorant binding protein 6 for volatile and nonvolatile host compounds in Adelphocoris lineolatus (Goeze). Insect Mol. Biol. 2017, 26, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Shields, V.D.C.; Hildebrand, J.G. Fine structure of antennal sensilla of the female sphinx moth, Manduca sexta (Lepidoptera: Sphingidae). II. Auriculate, coeloconic, and styliform complex sensilla. Can. J. Zool. 1999, 77, 302–313. [Google Scholar] [CrossRef]
- Xiao, S.; Sun, J.S.; Carlson, J.R. Robust olfactory responses in the absence of odorant binding proteins. eLife 2019, 8, e51040. [Google Scholar] [CrossRef]
- Lopes, O.; Barata, E.N.; Mustaparta, H.; Araújo, J. Fine structure of antennal sensilla basiconica and their detection of plant volatiles in the eucalyptus woodborer, Phoracantha semipunctata Fabricius (Coleoptera: Cerambycidae). Arthropod. Struct. Dev. 2002, 31, 1–13. [Google Scholar] [CrossRef]
- Clyne, P.; Grant, A.; O’Connell, R.; Carlson, J.R. Odorant response of individual sensilla on the Drosophila antenna. Invertebr. Neurosci. 1997, 3, 127–135. [Google Scholar] [CrossRef]
- Sukontason, K.; Sukontason, K.L.; Vogtsberger, R.C.; Boonchu, N.; Chaiwong, T.; Piangjai, S.; Disney, H. Ultrastructure of coeloconic sensilla on postpedicel and maxillary palp of Megaselia scalaris (Diptera: Phoridae). Ann. Entomol. Soc. Am. 2005, 98, 113–118. [Google Scholar] [CrossRef][Green Version]
- Jeong, S.A.; Kim, J.; Byun, B.K.; Oh, H.W.; Park, K.C. Morphological and ultrastructural characterization of olfactory sensilla in Drosophila suzukii: Scanning and transmission electron microscopy. J. Asia-Pac. Entomol. 2020, 23, 1165–1180. [Google Scholar] [CrossRef]
- Menuz, K.; Larter, N.K.; Park, J.; Carlson, J.R. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 2014, 10, e1004810. [Google Scholar] [CrossRef]
- Nemeth, D.C.; Ammagarahalli, B.; Layne, J.E.; Rollmann, S.M. Evolution of coeloconic sensilla in the peripheral olfactory system of Drosophila mojavensis. J. Insect Physiol. 2018, 110, 13–22. [Google Scholar] [CrossRef]
- Davis, E.E.; Sokolove, P.G. Temperature responses of antennal receptors of the mosquito Aedes aegypti. J. Comp. Physiol. 1975, 6, 223–236. [Google Scholar] [CrossRef]
- Bruyne, M.D.; Clyne, P.J.; Carlson, J.R. Odor coding in a model olfactory organ: The Drosophila maxillary palp. J. Neurosurg. Sci. 1999, 19, 4520–4532. [Google Scholar]
- Wasserman, S.; Itagaki, H. The olfactory responses of the antenna and maxillary palp of the fleshfly, Neobellieria bullata (Diptera: Sarcophagidae), and their sensitivity to blockage of nitric oxide synthase. J. Insect Physiol. 2003, 49, 271–280. [Google Scholar] [CrossRef]
- Kelling, F.J.; Biancaniello, G.; den Otter, C.J. Electrophysiological characterization of olfactory cell types in the antennae and palps of the housefly. J. Insect Physiol. 2002, 48, 997–1008. [Google Scholar] [CrossRef]

| Sex | Antenna | Maxillary Palps | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scape | Pedicel | Flagellum | ||||||||
| St | Sc | St | Sc | St | Sb | Sco | Sc | St | Sb | |
| ♂ | 321 ± 13 | 19 ± 1 | 793 ± 30 | 70 ± 5 | 251 ± 17 | 90 ± 5 | 12 ± 1 | 10 ± 2 | 501 ± 15 * | 70 ± 9 |
| ♀ | 305 ± 17 | 20 ± 1 | 866 ± 36 * | 68 ± 5 | 250 ± 10 | 87 ± 6 | 12 ± 1 | 10 ± 2 | 421 ± 28 | 69 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Hu, T.; Guo, H.-W.; Liang, X.-F.; Cheng, Y.-Q. Ultrastructure of the Olfactory Sensilla across the Antennae and Maxillary Palps of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2021, 12, 289. https://doi.org/10.3390/insects12040289
Liu Z, Hu T, Guo H-W, Liang X-F, Cheng Y-Q. Ultrastructure of the Olfactory Sensilla across the Antennae and Maxillary Palps of Bactrocera dorsalis (Diptera: Tephritidae). Insects. 2021; 12(4):289. https://doi.org/10.3390/insects12040289
Chicago/Turabian StyleLiu, Zhao, Ting Hu, Huai-Wang Guo, Xiao-Fei Liang, and Yue-Qing Cheng. 2021. "Ultrastructure of the Olfactory Sensilla across the Antennae and Maxillary Palps of Bactrocera dorsalis (Diptera: Tephritidae)" Insects 12, no. 4: 289. https://doi.org/10.3390/insects12040289
APA StyleLiu, Z., Hu, T., Guo, H.-W., Liang, X.-F., & Cheng, Y.-Q. (2021). Ultrastructure of the Olfactory Sensilla across the Antennae and Maxillary Palps of Bactrocera dorsalis (Diptera: Tephritidae). Insects, 12(4), 289. https://doi.org/10.3390/insects12040289






