Simple Summary
Desiccation resistance and physiological plasticity are key traits for species persistence in the context of aridification under global change. One of the main mechanisms of desiccation resistance in insects is the control of cuticular transpiration through changes in the quantity and composition of epicuticular hydrocarbons (CHCs), which have been well studied in terrestrial insects. Our study provides novel information regarding the capacity to cope with desiccation stress through plastic changes in the composition of cuticle hydrocarbons in aquatic insects from saline intermittent streams. We demonstrate that the plasticity of CHCs is an effective mechanism to regulate water loss rate under desiccation stress in the most saline-tolerant species studied. These traits, so far largely unexplored in aquatic insects, are relevant to understanding different biochemical adaptations to deal with drought stress in inland saline waters in an evolutionary and ecological context.
Abstract
In the context of aridification in Mediterranean regions, desiccation resistance and physiological plasticity will be key traits for the persistence of aquatic insects exposed to increasing desiccation stress. Control of cuticular transpiration through changes in the quantity and composition of epicuticular hydrocarbons (CHCs) is one of the main mechanisms of desiccation resistance in insects, but it remains largely unexplored in aquatic ones. We studied acclimation responses to desiccation in adults of two endemic water beetles from distant lineages living in Mediterranean intermittent saline streams: Enochrus jesusarribasi (Hydrophilidae) and Nebrioporus baeticus (Dytiscidae). Cuticular water loss and CHC composition were measured in specimens exposed to a prior non-lethal desiccation stress, allowed to recover and exposed to a subsequent desiccation treatment. E. jesusarribasi showed a beneficial acclimation response to desiccation: pre-desiccated individuals reduced cuticular water loss rate in a subsequent exposure by increasing the relative abundance of cuticular methyl-branched compounds, longer chain alkanes and branched alkanes. In contrast, N. baeticus lacked acclimation capacity for controlling water loss and therefore may have a lower physiological capacity to cope with increasing aridity. These results are relevant to understanding biochemical adaptations to drought stress in inland waters in an evolutionary and ecological context.
1. Introduction
Among fitness-related physiological traits, those associated with water balance are highly relevant for insects in natural habitats [1]. The success of insects in terrestrial environments is largely due to the waterproofing properties of their cuticles to resist desiccation stress, which has been the subject of many studies on terrestrial arthropods (e.g., [2,3,4,5]), but is relatively unexplored in aquatic insects that experience desiccating conditions during drought events. Drought is a critical stressor in many freshwater ecosystems [6,7]. In intermittent systems of arid and semiarid areas, flow connectivity is disrupted and some water bodies may remain completely dry for long periods during seasonal droughts. Adults of some aquatic insects, like water beetles, fly from drying sites to more favorable wet habitats, experiencing important water loss because of the exposure to air and the extra dehydration associated with flight activity [8,9,10]. Therefore, the desiccation resistance of the adult (dispersive stage) may constrain the dispersal and survival strategies of these species [11,12]. In Mediterranean regions, where dry events are becoming more intense, prolonged and unpredictable with ongoing climate change [13], desiccation resistance will be a key trait for the persistence of many aquatic species. However, our understanding of the specific mechanisms by which aquatic insects deal with desiccation stress is still very limited.
One of the main mechanisms of desiccation resistance in insects is the control of cuticular transpiration [14,15], which generally accounts for the greatest proportion of body water loss [16,17]. The variation of cuticular water loss is driven by the permeability of the epicuticular hydrocarbons (CHCs) [15,17,18]. In general, a higher amount of CHCs with longer carbon chains, higher linearity and saturation confer a better waterproofing capacity to the cuticle [4,19,20]. In some terrestrial insects, CHC profiles show relatively rapid plastic changes in composition to adjust to the current environment, for example in response to changing temperature or desiccation conditions, (e.g., [5,21,22]). The overall CHC amount can be also increased in response to drought stress (e.g., [22,23,24,25,26]). Such changes have been proven to confer increased desiccation resistance (e.g., [2,4,27,28,29]).
Beetles from intermittent saline waters represent an interesting study case to assess whether the CHC’s composition and plasticity also play a relevant role in the desiccation resistance in aquatic insects, for several reasons. First, salinity and desiccation are co-occurring stressors in their habitats, and both induce osmotic stress and alter water balance, producing cuticular water loss. Indeed, some species have shown cross-tolerance (as defined by [30]) to these stressors [31]. These habitats are also characterized by a high climatic and hydrological variability, which may select for the evolution of high physiological plasticity [32,33,34]. Accordingly, it has been demonstrated that acclimation to high salinity induces changes in CHC composition that reduce cuticle permeability in saline water beetles [35]. Therefore, it is likely that desiccation induces plastic changes in CHCs similar to those elicited by salinity, and the modulation of cuticular permeability could be one of the possible shared protective mechanisms underlying cross-tolerance to both stressors. Finally, it has been suggested that desiccation resistance provided the physiological basis for the development of osmoregulation ability in some water beetle lineages, enabling the colonization and diversification across meso- and hypersaline habitats [36,37]. Then, a better knowledge of the specific strategies by which saline aquatic beetles cope with desiccation stress might shed light on the mechanistic and evolutionary links between these correlated stressors and contribute to understanding the success of some particular insect lineages in highly stressful environments such as intermittent saline waters [38].
The objective of this study was to explore if saline beetle species representative of independent evolutionary lineages that colonized such naturally stressful habitats show plastic variation in cuticular permeability (cuticular water loss rate) and CHCs quantity and composition in response to desiccation stress. For that purpose, we focused on the Iberian endemic water beetle species Enochrus jesusarribasi Arribas and Millán, 2013 (Polyphaga, Hydrophilidae) and Nebrioporus baeticus (Schaum 1864) (Adephaga, Dytiscidae), common inhabitants of Mediterranean saline running waters [39]. Both species are effective osmoregulators, their basal tolerance to salinity and desiccation resistance have been well documented [31,40,41] and they have shown an extraordinary ability to rapidly adjust CHC composition in response to environmental salinity changes compared with freshwater congeneric species [35]. We predicted that these species, adapted to osmotic stress, will also show CHC plasticity in response to desiccation, and so, exposure to non-lethal desiccation conditions would enhance desiccation resistance under a subsequent desiccation stress. To test this hypothesis, we measured cuticular water loss rate and multiple CHC traits (proportions of different CHC classes, median chain lengths of each class and the whole CHC profiles). Under the hypothesized beneficial acclimation response, we would expect that prior desiccation exposure reduces cuticular permeability in a subsequent exposure and elicits changes in either CHC amount and/or composition associated with enhanced waterproofing capacity in insects (e.g., increase in total CHC amount, in the proportion of longer chain n-alkanes and branched compounds and decrease in the proportion of unsaturated ones).
2. Materials and Methods
2.1. Study Species, Field Collection and Maintenance
Enochrus jesusarribasi and N. baeticus are Iberian endemic species that inhabit intermittent saline streams in South Spain [39]. These species face frequent seasonal droughts in their localities by flying from drying to wet sites, and no other desiccation resistance strategies (e.g., diapause, desiccation-resistant eggs or larvae) are known [39]. Experimental studies have shown that E. jesusarribasi is more tolerant to osmotic stress (both salinity and desiccation) than N. baeticus [31,40,41], and they also differ in body size, morphology and microhabitat. Nebrioporus baeticus (6.4 ± 0.28 mg of fresh mass), with a hydrodynamic body shape, is a strong swimmer frequently found in the water column [42]. E. jesusarribasi is slightly larger (7.20 ± 0.64 mg of fresh mass), has a more round and convex body shape and is a “walker” species mostly associated with the shorelines [39].
Adult individuals of each species were collected in October 2016 from two intermittent saline streams located in Murcia (SE Spain): Rambla Salada (38°07’22.0” N, 1°06’39.5” W) (E. jesusarribasi) and Río Chícamo (38°12’43.7” N, 1°03’07.9” W) (N. baeticus). Electrical conductivity values, measured in situ with a conductivity-meter (HACH/Hq40d), were 84 and 20 mS cm−1, respectively.
In the laboratory, specimens were placed in aerated 4 L aquaria with saline water at the salinity conditions measured in the collection localities of each species (20 mS cm−1 for N. baeticus and 80 mS cm−1 for E. jesusarribasi). Such conductivities are within the optimal range for these species in nature [41] and were chosen to avoid additional osmotic stress previous to the experiments. Saline water was prepared by dissolving an appropriate quantity of marine salt (Ocean Fish, Prodac, Cittadella, Padua, Italy) in distilled water. They were kept at 20 °C and a 12:12 L:D cycle in an environmental chamber (SANYO MLR-351, Sanyo Electric Co., Ltd., Moriguchi City, Osaka, Japan) for 5 days prior to the experiments, for habituation to laboratory conditions. Food was provided daily (frozen chironomid larvae for N. baeticus and Ruppia sp. for E. jesusarribasi).
2.2. Desiccation Experimental Procedures
Groups of 50 individuals of each species were randomly assigned to either a water control group (C) or to a prior desiccation group (Figure 1). For this prior desiccation exposure, specimens were gently dried on blotting paper and then individually placed in open glass vials and exposed to 40 ± 5% RH and 20 °C for 6 h (E. jesusarribasi) or 3 h (N. baeticus) in the environmental chamber, at the constant temperature and light cycle conditions mentioned above (Figure 1). A relative humidity of 40% was selected because it represents the minimum air humidity in the natural habitats of these species. Temperature was set to 20 °C to avoid additional thermal stress in the experiment. Exposure times were set based on the differential survival times under desiccation shown by these species in a previous study [31], in which N. baeticus was more sensitive to desiccation than E. jesusarribasi. After prior desiccation, specimens were allowed to recover for 24 h by immersing them in water at their corresponding field optimum salinity. The control groups were simultaneously maintained in aquaria at the conditions described above. Then, specimens from both the control and prior desiccation groups were exposed simultaneously to subsequent desiccation of the same intensity and duration as the first desiccation treatment (40 ± 5% RH for 3 h in N. baeticus and 6h in E. jesusarribasi). These experimental groups are referred to as D1 (one desiccation phase) and D2 treatments (two desiccation phases), respectively (Figure 1). Survival was monitored after each experimental phase (i.e., prior desiccation, recovery underwater and subsequent desiccation).
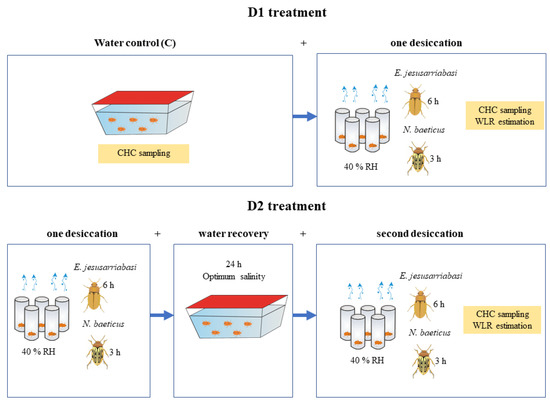
Figure 1.
Schematic representation of the experimental design showing the different stress exposure steps and timings for cuticular hydrocarbons (CHCs) sampling and total water loss rate (WLR) estimation.
2.3. CHC Extraction
To determine changes in CHC associated with desiccation, six individuals were randomly removed from the water control (C), and directly after desiccation (i.e., one desiccation in D1 treatment and the second desiccation in D2 treatment; see Figure 1), and immediately freeze-killed at −80 °C. CHC extraction, identification and quantification were made using gas chromatography–mass spectrometry (GC–MS) (see Botella-Cruz et al. 2017 [35], 2019 [43], for details on analytical procedures). The basic characterization of CHC structures was conducted by interpreting their EI mass spectra. N-alkanes were identified by comparison of retention times with n-alkane standards (C10-C40; Sigma Aldrich, St. Louis, MO, USA) and adjustments were made to peak time based on the time and area of the octadecane standard as indicated in [35] (p. 3) and [43] (p. 4). From each individual CHC sample, the following variables were calculated: (i) the total amount of CHCs, (ii) the relative abundance of each CHC as the proportion of its adjusted peak area on the total adjusted areas (i.e., the sum of all the CHCs in the corresponding sample), (iii) the proportion of the main CHC classes (n-alkanes, branched alkanes and unsaturated) and (iv) median chain length of each CHC class. After CHC extraction, adults were sexed by examining genitalia in a stereomicroscope (Leica M165C with a Leica MEB10 fiber optic illuminator).
2.4. Cuticular Water Loss
Individuals from D1 and D2 treatments were weighed before and after the desiccation exposure in an analytical balance (0.01 mg accuracy) to determine gravimetrically the rate of total water loss (WLR; mg h−1), i.e., the sum of cuticular and respiratory water loss (Figure 1).
The proportion of WLR accounted for by cuticular transpiration was estimated by measuring cuticular and respiratory water loss in an independent experiment. For this, we used the hyperoxic switch method by a non-invasive technique that modulates spiracular opening by gas composition [44].
Twenty adult specimens of each species, previously acclimated in the laboratory at their optimum salinity conditions (see above), were individually placed in empty, hermetically sealed 100 mL chambers coupled with microsensors (BME280, Bosch Sensortec, Germany) that monitor the air temperature, pressure, and relative humidity every 60s while movement was controlled by a IR motion sensor (VCNL4010, Vishay, Chester County, PA, USA). Sensors were connected via an 8 channel MUX to an Arduino board running custom written software. The chambers were placed in an aquarium with a thermostat to keep temperature controlled at 20 °C. Relative humidity was also kept constant at 40 ± 5% by continuous bubble humidification connected to each individual chamber. In addition, the aquarium was covered with aluminum foil to avoid stress by light and minimize specimens’ movement. In each measurement, an empty chamber was also used as a control. After reaching a steady state, we started measuring H2O vapor for 45 min at normoxic conditions (used as a control); later, we infused the chambers with pure O2 for 45 min, which caused a temporary decrease in spiracular area and a coincident, transient drop in CO2 output and H2O vapor loss. Those individuals which showed high activity during the trials were discarded for further analysis.
We calculated the respiratory WLR from the proportional decrease of H2O vapor under hyperoxic conditions and estimated cuticular WLR by subtracting respiratory WLR from total WL with the equations:
Respiratory water loss (RWL): 100 (∆WL/WLR normoxia)
∆WL: WLR normoxia − WLR hyperoxia
Cuticle water loss (CWL): 100-RWL
Given the possibility that specimens did not maintain the spiracles fully closed during the complete hyperoxia period, we compared these estimates with those obtained from the first 15 min exposure. For simplicity, 45 min estimates were used because rates were similar.
The percentage of CWL estimated by this experiment was applied to the total WLR measured gravimetrically in the desiccation experiment.
2.5. Data Analysis
We compared the cuticular WLR during desiccation exposure between D1 and D2 treatments (see Figure 1). CHC traits and profiles were compared between both desiccation treatments plus the samples subtracted from the water control (C), as a reference of CHC characteristics in the absence of desiccation stress. The analyses were made for each species separately to avoid direct comparisons of WLR and CHC estimates in two distant species with different salinity optima and exposure times to desiccation. We compared instead the capacity of the species to display an acclimation response after prior desiccation exposure. Differences in water loss rates, total amount of CHCs, the relative abundances of the major CHC classes and median chain length of each class among treatments were assessed by analyses of variance (ANOVA) and Bonferroni post hoc tests, using R v.3.3.2 (R Development Core Team, 2019). Sex was initially included as a covariate and, for simplification, removed from further analyses because its effect was not significant. Normality and homoscedasticity assumptions were validated on model residuals by graphical inspection [45]. Variation in CHC profiles was investigated by a Partial-Least Squares Discriminant Analysis (PLS-DA). The multiple correlation coefficient (R2) and cross-validated R2 (Q2) were used to confirm the predictive power of the fitted models. The statistical significance of the PLS-DA was also assessed with permutation tests (1000 permutations). Variable Importance in Projection (VIP) scores, which are the weighted sums of the squares of the PLS loadings, were obtained by the PLS-DA to identify the hydrocarbons that contributed the most to the CHC profile differences (VIP > 1.5). The concentrations of CHCs were log-transformed. These analyses were conducted using the statistical software MetaboAnalyst 4.0 [46].
3. Results
3.1. Variation in Cuticular Water Loss Rates
The proportion of cuticular water loss over the total water loss at 40% RH represented 59% ± 3.78 (N = 6) in E. jesusarribasi and 90% ± 1.86 (N = 10) in N. baeticus. In E. jesusarribasi, prior desiccation induced a positive acclimation response: cuticular WLR in the D2 treatment measured after the second desiccation was 17% lower than that measured at the same time in the D1 treatment (i.e., individuals not previously desiccated) (p < 0.01, Figure 2). In N. baeticus, no significant differences between treatments were found (p > 0.05, Figure 2).

Figure 2.
Cuticular water loss rate measured in D1 (individuals from the control exposed to desiccation) and D2 (individuals from the prior desiccation exposed to a subsequent desiccation). Lowercase letters indicate significant differences between treatments in Bonferroni post hoc tests (p < 0.05).
3.2. CHC Traits
In E. jesusarribasi, a total of 52 different compounds were identified, ranging from 40 to 42 among treatments (Table 1 and Table S1). Hexatriacontane (n-C36) was the longest-chain CHC identified in this species, and the most abundant compound was an unsaturated one (n-C21) in all treatments.

Table 1.
Total abundance of cuticular hydrocarbons (CHC), number of CHC compounds, median chain length (CL) (total and for each CHC main class) and number and proportion of compounds of each class (number of CHC of the corresponding class over the total) for the studied species. D1: individuals from the water control exposed to desiccation, D2: individuals from the prior desiccation exposed to a subsequent desiccation treatment.
Individuals from the D2 treatment showed significantly longer chain lengths of n-alkanes and branched alkanes than those from D1 and C treatments (Table 1). The greatest amount of CHCs was found in D1 samples (Figure 3a, Table 1). D2 individuals presented significantly higher proportion of branched alkanes and a lower relative abundance of unsaturated compounds than the D1 and C groups (Figure 3, Table 1).

Figure 3.
Total amount of cuticular hydrocarbons (CHCs) (Mean ± S.E.) (a,b), and mean relative abundance of the major CHC classes (c,d) in the different treatments (C: water control, D1: individuals from the control exposed to desiccation and D2: individuals from the prior desiccation exposed to a subsequent desiccation) of Enochrus jesusarribasi (left) and Nebrioporus baeticus (right). Lowercase letters indicate significant differences in Bonferroni post hoc tests (p < 0.05) between treatments (a,b) and between treatments within each major CHC class (c,d).
In N. baeticus, 54 different compounds were identified, ranging from 41 to 48 among treatments (Table 1 and Table S1). The CHC with the longest carbon chain was hentriacontane (n-C31), and the most abundant one was an unsaturated compound (n-C21) in the control and tricosane in both desiccation treatments. The median chain length of unsaturated compounds was slightly but significantly longer in individuals from D2 than D1 and C treatments (p < 0.05, Table 1). CHC amount differed significantly among all treatments, being the highest in the control and the lowest in D2 individuals (p < 0.05, Figure 3, Table 1). No significant differences were found in the relative abundances of any CHC class among treatments (Table 1, Figure 3b,d).
3.3. CHC Profiles
In both species, the PLS-DA revealed significant differences in CHC profiles among treatments (permutation tests; p < 0.05, 0/1000). The PLS–DA analysis returned the first (LD1) and second (LD2) linear discriminant axes, which accounted for 36.1% and 24%, and 26.1% and 19% of the total variance in E. jesusarribasi and N. baeticus, respectively (Figure 4a,b). In both species, CHC profiles of individuals of the C group were located on the negative side of the first axis (LD1) and those from D2 were on the positive side, with D1 individuals in an intermediate position.

Figure 4.
Score plots of the two principal components of cuticular hydrocarbons (CHC) concentration in Enochrus jesusarribasi (a) and Nebrioporus baeticus (b) (each dot represents an individual sample, ellipses represent the 95% confidence region for each species sample group, and the explained variances are shown in both axes in brackets). The variable importance plots (VIP) show the CHC compounds that contributed the most to the first axis based on their VIP scores for E. jesusarribasi (c) and N. baeticus (d) (colored squares represent the relative CHC concentrations of the corresponding compounds in each species; the corresponding ID number in Table S1 is indicated in brackets). C: water control, D1: individuals from the control exposed to desiccation, D2: individuals from the prior desiccation exposed to subsequent desiccation.
In E. jesusarribasi, the VIP scores analyses showed that differences in CHC composition among treatments were principally due to the abundance increase of long-chain n- alkanes (hexatriacontane, triacontane) and branched alkanes (3-methyl-heptacosane, compound 67; Table S1) along desiccation treatments, and the biosynthesis of new long branched alkanes (10-methyl-octacosane and compounds 59, 71; Table S1) in the individuals exposed to D2 (Figure 4c). In N. baeticus, the abundance of 11/13-methyl-heptacosane was higher in individuals from D1, and nonacosane was only present in the individuals from D2. Other compounds that contributed to profile differences were n-C21 unsaturated (compounds 3 and 5) and a n-C29 branched alkane (compound 58; Table S1), showing the maximum abundance in the control and being absent in those individuals from D2 desiccation treatment (Figure 4d).
4. Discussion
Our study shows that desiccation exposure induces changes in CHCs in two aquatic beetles living in intermittent saline streams from semi-arid regions that represent distant evolutionary lineages of aquatic Coleoptera. However, the chemical strategies to cope with desiccation and acclimation capacity differed between the studied species.
E. jesusarribasi showed a clear beneficial acclimation response, increasing its desiccation resistance after a prior non-lethal desiccation hardening, in agreement with its high tolerance to salinity and desiccation reported in previous studies [31,40,41]. Such acute response to desiccation stress was associated with a change in cuticular hydrocarbons, as has been observed in some terrestrial insects, like Drosophila melanogaster [4]. In N. baeticus, the acclimation response was not found, as WLR did not differ between treatments (pre-desiccated and not pre-desiccated individuals) in this species (Figure 2).
The differential variation pattern in CHC traits shown by the two species (Figure 3) is congruent with such contrasting acclimation response to desiccation stress. In E. jesusarribasi, individuals from D2 displayed some of the expected CHC changes associated with increasing waterproofing capacity in insects: a higher abundance of branched alkanes, higher median chain length of alkanes and branched alkanes, and a lower abundance of unsaturated compounds than those not subjected to prior desiccation. Likewise, the most common responses in terrestrial insects subject to desiccation are the increase of the proportion of n-alkanes and the reduction of unsaturated CHCs, as seen, for example in ants [5], mosquitoes [3] and flies, even after a few hours of exposure to desiccation [2,4]. Branched alkanes also have an essential role in the waterproofing properties of the cuticle. They are quite common in terrestrial insect species from dry areas [47] and the predominant class of CHCs in desert Tenebrionidae, with an exceptionally thick and impermeable epicuticular wax layer, and generally with more carbon atoms than n-alkanes [48]. In addition, a recent study showed that a gene related to the synthesis of methyl-branched hydrocarbons is a determinant for the waterproofing properties of Rhodnius prolixus cuticle under desiccation [49].
E. jesusarribasi also increased the total CHC amount under desiccation (but only in the D1 desiccation treatment) as found in response to acclimation at high salinity in this species [35] and also in different terrestrial insects exposed to desiccation stress (e.g., [22,23,24,25,50,51]). However, in the subsequent desiccation (D2), a reduction of the total amount of CHC was observed, possibly indicating the responses to cope with desiccation varies with exposure time and under repeated exposures. Increasing the amount of the available CHCs could be a more immediate response to rapidly reduce cuticular water loss, while under longer or repeated stress, the strategy might shift towards a more complex response involving the synthesis of new compounds.
In N. baeticus, the changes in CHC typically related to improved waterproofing capacity were not observed. This result was consistent with the inability of this species to reduce WLR when exposed to desiccation after a prior desiccation. In this species, the total amount of CHCs decreased (a pattern also observed in response to acclimation at high salinity in [35] (pp. 4–5)), and the median chain length of unsaturated hydrocarbon increased. Therefore, these changes may reflect a physical response of the CHC blend to dryness rather than a physiological mechanism to control water loss. Indeed, these CHC changes would reduce overall waterproofing, although some authors found that alkenes also increase with temperature [22,52].
Although they exhibited different patterns in quantitative terms, both species showed changes in CHC profiles associated with desiccation, which involved the increase of the abundance of some CHCs and the biosynthesis of new hydrocarbons (Figure 4). Adjustments in CHC composition that involve the synthesis of new saturated n-alkanes and longer-chain branched alkanes were also common in the acclimation and acclimatization responses to high-salinity conditions in these species [35]. As we found, the largest acclimation effects often concern strongly aggregating (e.g., n-alkanes) and strongly disruptive (e.g., multiply methyl-branched alkanes) compounds [26]. In the two studied species, salinity and desiccation induce changes in the cuticle, suggesting a pivotal role of this protective structure to deal with multiple stressors and a possible driver of cross-tolerance responses [31]. However, the specific changes in abundance of CHCs in response to salinity or desiccation are different [35], as well as their effectiveness in reducing cuticle permeability, which reflects the complexity of the studied responses and likely an important interplay between exposure time, stress type and intensity. This opens emerging interesting research questions to further explore the relationship between cuticle chemistry and waterproofing capacity in insects exposed in nature to different sources of osmotic stress, such as saline water beetles.
The differences in desiccation resistance between the studied species could reflect their different evolutionary histories [37,53,54]) and their different adaptations to desiccation to face current and future aridification in the context of climate change. Within the Coleoptera, the evolution pattern of genes related to CHC synthesis to face desiccation is more variable than in other groups of insects [55]. It is possible that the Enochrus species studied here belongs to a lineage with more recent terrestrial (and desiccation-resistant) ancestors than the Nebrioporus one, despite Hydroporinae (to which Nebrioporus genus belongs, see [56]) being a younger lineage than Hydrophilinae [53]. This hypothesis is supported by ancestral reconstruction analyses that suggest that desiccation resistance was ancestrally high in the subgenus Lumetus (including E. jesusarribasi) [37] and by the frequent secondary colonisations of the terrestrial medium (and back to water again) within the family Hydrophilidae [57,58,59], apparently not common within the suborder Adephaga [53,60]. Thus, E. jesusarribasi, could have a better physiological capacity to deal with increasing aridification and be less vulnerable to climate change than N. baeticus.
In an ecological context, the differences in desiccation resistance and physiological acclimation capacity between the studied species could be also related to different microhabitat occupations and behavioral plasticity. Both species occupy habitats characterized by a high daily and seasonal climatic variation, which is generally associated with high acclimation capacity [32,33,34]. However, such variability might differ at a microhabitat scale. Dytiscids as Nebrioporus species are strong swimmers, which might confer them a better ability for behavioral stress avoidance by accessing a wide range of microhabitats within the water column. Conversely, the hydrophilid Enochrus species are poor swimmers and mostly associated with the shorelines of aquatic environments [39], where environmental conditions are more extreme and fluctuating, and thus where they could be exposed to higher desiccation pressure. A limited potential for behavioral plasticity in E. jesusarribasi may have favored the evolution of greater plasticity in physiological traits [61], in consistency with the “Bogert effect” [62,63].
Further studies of desiccation resistance traits of saline and freshwater species within each genus, of well-known taxonomy and habitat preference, and accounting for phylogenetic relationships, would shed light on these questions. Besides, transcriptomic studies of the gene expression patterns associated with these CHC changes and their function would help to elucidate the evolutionary constraints on these traits in these water beetle lineages.
5. Concluding Remarks
We have demonstrated that insect cuticle composition and its plasticity play a fundamental and rather complex protective function against desiccation stress in saline aquatic insect species. Species representative of two different aquatic Coleoptera suborders (Adephaga and Polyphaga) showed changes in cuticular hydrocarbons composition and quantity from a relatively short exposure to desiccation, but the variation pattern differed between them. Prior desiccation in E. jesusarribasi induced a positive acclimation response through CHC changes similar to those found in response to salinity stress, mainly the increase in the relative abundance of methyl-branched compounds and higher chain length of alkanes and branched alkanes, showing that they were effective in decreasing cuticular permeability to avoid water loss. In N. baeticus, the observed CHC changes did not increase the waterproofing capacity, so their ecological function remains to be further explored. These interspecific differences reflect the importance of the evolutionary history and ecology in driving responses of species exposed to similar stressors (salinity and desiccation). Our results suggest that E. jesusarribasi, the species with higher tolerance to salinity and desiccation and acclimation capacity, could have a better physiological capacity to deal with increasing aridification and be less vulnerable to climate change than N. baeticus.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects12040285/s1, Table S1: Absolute (ng/specimen) and relative abundance (%) of the cuticular hydrocarbons (CHCs) identified by GC/MS in the different treatments of the species studied. KI: Kovat index; n: number of samples of the total analized where the compound is present.
Author Contributions
Conceptualization, all authors; methodology (desiccation experiments) M.B.-C., S.P. and (cuticular water loss experiments) S.H. and M.B.-C.; formal analysis, M.B.-C., S.P., A.M. and J.V.; writing—original draft preparation, M.B.-C., J.V. and S.P.; writing—review and editing, all authors.; supervision, A.M., J.V. and S.P.; project administration, A.M., J.V.; funding acquisition, A.M., J.V. and M.B.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the R&D&I Project CGL2013-48950-C2-2-P (J.V. and A.M.) (Spanish Ministry of Economy and Competitiveness), co-financed by FEDER funds, and a PhD Grant from the University of Murcia to MBC. S.P is funded by a postdoctoral grant from the Juan de la Cierva-Formación program (FJC2018-035577-I) by the Spanish Ministry of Science and Innovation.
Institutional Review Board Statement
Specimens were collected under collection permission number 201600150115 from the Consejeria de Agua, Agricultura y Medio Ambiente, Región de Murcia.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We thank José Rodríguez and the technicians from the SAI (Servicio de Apoyo a la Investigación de la Universidad de Murcia) for their technical support for the GC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chown, S.L.; Sørensen, J.G.; Terblanche, J.S. Water loss in insects: An environmental change perspective. J. Insect Physiol. 2011, 57, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, A.L.; Marshall, K.E.; MacMillan, H.A.; Williams, C.M.; Sinclair, B.J. Rapid changes in desiccation resistance in Drosophila melanogaster are facilitated by changes in cuticular permeability. J. Insect Physiol. 2010, 56, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Reidenbach, K.R.; Cheng, C.; Liu, F.; Liu, C.; Besansky, N.J.; Syed, Z. Cuticular differences associated with aridity acclimation in African malaria vectors carrying alternative arrangements of inversion 2La. Parasites Vectors 2014, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stinziano, J.R.; Sové, R.J.; Rundle, H.D.; Sinclair, B.J. Rapid desiccation hardening changes the cuticular hydrocarbon profile of drosophila melanogaster. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 180, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Menzel, F.; Zumbusch, M.; Feldmeyer, B. How ants acclimate: Impact of climatic conditions on the cuticular hydrocarbon profile. Funct. Ecol. 2018, 32, 657–666. [Google Scholar] [CrossRef]
- Bond, N.R.; Lake, P.S.; Arthington, A.H. The impacts of drought on freshwater ecosystems: An Australian perspective. Hydrobiologia 2008, 1–14. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Millan, A.; Url, S. Insect Dispersal in a Drying Desert Stream: Effects of Temperature and Water Loss. Naturalist 1998, 43, 80–87. [Google Scholar]
- Dudley, R. The Biomechanics of Insect Flight: Form, Function, Evolution; Princeton University Press: Princeton, NJ, USA, 2000; ISBN 9780691094915. [Google Scholar]
- Strachan, S.R.; Chester, E.T.; Robson, B.J. Freshwater Invertebrate Life History Strategies for Surviving Desiccation. Springer Sci. Rev. 2015, 3, 57–75. [Google Scholar] [CrossRef]
- Chester, E.T.; Miller, A.D.; Valenzuela, I.; Wickson, S.J.; Robson, B.J. Drought survival strategies, dispersal potential and persistence of invertebrate species in an intermittent stream landscape. Freshw. Biol. 2015, 60, 2066–2083. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Gutiérrez-Cánovas, C.; Acosta, R.; Castro-López, D.; Cid, N.; Fortuño, P.; Munné, A.; Múrria, C.; Pimentão, A.R.; Sarremejane, R.; et al. As time goes by: 20 years of changes in the aquatic macroinvertebrate metacommunity of Mediterranean river networks. J. Biogeogr. 2020, 47, 1861–1874. [Google Scholar] [CrossRef]
- Hering, D.; Haidekker, A.; Schmidt-Kloiber, A.; Barker, T.; Buisson, L.; Graf, W.; Grenouillet, G.; Lorenz, A.; Sandin, L.; Stendera, S. Monitoring the Responses of Freshwater Ecosystems to Climate Change. In Climate Change Impacts on Freshwater Ecosystems; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 84–118. ISBN 9781405179133. [Google Scholar]
- Chown, S.L.; Nicolson, S. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004; ISBN 9788578110796. [Google Scholar]
- Blomquist, G.J.; Bagnères, A.G. Insect Hydrocarbons Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780511711909. [Google Scholar]
- Hadley, N. Water Relations of Terrestrial Arthropods; Academic Press: San Diego, CA, USA, 1994; ISBN 9780080918525. [Google Scholar]
- Gibbs, A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002, 48, 391–400. [Google Scholar] [CrossRef]
- Gibbs, A.; Pomonis, J.G. Physical properties of insect cuticular hydrocarbons: The effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 243–249. [Google Scholar] [CrossRef]
- Benoit, J.B. Water Management by Dormant Insects: Comparisons between Dehydration Resistance During Summer Aestivation and Winter Diapause. In Aestivation Molecular and Physiological Aspects; Springer: New York, NY, USA, 2010; ISBN 9783642024207. [Google Scholar]
- Gibbs, A.G.; Rajpurohit, S. Cuticular lipids and water balance Cuticular lipids and water balance. Insect Hydrocarb. Biol. Biochem. Chem. Ecol. 2010, 100–120. [Google Scholar] [CrossRef]
- Rajpurohit, S.; Hanus, R.; Vrkoslav, V.; Behrman, E.L.; Bergland, A.O.; Petrov, D.; Cvačka, J.; Schmidt, P.S. Adaptive dynamics of cuticular hydrocarbons in Drosophila. J. Evol. Biol. 2017, 30, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, P.P.; Burkert, L.H.; Abou, B.; Federle, W.; Menzel, F. Coping with climate: Cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 2018, 221, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Hadley, N. Epicuticular lipids of the desert tenebrionid beetle, Eleodes armata: Seasonal and acclimatory effects on composition. Insect Biochem. 1977, 7, 277–283. [Google Scholar] [CrossRef]
- Noorman, N.; Den Otter, C.J. Effects of relative humidity, temperature, and population density on production of cuticular hydrocarbons in housefly Musca domestica L. J. Chem. Ecol. 2002, 28, 1819–1829. [Google Scholar] [CrossRef]
- Gefen, E.; Talal, S.; Brendzel, O.; Dror, A.; Fishman, A. Variation in quantity and composition of cuticular hydrocarbons in the scorpion Buthus occitanus (Buthidae) in response to acute exposure to desiccation stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 182, 58–63. [Google Scholar] [CrossRef]
- Sprenger, P.P.; Menzel, F. Cuticular hydrocarbons in ants (Hymeno ptera: Formicidae) and other insects: How and why they differ among individuals, colonies, and species. Myrmecol. News 2020, 29, 1–26. [Google Scholar] [CrossRef]
- Hoffmann, A.A. Acclimation for desiccation resistance in Drosophila melanogaster and the association between acclimation responses and genetic variation. J. Insect Physiol. 1990, 36, 885–891. [Google Scholar] [CrossRef]
- Hoffmann, A.A. Acclimation for desiccation resistance in Drosophila: Species and population comparisons. J. Insect Physiol. 1991, 37, 757–762. [Google Scholar] [CrossRef]
- Aggarwal, D.D.; Ranga, P.; Kalra, B.; Parkash, R.; Rashkovetsky, E.; Bantis, L.E. Rapid effects of humidity acclimation on stress resistance in Drosophila melanogaster. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Ferguson, L.V.; Salehipour-Shirazi, G.; Macmillan, H.A. Cross-tolerance and cross-talk in the cold: Relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 2013, 53, 545–556. [Google Scholar] [CrossRef]
- Pallarés, S.; Arribas, P.; Bilton, D.T.; Millán, A.; Velasco, J.; Ribera, I. The chicken or the egg? Adaptation to desiccation and salinity tolerance in a lineage of water beetles. Mol. Ecol. 2017, 26, 5614–5628. [Google Scholar] [CrossRef]
- Janzen, D.H. Why Mountain Passes are Higher in the Tropics. Am. Nat. 1967, 101, 233–249. [Google Scholar] [CrossRef]
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Rohr, J.R.; Civitello, D.J.; Cohen, J.M.; Roznik, E.A.; Sinervo, B.; Dell, A.I. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 2018, 21, 1425–1439. [Google Scholar] [CrossRef]
- Botella-Cruz, M.; Pallarés, S.; Millán, A.; Velasco, J. Role of cuticle hydrocarbons composition in the salinity tolerance of aquatic beetles. J. Insect Physiol. 2019, 117, 103899. [Google Scholar] [CrossRef]
- Arribas, P.; Andújar, C.; Abellán, P.; Velasco, J.; Millán, A.; Ribera, I. Tempo and mode of the multiple origins of salinity tolerance in a water beetle lineage. Mol. Ecol. 2014, 23, 360–373. [Google Scholar] [CrossRef]
- Pallarés, S.; Botella-Cruz, M.; Arribas, P.; Millán, A.; Velasco, J. Aquatic insects in a multistress environment: Cross-tolerance to salinity and desiccation. J. Exp. Biol. 2017, 220, 1277–1286. [Google Scholar] [CrossRef]
- Bilton, D.T.; Ribera, I.; Short, A.E.Z. Water Beetles as Models in Ecology and Evolution. Annu. Rev. Entomol. 2019, 64, 359–377. [Google Scholar] [CrossRef]
- Millán, A.; Sánchez-fernández, D.; Abellán, P.; Picazo, F.; Carbonell, J.A.; Lobo, J.M.; Ribera, I. Atlas de Los Coleópteros Acuáticos de España Peninsular; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2014; ISBN 9788449114182.
- Céspedes, V.; Pallarés, S.; Arribas, P.; Millán, A.; Velasco, J. Water beetle tolerance to salinity and anionic composition and its relationship to habitat occupancy. J. Insect Physiol. 2013, 59, 1076–1084. [Google Scholar] [CrossRef]
- Pallarés, S.; Arribas, P.; Bilton, D.T.; Millán, A.; Velasco, J. The comparative osmoregulatory ability of two water beetle genera whose species span the fresh-hypersaline gradient in inland waters (Coleoptera: Dytiscidae, Hydrophilidae). PLoS ONE 2015, 10, e0124299. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A. Ecology, Systematics, and the Natural History of Predaceous Diving Beetles (Coleoptera: Dytiscidae); Yee, D.A., Ed.; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789401791083. [Google Scholar]
- Botella-Cruz, M.; Villastrigo, A.; Pallarés, S.; López-Gallego, E.; Millán, A.; Velasco, J. Cuticle hydrocarbons in saline aquatic beetles. PeerJ 2017, 5, e3562. [Google Scholar] [CrossRef] [PubMed]
- Schilman, P.E.; Lighton, J.R.B.; Holway, D.A. Respiratory and cuticular water loss in insects with continuous gas exchange: Comparison across five ant species. J. Insect Physiol. 2005, 51, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. ISBN 978-0-387-87457-9. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, 1–128. [Google Scholar] [CrossRef]
- Menzel, F.; Blaimer, B.B.; Schmitt, T. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef]
- Crowson, R. The Biology of the Coleoptera; Academic Press: London, UK, 1981; ISBN 0121960501. [Google Scholar]
- Dulbecco, A.B.; Moriconi, D.E.; Lynn, S.; McCarthy, A.; Juárez, M.P.; Girotti, J.R.; Calderón-Fernández, G.M. Deciphering the role of Rhodnius prolixus CYP4G genes in straight and methyl-branched hydrocarbon formation and in desiccation tolerance. Insect Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Engl, T.; Eberl, N.; Gorse, C.; Krüger, T.; Schmidt, T.H.P.; Plarre, R.; Adler, C.M. Kaltenpoth Ancient symbiosis confers desiccation resistance to stored grain pest beetles. Mol. Ecol. 2018, 27, 2095–2108. [Google Scholar] [CrossRef]
- Leeson, S.A.; Kennington, W.J.; Evans, T.A.; Simmons, L.W. Phenotypic plasticity but no adaptive divergence in cuticular hydrocarbons and desiccation resistance among translocated populations of dung beetles. Evol. Ecol. 2020. [Google Scholar] [CrossRef]
- Buellesbach, J.; Whyte, B.A.; Cash, E.; Gibson, J.D.; Scheckel, K.J.; Sandidge, R.; Tsutsui, N.D. Desiccation Resistance and Micro-Climate Adaptation: Cuticular Hydrocarbon Signatures of Different Argentine Ant Supercolonies Across California. J. Chem. Ecol. 2018, 44, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.; Bergsten, J.; Levkanicova, Z.; Papadopoulou, A.; St. John, O.; Wild, R.; Hammond, P.M.; Ahrens, D.; Balke, M.; Caterino, M.S.; et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 2007, 318, 1913–1916. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, V.; Hoffmann, A.A.; Overgaard, J.; Loeschcke, V.; Sgrò, C.M. Plasticity for desiccation tolerance across drosophila species is affected by phylogeny and climate in complex ways. Proc. R. Soc. B Biol. Sci. 2018, 285, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Origin and evolution of the CYP4G subfamily in insects, cytochrome P450 enzymes involved in cuticular hydrocarbon synthesis. Mol. Phylogenet. Evol. 2020, 143. [Google Scholar] [CrossRef] [PubMed]
- Abellán, P.; Sánchez-Fernández, D.; Picazo, F.; Millán, A.; Lobo, J.M.; Ribera, I. Preserving the evolutionary history of freshwater biota in Iberian National Parks. Biol. Conserv. 2013, 162, 116–126. [Google Scholar] [CrossRef]
- Bernhard, D.; Schmidt, C.; Korte, A.; Fritzsch, G.; Beutel, R.G. From terrestrial to aquatic habitats and back again—Molecular insights into the evolution and phylogeny of Hydrophiloidea (Coleoptera) using multigene analyses. Zool. Scr. 2006, 35, 597–606. [Google Scholar] [CrossRef]
- Short, A.E.Z.; Fikáček, M. Molecular phylogeny, evolution and classification of the Hydrophilidae (Coleoptera). Syst. Entomol. 2013, 38, 723–752. [Google Scholar] [CrossRef]
- Bloom, D.D.; Fikáček, M.; Short, A.E.Z. Clade age and diversification rate variation explain disparity in species richness among water scavenger beetle (Hydrophilidae) lineages. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Shull, V.L.; Vogler, A.P.; Baker, M.D.; Maddison, D.R.; Hammond, P.M. Sequence alignment of 18S ribosomal RNA and the basal relationships of adephagan beetles: Evidence for monophyly of aquatic families and the placement of trachypachidae. Syst. Biol. 2001, 50, 945–969. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Armstrong, E.J.; Stillman, J.H. Multiple Stressors in a Changing World: The Need for an Improved Perspective on Physiological Responses to the Dynamic Marine Environment. Ann. Rev. Mar. Sci. 2016, 8, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Bogert, C.M. Thermoregulation in reptiles; a factor in evolution. Evolution 1949, 3, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Hertz, P.E.; Sinervo, B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am. Nat. 2003, 161, 357–366. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).