Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
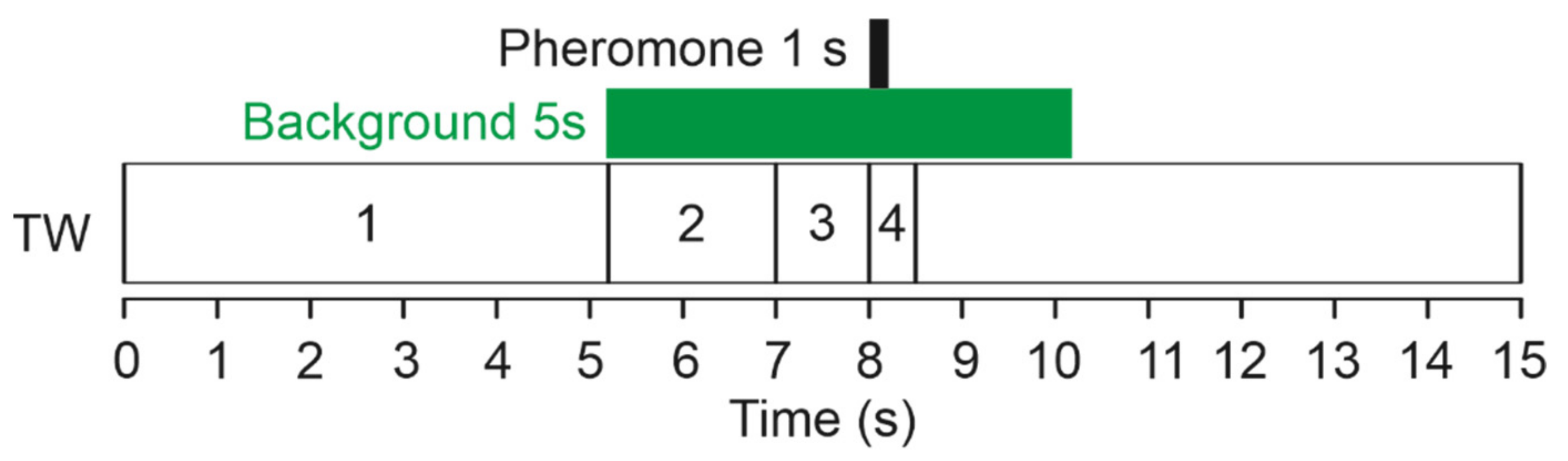
2.3. Odor Stimulus Delivery
2.4. Odor Stimuli
2.5. Electrophysiology
2.6. Calcium Imaging in Antennal Lobes
2.7. Analysis of Electrophysiological Data
3. Results
3.1. Calcium Responses to Single VPCs in the MGC
3.2. Some Single VPCs Activate MGC Neurons and Affect Their Responses to Z7-12:Ac
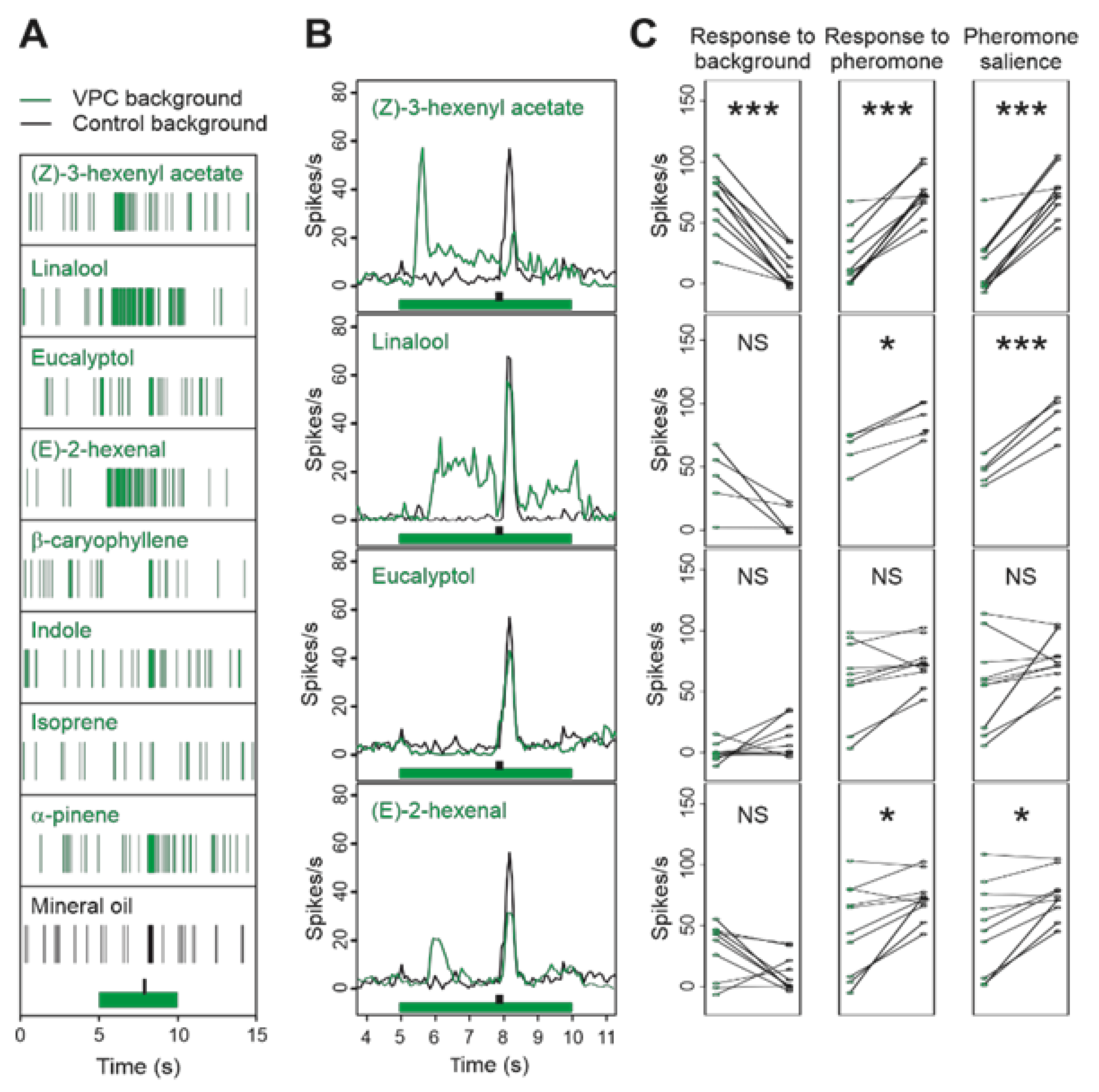
3.3. Single VPCs Modulate the Z7-ORN Spontaneous Firing and Affect Their Responses to Pheromone
3.4. Binary Blends Produce the Effects of Their Most Active Component
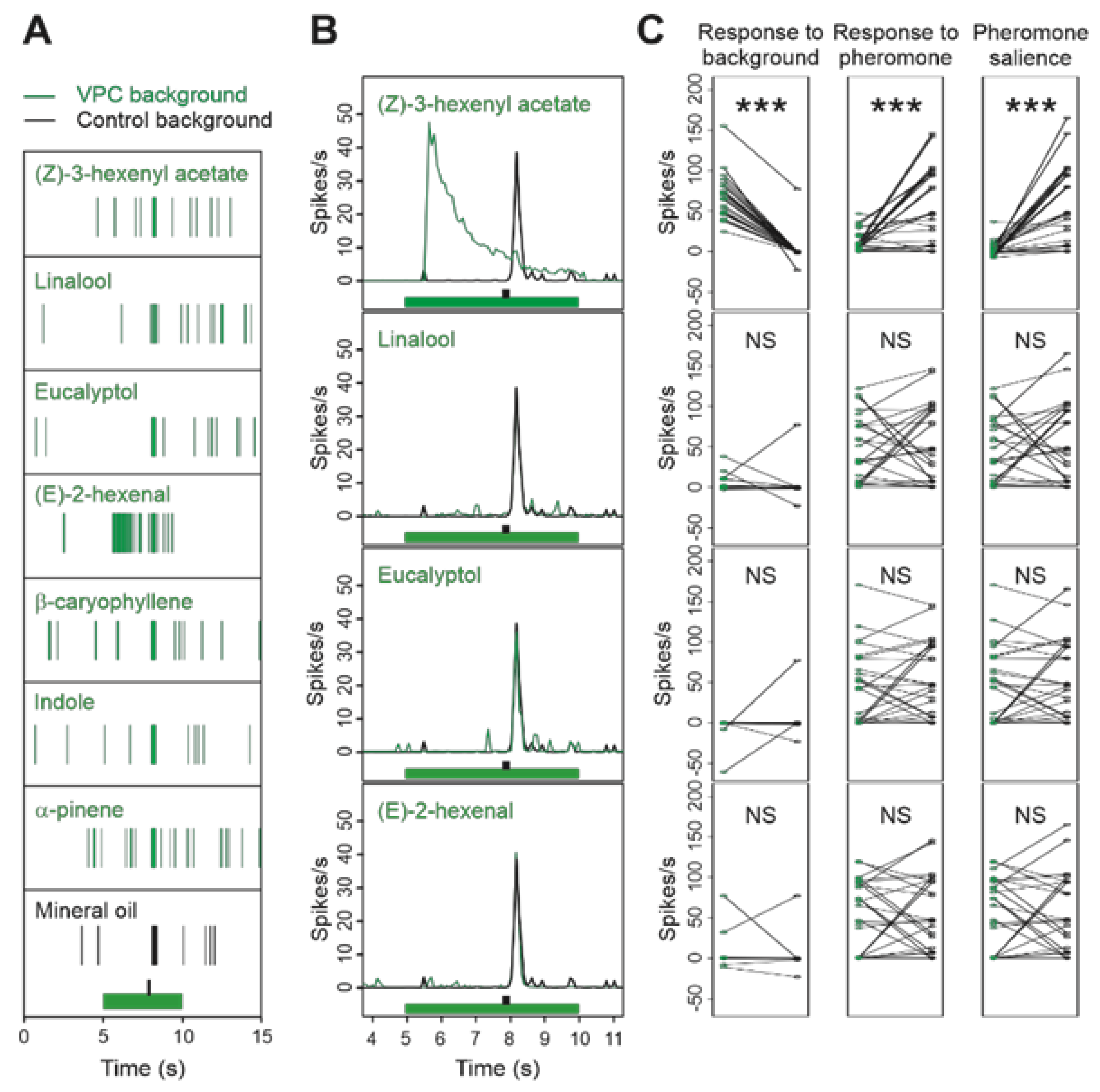
3.5. Multi Component Blends Reproduced the Activity of Salient Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conchou, L.; Anderson, P.; Birgersson, G. Host plant species differentiation in a polyphagous moth: Olfaction is enough. J. Chem. Ecol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kantsa, A.; Raguso, R.A.; Lekkas, T.; Kalantzi, O.I.; Petanidou, T. Floral volatiles and visitors: A meta-network of associations in a natural community. J. Ecol. 2019, 1–13. [Google Scholar] [CrossRef]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef]
- Knudsen, G.K.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Bergstrom, G.L. Floral scents—A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Riffell, J.A.; Lei, H.; Abrell, L.; Hildebrand, J.G. Neural basis of a pollinator’s buffet: Olfactory specialization and learning in Manduca sexta. Science 2013, 339, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Riffell, J.A.; Shlizerman, E.; Sanders, E.; Abrell, L.; Medina, B.; Hinterwirth, A.J.; Kutz, J.N. Sensory biology. Flower discrimination by pollinators in a dynamic chemical environment. Science 2014, 344, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Kuhn, U.; Wolf, A.; Andreae, M.O.; Ciccioli, P.; Brancaleoni, E.; Frattoni, M.; Guenther, A.; Greenberg, J.; De Castro Vasconcellos, P.; et al. Atmospheric volatile organic compounds (VOC) at a remote tropical forest site in central Amazonia. Atmos. Environ. 2000, 34, 4063–4072. [Google Scholar] [CrossRef]
- Wiedenmyer, C.; Friedfeld, S.; Baugh, W.; Greenberg, J.; Guenther, A.; Fraser, M.; Allen, D. Measurement and analysis of atmospheric concentrations of isoprene and its reaction products in central Texas. Atmos. Environ. 2011, 35, 1001–1013. [Google Scholar] [CrossRef]
- De Bruyne, M.; Baker, T.C. Odor detection in Insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: Receptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef]
- Hansson, B.S.; Anton, S. Function and morphology of the antennal lobe: New developments. Annu. Rev. Entomol. 2000, 45, 203–231. [Google Scholar] [CrossRef]
- Jarriault, D.; Gadenne, C.; Rospars, J.P.; Anton, S. Quantitative analysis of sex-pheromone coding in the antennal lobe of the moth Agrotis ipsilon: A tool to study network plasticity. J. Exp. Biol. 2009, 212, 1191–1201. [Google Scholar] [CrossRef]
- Jarriault, D.; Gadenne, C.; Lucas, P.; Rospars, J.P.; Anton, S. Transformation of the sex pheromone signal in the noctuid moth Agrotis ipsilon: From peripheral input to antennal lobe output. Chem. Senses 2010, 35, 705–715. [Google Scholar] [CrossRef]
- Rospars, J.P.; Gremiaux, A.; Jarriault, D.; Chaffiol, A.; Monsempes, C.; Deisig, N.; Anton, S.; Lucas, P.; Martinez, D. Heterogeneity and convergence of olfactory first-order neurons account for the high speed and sensitivity of second-order neurons. PLoS Comput. Biol. 2014, 10, e1003975. [Google Scholar] [CrossRef]
- Martin, J.P.; Lei, H.; Riffell, J.A.; Hildebrand, J.G. Synchronous firing of antennal-lobe projection neurons encodes the behaviorally effective ratio of sex-pheromone components in male Manduca sexta. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2013, 199, 963–979. [Google Scholar] [CrossRef]
- Wu, W.; Anton, S.; Löfstedt, C.; Hansson, B.S. Discrimination among pheromone component blends by interneurons in male antennal lobes of two populations of the turnip moth, Agrotis segetum. Proc. Natl. Acad. Sci. USA 1996, 93, 8022–8027. [Google Scholar] [CrossRef]
- Anton, S.; Hansson, B.S. Sex-pheromone and plant-associated odor processing in antennal lobe interneurons of male Spodoptera littoralis (Lepidoptera, Noctuidae). J. Comp. Physiol. A 1995, 176, 773–789. [Google Scholar] [CrossRef]
- Levakova, M.; Kostal, L.; Monsempes, C.; Jacob, V.; Lucas, P. Moth olfactory receptor neurons adjust their encoding efficiency to temporal statistics of pheromone fluctuations. PLoS Comput. Biol. 2018, 14, e1006586. [Google Scholar] [CrossRef]
- Levakova, M.; Kostal, L.; Monsempes, C.; Lucas, P.; Kobayashi, R. Adaptive integrate-and-fire model reproduces the dynamics of olfactory receptor neuron responses in a moth. J. R. Soc. Interface 2019, 16, 20190246. [Google Scholar] [CrossRef] [PubMed]
- Jacob, V.; Monsempès, C.; Rospars, J.P.; Masson, J.B.; Lucas, P. Olfactory coding in the turbulent realm. PLoS Comput. Biol. 2017, 13, e1005870. [Google Scholar] [CrossRef] [PubMed]
- Gorur-Shandilya, S.; Demir, M.; Long, J.; Clark, D.A.; Emonet, T. Olfactory receptor neurons use gain control and complementary kinetics to encode intermittent odorant stimuli. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Borrero-Echeverry, F.; Bengtsson, B.; Nakamuta, K.; Peter Witzgall, P. Plant odor and sex pheromone are integral elements of specific mate recognition in an insect herbivore. Evolution 2018, 72, 2225–2233. [Google Scholar] [CrossRef]
- Ian, E.; Kirkerud, N.H.; Galizia, C.G.; Berg, B.G. Coincidence of pheromone and plant odor leads to sensory plasticity in the heliothine olfactory system. PLoS ONE 2017, 12, e0175513. [Google Scholar] [CrossRef]
- Ochieng, S.A.; Park, K.C.; Baker, T.C. Host plant volatiles synergise responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J. Comp. Physiol. A 2002, 188, 325–333. [Google Scholar] [CrossRef]
- Rouyar, A.; Deisig, N.; Dupuy, F.; Limousin, D.; Wycke, M.-A.; Renou, M.; Anton, S. Unexpected plant odor responses in a moth pheromone system. Front. Physiol. 2015, 6, 148. [Google Scholar] [CrossRef]
- Party, V.; Hanot, C.; Said, I.; Rochat, D.; Renou, M. Plant terpenes affect intensity and temporal parameters of pheromone detection in a moth. Chem. Senses 2009, 34, 763–774. [Google Scholar] [CrossRef]
- Dupuy, F.; Rouyar, A.; Deisig, N.; Bourgeois, T.; Limousin, D.; Wycke, M.A.; Anton, S.; Renou, M. A background of a volatile plant compound alters neural and behavioral responses to the sex pheromone blend in a moth. Front. Physiol. 2017, 8, 79. [Google Scholar] [CrossRef]
- Deisig, N.; Kropf, J.; Vitecek, S.; Pevergne, D.; Rouyar, A.; Sandoz, J.C.; Lucas, P.; Gadenne, C.; Anton, S.; Barrozo, R. Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE 2012, 7, e33159. [Google Scholar] [CrossRef]
- Chaffiol, A.; Dupuy, F.; Barrozo, R.; Kropf, J.; Renou, M.; Rospars, J.-P.; Anton, S. Pheromone modulates plant odour responses in the antennal lobe of a moth. Chem. Senses 2014, 39, 451–463. [Google Scholar] [CrossRef]
- Chaffiol, A.; Kropf, J.; Barrozo, R.B.; Gadenne, C.; Rospars, J.P.; Anton, S. Plant odour stimuli reshape pheromonal representation in neurons of the antennal lobe macroglomerular complex of a male moth. J. Exp. Biol. 2012, 215, 1670–1680. [Google Scholar] [CrossRef]
- Trona, F.; Anfora, G.; Bengtsson, M.; Witzgall, P.; Ignell, R. Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydia pomonella. J. Exp. Biol. 2010, 213, 4291–4303. [Google Scholar] [CrossRef]
- Pregitzer, P.; Schubert, M.; Breer, H.; Hansson, B.S.; Sachse, S.; Krieger, J. Plant odorants interfere with detection of sex pheromone signals by male Heliothis virescens. Front. Cell Neurosci. 2012, 6, 42. [Google Scholar] [CrossRef]
- Bengtsson, J.M.; Gonzalez, F.; Cattaneo, A.M.; Montagné, N.; Walker, W.B.; Bengtsson, M.; Anfora, G.; Ignell, R.; Jacquin-Joly, E.; Witzgall, P. A predicted sex pheromone receptor of codling moth Cydia pomonella detects the plant volatile pear ester. Front. Ecol. Evol. 2014, 2, 33. [Google Scholar] [CrossRef]
- Badeke, E.; Haverkamp, A.; Hansson, B.S.; Sachse, S. A challenge for a male noctuid moth? Discerning the female sex pheromone against the background of plant volatiles. Front. Physiol. 2016, 7, 143. [Google Scholar] [CrossRef]
- Penuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef]
- Lathière, J.; Hewitt, C.N.; Beerling, D.J. Sensitivity of isoprene emissions from the terrestrial biosphere to 20th century changes in atmospheric CO2 concentration, climate, and land use. Glob. Biogeochem. Cycles 2010, 24, GB1004. [Google Scholar] [CrossRef]
- Zhu, Y.; Keaster, A.J.; Gerhard, K.O. Field observations on attractiveness of selected blooming plants to noctuid moths and electroantennogram responses of black cutworm (Lepidoptera: Noctuidae) moths to flower volatiles. Environ. Entomol. 1993, 22, 162–166. [Google Scholar] [CrossRef]
- Wynne, J.W.; Keaster, A.J.; Gerhardt, K.O.; Krause, G.F. Plant species identified as food sources for adult black cutworm (Lepidoptera:Noctuidae) in Northern Missouri. J. Kans. Entomol. Soc. 1991, 64, 381–387. [Google Scholar]
- Deisig, N.; Dupuy, F.; Anton, S.; Renou, M. Responses to pheromones in a complex odor world: Sensory processing and behavior. Insects 2014, 5, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Gemeno, C.; Haynes, K.F. Chemical and behavioral evidence for a third pheromone component in a north american population of the black cutworm moth, Agrotis ipsilon. J. Chem. Ecol. 1998, 24, 999–1011. [Google Scholar] [CrossRef]
- Picimbon, J.F.; Gadenne, C.; Becard, J.M.; Clement, J.L.; Sreng, L. Sex pheromone of the French black cutworm moth, Agrotis ipsilon (Lepidoptera: Noctuidae): Identification and regulation of a multicomponent blend. J. Chem. Ecol. 1997, 23, 211–230. [Google Scholar] [CrossRef]
- Renou, M.; Gadenne, C.; Tauban, D. Electrophysiological investigations of pheromone-sensitive sensilla in the hybrids between two moth species. J. Insect Physiol. 1996, 42, 267–277. [Google Scholar] [CrossRef]
- Gadenne, C.; Picimbon, J.-F.; Becard, J.-M.; Lalanne-Cassou, B.; Renou, M. Development and pheromone communication systems in hybrids of Agrotis ipsilon and Agrotis segetum (Lepidoptera: Noctuidae). J. Chem. Ecol. 1997, 23, 191–209. [Google Scholar] [CrossRef]
- Greiner, B.; Gadenne, C.; Anton, S. Central processing of plant volatiles in Agrotis ipsilon males is age-independent in contrast to sex pheromone processing. Chem. Senses. 2002, 27, 45–48. [Google Scholar] [CrossRef][Green Version]
- Poitout, S.; Bues, R. Elevage de chenilles de 28 espèces de Lépidoptères Noctuidae et de 2 espèces d’Arctiidae sur milieu artificiel simple. Particularités de l’élevage selon les espèces. Ann. Zool. Ecol. Anim. 1974, 6, 431–441. [Google Scholar]
- Degen, T.; Dillmann, C.; Marion Poll, F.; Turlings, T.C.J. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol 2004, 135, 1928–1938. [Google Scholar] [CrossRef]
- Staudt, M.; Bertin, N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak (Quercus ilex L.) leaves. Plant Cell Environ 1998, 21, 385–395. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Louis, S.; Behnke, K.; Loivamäki, M. Poplar volatiles—Biosynthesis, regulation and (eco)physiology of isoprene and stress-induced isoprenoids. Plant Biol. 2010, 12, 302–316. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D. Where do herbivore-induced plant volatiles go? Front. Plant Sci. 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Jerkovic, I.; Mastelic, J.; Milos, M.; Juteau, F.; Masotti, V.; Viano, J. Chemical variability of Artemisia vulgaris L. essential oils originated from the Mediterranean area of France and Croatia. Flavour. Fragr. J. 2003, 18, 436–440. [Google Scholar] [CrossRef]
- Munch, D.; Schmeichel, B.; Silbering, A.F.; Galizia, C.G. Weaker ligands can dominate an odor blend due to syntopic interactions. Chem. Senses 2013, 38, 293–304. [Google Scholar] [CrossRef]
- Cometto-Muniz, J.E.; Cain, W.S.; Abraham, M.H. Quantification of chemical vapors in chemosensory research. Chem. Senses 2003, 28, 467–477. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 29 April 2021).
- Blejec, A. Statistical method for detection of firing rate changes in spontaneously active neurons. Neurocomputing 2005, 65–66, 557–563. [Google Scholar] [CrossRef]
- Jari Oksanen, F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; Dan McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-6; Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Anderson, M. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Rospars, J.P.; Lansky, P.; Chaput, M.; Duchamp-Viret, P. Competitive and non competitive odorant interactions in the early neural coding of odorant mixtures. J. Neurosci. 2008, 28, 2659–2666. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Jarriault, D.; Deisig, N.; Gemeno, C.; Monsempes, C.; Lucas, P.; Gadenne, C.; Anton, S. Mating-induced differential coding of plant odour and sex pheromone in a male moth. Eur. J. Neurosci. 2011, 33, 1841–1850. [Google Scholar] [CrossRef]
- Rouyar, A.; Party, V.; Prešern, J.; Blejec, A.; Renou, M. A general odorant background affects the coding of pheromone stimulus intermittency in specialist olfactory receptor neurones. PLoS ONE 2011, 6, e26443. [Google Scholar] [CrossRef]
- Renou, M.; Party, V.; Rouyar, A.; Anton, S. Olfactory signal coding in an odor background. Biosystems 2015, 136, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Deisig, N.; Giurfa, M.; Lachnit, H.; Sandoz, J.C. Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur. J. Neurosci. 2006, 24, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Gorur-Shandilya, S.; Martelli, C.; Demir, M.; Emonet, T. Controlling and measuring dynamic odorant stimuli in the laboratory. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed]
- Martelli, C.; Carlson, J.R.; Emonet, T. Intensity invariant dynamics and odor-specific latencies in olfactory receptor neuron response. J. Neurosci. Methods 2013, 33, 6285–6297. [Google Scholar] [CrossRef]
- Noldus, L.P.J.J.; Potting, R.P.J.; Barendregt, H.E. Moth sex pheromone adsorption to leaf surface: Bridge in time for chemical spies. Physiol. Entomol. 1991, 16, 329–344. [Google Scholar] [CrossRef]
- Trona, F.; Anfora, G.; Balkenius, A.; Bengtsson, M.; Tasin, M.; Knight, A.; Janz, N.; Witzgall, P.; Ignell, R. Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc. Biol. Sci. 2013, 280, 20130267. [Google Scholar] [CrossRef]
- Namiki, S.; Iwabuchi, S.; Kanzaki, R. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J. Comp. Physiol. A 2008, 194, 501–515. [Google Scholar] [CrossRef]
- Schmidt-Büsser, D.; von Arx, M.; Guerin, P.M. Host plant volatiles serve to increase the response of male European grape berry moths, Eupoecilia ambiguella, to their sex pheromone. J. Comp. Physiol. A 2009, 195, 853–864. [Google Scholar] [CrossRef]
- Clifford, M.R.; Riffell, J.A. Mixture and odorant processing in the olfactory systems of insects: A comparative perspective. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2013, 199, 911–928. [Google Scholar] [CrossRef]
- Masse, N.Y.; Turner, G.C.; Jefferis, G.S. Olfactory information processing in Drosophila. Curr. Biol. 2009, 19, R700–R713. [Google Scholar] [CrossRef]
- Bhandawat, V.; Olsen, S.R.; Gouwens, N.W.; Schlief, M.L.; Wilson, R.I. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 2007, 10, 1474–1482. [Google Scholar] [CrossRef]
- Murlis, J.; Jones, C.D. Fine-scale structure of odour plumes in relation to distant pheromone and other attractant sources. Physiol. Entomol. 1981, 6, 71–86. [Google Scholar] [CrossRef]
- Murlis, J. The structure of odour plumes. In Mechanisms in Insect Olfaction; Payne, T.L., Birch, M.C., Kennedy, C.E.J., Eds.; Clarendon: Oxford, UK, 1986; pp. 27–38. [Google Scholar]
- Root, C.M.; Masuyama, K.; Green, D.S.; Enell, L.E.; Nassel, D.R.; Lee, C.H.; Wang, J.W. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron 2008, 59, 311–321. [Google Scholar] [CrossRef]
- Wilson, R.I. Early olfactory processing in Drosophila: Mechanisms and principles. Annu. Rev. Neurosci. 2013, 36, 217–241. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef]
- Shen, K.; Tootoonian, S.; Laurent, G. Encoding of mixtures in a simple olfactory system. Neuron 2013, 80, 1246–1262. [Google Scholar] [CrossRef]
- Ache, B.W.; Gleeson, R.A.; Thompson, H.A. Mechanisms for mixture suppression in olfactory receptors of the spiny lobster. Chem. Senses 1988, 13, 425–434. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate change effects on secondary compounds of forest trees in the Northern hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef]
- Ghirardo, A.; Lindstein, F.; Koch, K.; Buegger, F.; Schloter, M.; Albert, A.; Michelsen, A.; Winkler, J.B.; Schnitzler, J.P.; Rinnan, R. Origin of volatile organic compound emissions from subarctic tundra under global warming. Glob. Chang. Biol. 2020, 26, 1908–1925. [Google Scholar] [CrossRef]
- Knudsen, G.K.; Norli, H.R.; Tasin, M. The ratio between field attractive and background volatiles encodes host-plant recognition in a specialist moth. Front. Plant Sci. 2017, 8, 2206. [Google Scholar] [CrossRef]
- Conchou, L.; Lucas, P.; Deisig, N.; Demondion, E.; Renou, M. Effects of an odor background on moth pheromone communication: Constituent identity matters more than blend complexity. bioRxiv 2020. [Google Scholar] [CrossRef]
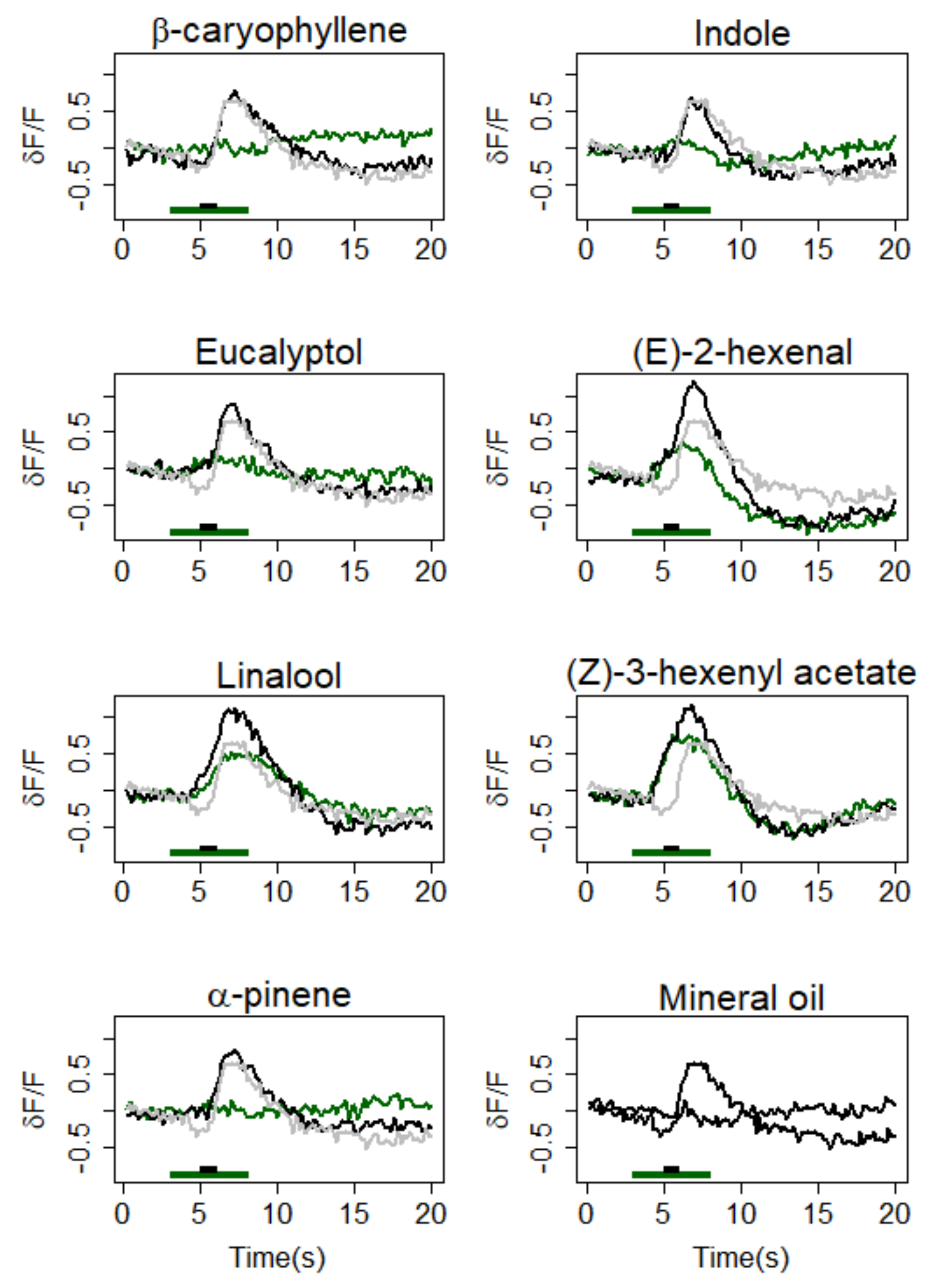
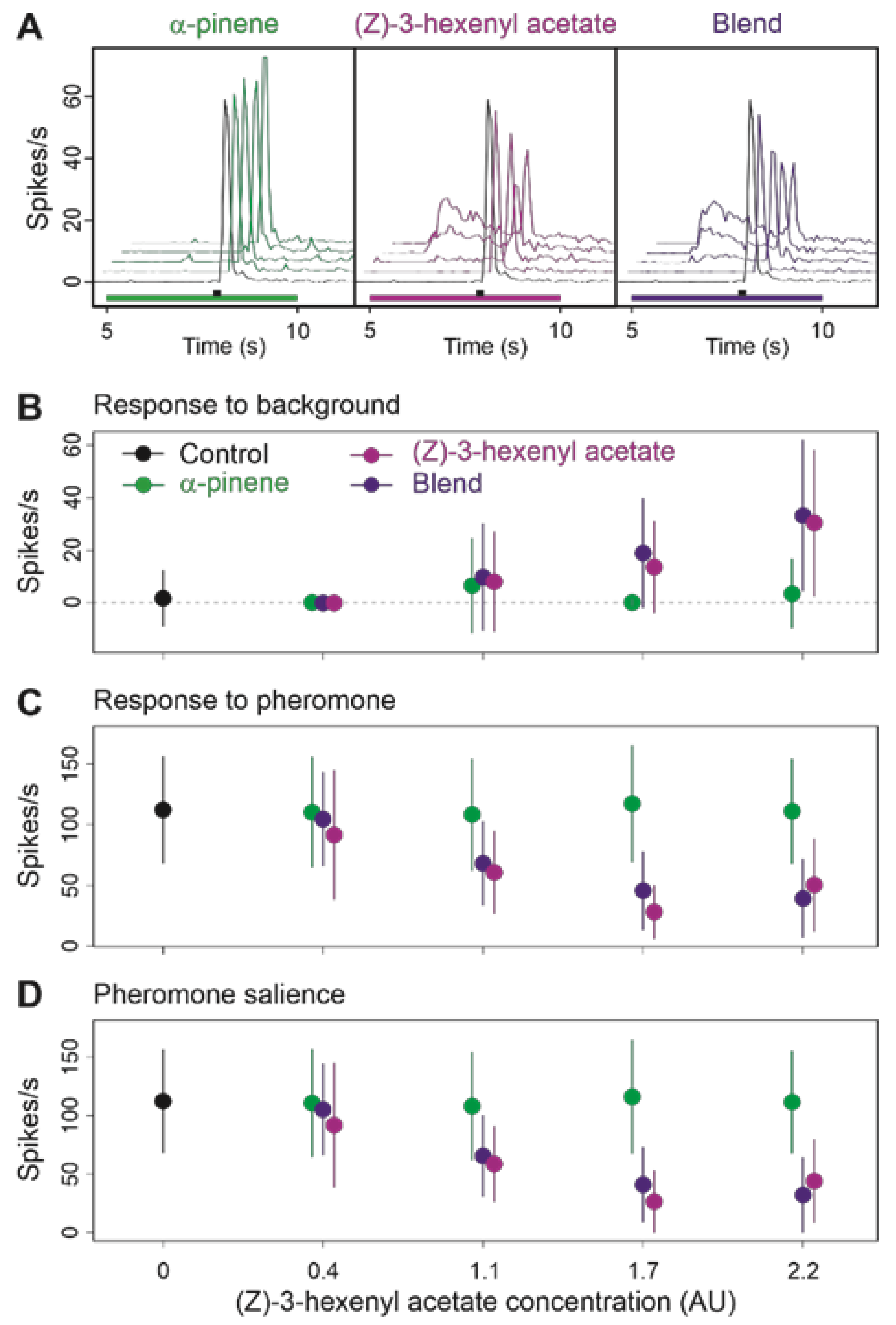

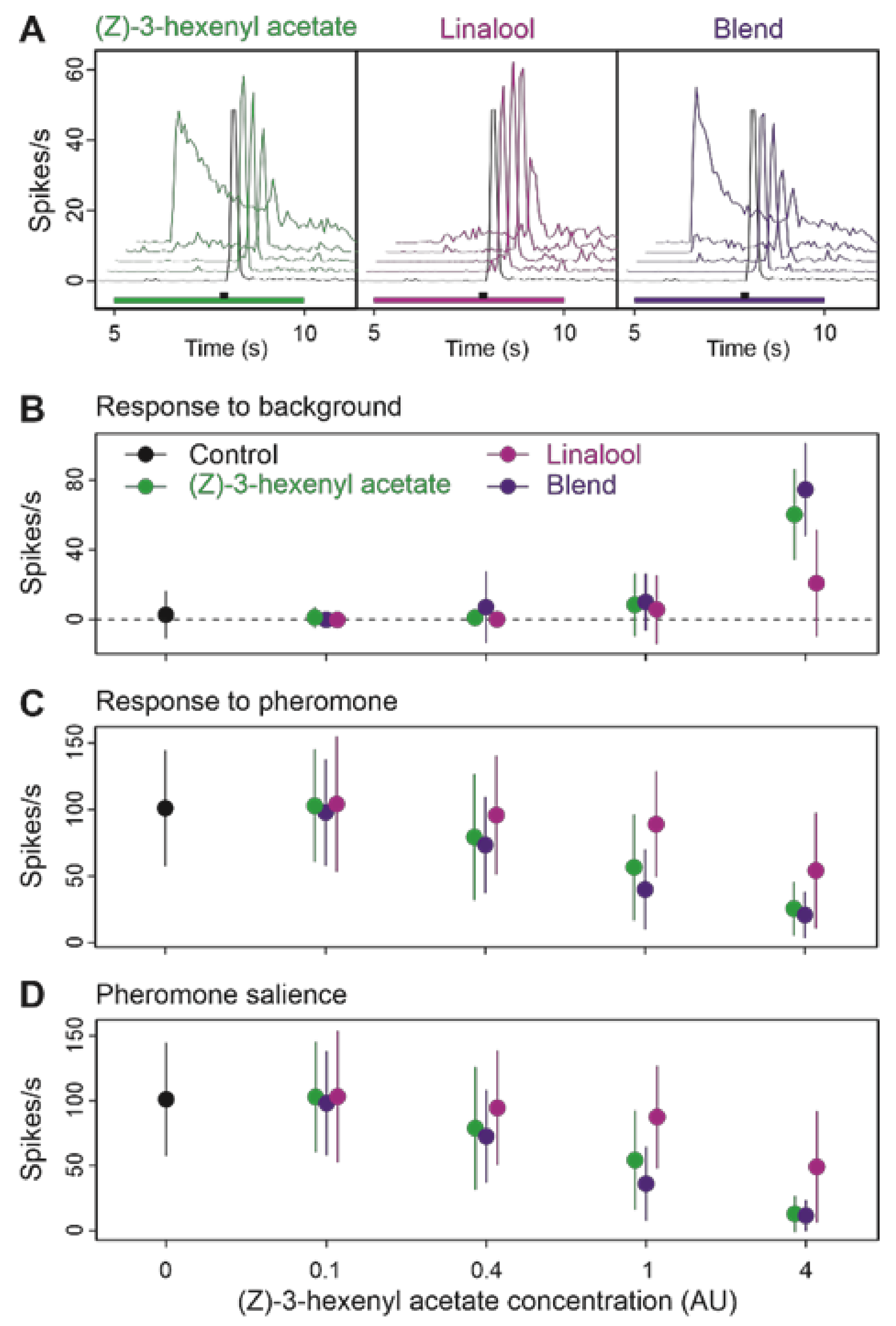
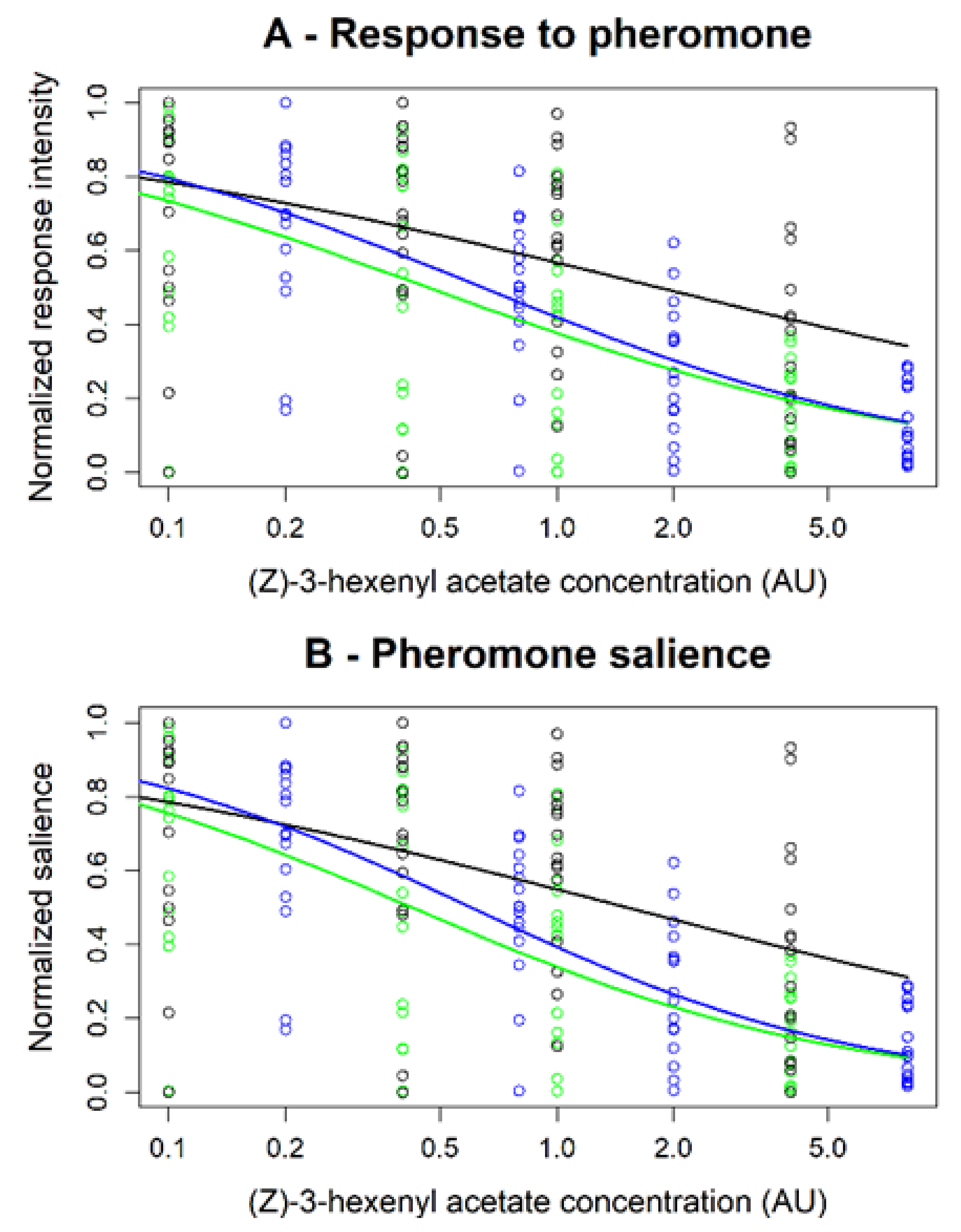
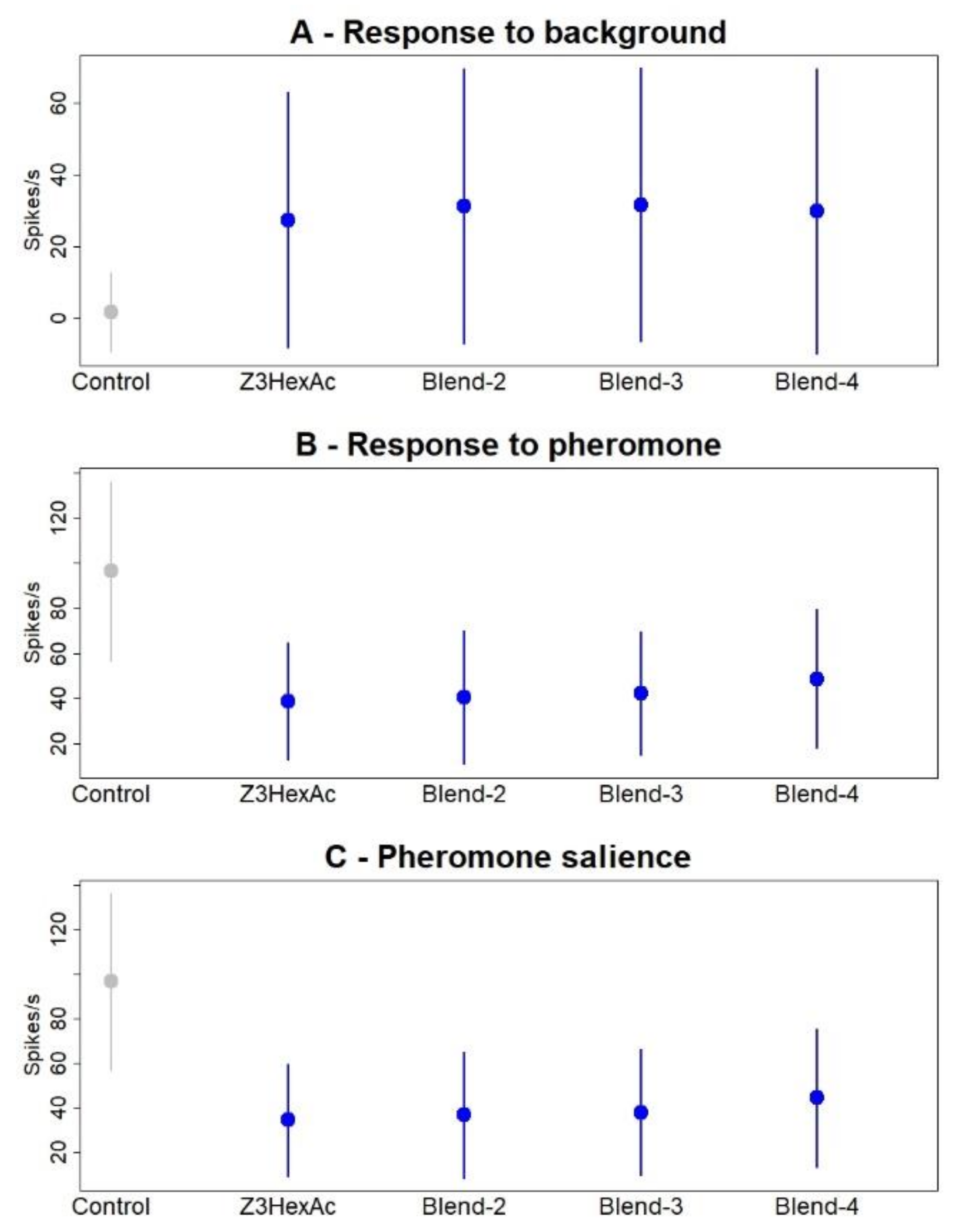
| Type of Background | δF/F Mean (SD) | Background vs. Z7-12:Ac in Background | Z7-12:Ac vs. Z7-12:Ac in Background | Z7-12:Ac vs. Background | ||||
|---|---|---|---|---|---|---|---|---|
| Background | Background + Z7-12:Ac | F | p | F | p | F | p | |
| 2-hexenal | 0.33 | 1.27 (0.19) | 15.4626 | 0.0005 | 7.60928 | 0.01012 | 2.63462 | 0.11576 |
| (0.14) | ||||||||
| Linalool | 0.48 | 1.15 (0.19) | 7.0363 | 0.01301 | 5.14534 | 0.03121 | 0.53650 | 0.46997 |
| (0.17) | ||||||||
| (Z)3-hexenyl acetate | 0.77 | 1.22 (0.15) | 5.7185 | 0.02375 | 8.69025 | 0.00639 | 0.60096 | 0.44471 |
| (0.11) | ||||||||
| α-pinene | 0.05 | 0.89 (0.15) | 21.8173 | 0.00007 | 1.61932 | 0.21365 | 13.73691 | 0.00092 |
| (0.1) | ||||||||
| Eucalyptol | 0.1 | 0.93 (0.15) | 22.1018 | 0.00006 | 2.17790 | 0.15116 | 12.51131 | 0.00143 |
| (0.08) | ||||||||
| Indole | 0.05 | 0.64 (0.15) | 12.8135 | 0.00128 | 0.00007 | 0.99343 | 18.21651 | 0.00020 |
| (0.06) | ||||||||
| β-caryophyllene | 0.05 | 0.76 (0.14) | 19.2145 | 0.00015 | 0.47821 | 0.49493 | 14.73026 | 0.00065 |
| (0.09) | ||||||||
| Mineral oil | −0.13 | 0.69 (0.14) | 27.2565 | 0.00002 | 0.09661 | 0.75824 | 27.14252 | 0.00002 |
| (0.08) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conchou, L.; Lucas, P.; Deisig, N.; Demondion, E.; Renou, M. Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception. Insects 2021, 12, 409. https://doi.org/10.3390/insects12050409
Conchou L, Lucas P, Deisig N, Demondion E, Renou M. Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception. Insects. 2021; 12(5):409. https://doi.org/10.3390/insects12050409
Chicago/Turabian StyleConchou, Lucie, Philippe Lucas, Nina Deisig, Elodie Demondion, and Michel Renou. 2021. "Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception" Insects 12, no. 5: 409. https://doi.org/10.3390/insects12050409
APA StyleConchou, L., Lucas, P., Deisig, N., Demondion, E., & Renou, M. (2021). Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception. Insects, 12(5), 409. https://doi.org/10.3390/insects12050409






