Simple Summary
Nosemosis is a disease in bees that causes severe problems for their vitality, reproduction and productivity. Traditionally, the treatment involves the application of fumagillin, an antibiotic with proven effect. Recently, fumagillin production and registration has faced problems worldwide, leading to the absence of adequate treatment. Motivated by the reported health issues and the occurrence of residues after fumagillin application, scientists around the world have sought a medication or a supplement that could help beekeepers to control Nosema. Current trends include the search for alternative non-antibiotic treatments. In a laboratory (cage) experiment, we studied the effect of fumagillin and natural extract of mushroom Agaricus blazei on the survival of Nosema infected bees, Nosema spore loads and levels of immune-related gene expression and oxidative stress markers. The results undoubtedly confirmed the anti-Nosema effect of fumagillin, as seen in better bee survival and monitored parameters; its application without Nosema infection (preventive) caused disturbance in some of the parameters. The application of A. blazei extract, however, showed positive effects in both preventive and curative applications. These beneficial properties of A. blazei extract indicate a potential that needs to be further investigated.
Abstract
Depending on the infection level and colony strength, Nosema ceranae, a microsporidian endoparasite of the honey bee may have significant consequences on the health, reproduction and productivity of bee colonies. Despite exerting some side effects, fumagillin is most often used for Nosema control. In this study, in a cage experiment, N. ceranae infected bees were treated with fumagillin or the extract of Agaricus blazei mushroom, a possible alternative for Nosema control. Bee survival, Nosema spore loads, the expression levels of immune-related genes and parameters of oxidative stress were observed. Fumagillin treatment showed a negative effect on monitored parameters when applied preventively to non-infected bees, while a noticeable anti-Nosema effect and protection from Nosema-induced immunosuppression and oxidative stress were proven in Nosema-infected bees. However, a protective effect of the natural A. blazei extract was detected, without any side effects but with immunostimulatory activity in the preventive application. The results of this research suggest the potential of A. blazei extract for Nosema control, which needs to be further investigated.
1. Introduction
Nosema ceranae is a microsporidian endoparasite of the European honey bee, Apis mellifera [1,2,3], which infects the midgut but is also detected in other tissues [4,5,6] and the haemolymph [7] without any confirmed pathologic impact out of the ventricular epithelium [8]. N. ceranae is the dominant species in Europe [9,10,11,12], including Serbia [13,14,15,16]. Depending on the infection level, it could exert significant consequences on bee health [17,18,19], reproduction and the productivity of bee colonies [20,21,22]. Moreover, in the majority of laboratory experiments with artificially infected bees, N. ceranae decreased bees’ lifespan (reviewed in [18]). The impact of N. ceranae on honey bee immunity has been investigated more thoroughly in recent years. Some conclusions of the research underline N. ceranae–induced suppression of immune-related genes [23,24,25,26,27,28], proving its immunosuppressive impact. N. ceranae infection induced disorder in the expression of genes involved in homeostasis and renewal of intestinal tissues [29] and genes related to the host cell’s cycle and apoptosis [30,31]. This was reflected in the prevention of the apoptosis and self-renewal of ventricular epithelial cells [21,29,30,31,32]. Other researchers reported alterations in the carbohydrate metabolism in N. ceranae infected honey bees, which induced nutritional and energetic stress [19,33,34,35]. Energetic stress was reported in infected forager bees, which were hungrier than their uninfected counterparts [36] and consumed more sugar [37,38,39]. Moreover, the increase in oxidative stress was recorded through disturbed antioxidant enzyme levels as a response to N. ceranae infection [29,39,40].
The treatment for nosemosis includes the use of fumagillin, an antibiotic obtained from the fungus Aspergillus fumigatus. Soon after its discovery [41], fumagillin was proven to be effective in Nosema control [42,43]. It is available in a few commercial formulations (Fumagilin-B, Fumidil B, Fumagilin DCH, etc.) registered in the USA [44], Canada [45] and Argentina [46], while there are no registered formulations in Europe [46]. Due to severe bee losses and the high prevalence of N. ceranae in several European countries: United Kingdom, Spain, Belgium, Greece, Hungary, Romania, etc., provisional approvals were obtained for the use of fumagillin under veterinary supervision for the treatment of Nosema-positive colonies [44]. However, negative effects of fumagillin were described [47,48,49,50,51,52,53] as well as the risk of residues in bee products [54,55,56,57,58], which is why researchers have been looking for alternatives that could replace fumagillin [50]. Among the tested alternatives, some natural-based treatments and dietary supplements showed promising effects [28,59,60,61,62,63,64]. Polysaccharide-rich extracts from algae showed potential for Nosema control [59,60], the extract of mushroom mycelia was effective against bee viruses [65], and Agaricus blazei mushroom extract increased colony strength [66]. In this study we conducted a laboratory/cage trial to investigate (1) if A. blazei extract has a beneficial effect in Nosema control and (2) the impact of fumagillin on the health of infected bees.
2. Materials and Methods
2.1. Bees
All bees used in the experiment originated from healthy Apis mellifera colonies belonging to the experimental apiary of the University of Belgrade—Faculty of Veterinary Medicine. The absence of Nosema infection in the colonies was proven with the methodology described by Stevanovic et al. [13]. There was also no evidence to suggest the presence of other bee diseases after following the methods described in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals published by Office International des Epizooties (OIE) [67] and the COLOSS BEEBOOK recommendations [68], except for Varroa infestation, which was kept at a low level. The apiary was monitored daily by a licensed veterinarian experienced in the field of bee diseases.
2.2. Test Preparations
The feeding solution was made in sucrose syrup (50% w/v) with antibiotic fumagillin dicyclohexylamine (CAS No. 41567-78-6) with a concentration of 26.4 mg/L [57], taking care to exclude factors affecting the efficacy and stability of fumagillin (fumagillin solution was prepared using demineralized water, kept in amber vials, and used immediately after the preparation).
Hot water extract of Agaricus blazei (syn. A. brasiliensis) mushroom strain M7700 (Mycelia bvba, Nevele, Belgium) was prepared according to previously described methods [66,69]. The extract was rich in polysaccharides (45.9 g/100 g), mostly glucans (40.1 g/100 g) (α-glucans 17.3 g/100 g and β-glucans 22.8 g/100 g [70,71,72]), phenols (1 g/100 g) and proteins (4.7 g/100 g) [73]. The feeding solution was made in sucrose syrup (50% w/v) with a concentration of 0.2 mg/g [66].
2.3. Experimental Design
Frames with a sealed brood prior to emergence were taken from the five chosen colonies, placed in net bags (to prevent the dissipation of emerged bees) and kept overnight in an incubator (Figure 1) with a constant temperature (34 ± 1 °C) and humidity (66 ± 1%). The following morning, newly emerged worker bees were randomly collected from different frames and allocated to cages. Eighty bees were placed in each cage (specially designed by Glavinic et al. [27] for this purpose). Two series of the whole experiment were performed and the merged data were processed.

Figure 1.
Frames with sealed brood placed in net bags [27].
All groups were fed 50% (w/v) sugar solution. The two controls, the non-infected (NI) and the infected (I), were not given anything else (Table 1). There were 4 groups of bees treated with either fumagillin or A. blazei extract, mixed in the diet and given as follows: from day 1 after emergence to non-infected bees (groups F and AB) and to infected bees (I-F1 and I-AB1) and to infected bees from day 3 (I-F3 and I-AB3) and from day 6 (I-F6 and I-AB6).

Table 1.
Experimental design.
2.4. Inoculum Preparation, Experimental Infection and Bee Sampling
The inoculum preparation and experimental infection were completed according to a previously described methodology [27]. In brief, the inoculum with a final concentration of 1 × 106 spores/ml in a 50% sucrose solution was freshly prepared using N. ceranae infected bees. PCR determination of Nosema species (absence of N. apis and presence of N. ceranae) was done as previously outlined [27]. On day 3 the infected control group (I) and all treatment groups (I-F1, I-F3, I-F6, I-AB1, I-AB3 and I-AB6) were infected (Table 1).
From each cage, on days 6, 9 and 15, five bees were sampled for the RNA extraction, five for the analyses of oxidative stress and 10 for Nosema spore counting. The remaining 20 bees in each cage served for survival control until the end of the experiment. Dead bees were removed daily and their numbers recorded for the evaluation of survival rates.
2.5. Nosema Spore Counting
Bee abdomens were individually placed in 1.5 mL tubes and homogenized in 1 mL of distilled water with 3 mm tungsten carbide beads (Qiagen, Germany) in a TissueLyser II (Qiagen, Germany) for 1 min at 25 Hz. N. ceranae spore was estimated for each bee using a haemocytometer according to Cantwell [74] and OIE [75].
2.6. Extraction of RNA and cDNA Synthesis
For the total RNA extraction, the Quick-RNA MiniPrep Kit (Zymo Research, USA) was used. Each single honey bee was placed in a sterile 1.5 mL polypropylene tube with 500 μL of Genomic Lysis Buffer and homogenized using a 3 mm tungsten carbide bead (Qiagen, Hilden, Germany) in a TissueLyser II (Qiagen, Hilden, Germany) for 1 min at 25 Hz. Other steps of extraction were performed according to the manufacturer’s instructions. During the extraction process the samples passed through “in-column DNase treatment” (treatment with DNase I Reaction Mix) in order to remove any contaminating DNA. The total extracted RNA was immediately used to generate cDNA using the RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Vilnius, Lithuania), according to the manufacturer’s instructions.
2.7. Real-Time Quantitative PCR
Quantitative PCR (qPCR) amplification was performed using the SYBR green method in a 20 μL reaction mixture with the FastGene® IC Green 2× qPCR Universal Mix (Nippon Genetics Europe, Düren, Germany) following the manufacturer’s instructions. For each gene a specific primer pair was used (Table 2). The qPCR reactions were carried out in a 36 well rotor using Rotor-Gene Q 5plex (Qiagen Inc., Hilden, Germany). The amplification was performed according to the following protocol: 95 °C for 2 min followed by 40 cycles of 95 ℃ for 5 s and annealing temperatures for 30 s. Quantification of gene expression levels was performed using the 2−ΔΔCT method as described in our previous works [27,76,77]. β-actin was used as an internal control gene, and the median value of the NI group was used as a calibrator.

Table 2.
Primer pairs used for qPCR analyses.
2.8. Oxidative Stress Parameters
The activities of antioxidative enzymes superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) as well as the concentrations of malondialdehyde (MDA) were determined by the spectrophotometric analyses described in Dubovskiy et al. [80] and adapted by Glavinic [28]. Pools of five bees collected from every cage on each sampling day (6, 9 and 15) were used and analyzed on a UV/VIS Spectrophotometer BK-36 S390 (Biobase Biodustry, Shanghai, China).
2.9. Statistical Methods
The survival dynamics in the groups of bees was presented with the Kaplan-Meier survival function. The significance of the differences in survival distribution between pairs of groups was compared with the log-rank test.
Gene expression and spore load data were heterogeneous, so the hypothesis of the equality of the medians of three or more groups was tested with the Kruskal-Wallis test. To determine the significance of the difference between the two averages, the Mann-Whitney U test was used.
The data on oxidative stress were homogeneous within the samples for each parameter, and the significance of the differences between three or more means was tested with ANOVA. Then, the difference between the means of sample pairs was tested with the Tukey test.
The statistical analyses of the results were done with Statistica Software (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Bee Survival
The χ2 statistics showed no significant differences in bee survival when all groups treated with fumagillin and control groups were compared (p = 0.083). Since the level of significance was close to the critical risk level, the survival of the bees between the two groups was compared using the log-rank test. The results revealed that mortality in group I was higher (Figure 2) than in the NI (p = 0.008), I-F1 (p = 0.038) and I-F3 (p = 0.039) groups.
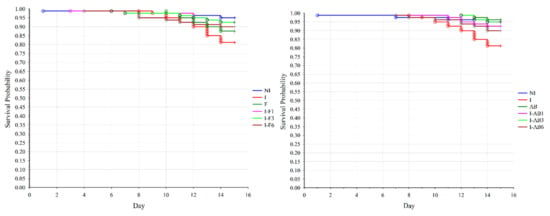
Figure 2.
Effects of treatment with fumagillin and A. blazei extract on the survival rate of N. ceranae infected bees. Survival rate was based on the daily accumulated mortality. The comparison was made between the non-infected control (NI), N. ceranae infected control (I) and groups infected and treated with fumagillin from day 1 (I-F1), day 3 (I-F3) and day 6 (I-F6) or A. blazei extract from day 1 (I-AB1), day 3 (I-AB3) and day 6 (I-AB6). Group names are indicated in Table 1.
A significant difference in bee survival (p = 0.006) was affirmed when comparing groups treated with A. blazei extract and control groups using χ2 statistics. The log-rank test revealed that mortality was higher in group I (Figure 2) than in the NI (p = 0.008), AB (p = 0.003), and I-AB1 and I-AB3 (p = 0.037) groups.
When corresponding groups (groups in which the treatment began on the same day) from the two treatments (fumagillin/A. blazei extract) were compared, the log-rank test showed that bee survival rates differed significantly (p = 0.041) only between the groups treated with fumagillin (F) and the group treated with A. blazei extract (AB) without infection.
3.2. Quantification of N. ceranae Spores
Samples from the non-infected control (NI) and non-infected treated groups (F and AB) and samples collected on day 6 remained negative for N. ceranae spores. The Kruskal-Wallis test showed significant differences in the numbers of N. ceranae spores on days 9 and 15 (p < 0.001) between the groups (Figure 3).
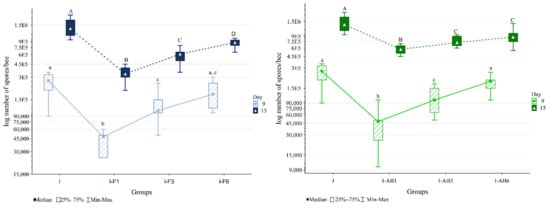
Figure 3.
Nosema spore loads in infected control (I) and groups infected and treated with fumagillin from day 1 (I-F1), day 3 (I-F3) and day 6 (I-F6) or A. blazei extract from day 1 (I-AB1), day 3 (I-AB3) and day 6 (I-AB6). Group names are indicated in Table 1. Groups labelled with the same letter did not differ significantly. The same font size (lowercase or uppercase) refers to the same time point.
The comparison of groups treated with A. blazei extract and control groups (Figure 3) in the Mann-Whitney U test on day 15 revealed higher spore loads in group I compared to other groups: I-AB1 (p < 0.001), I-AB3 (p < 0.001) and I-AB6 (p = 0.005). In addition, the spore load was lower in group I-AB1 than in I-AB3 (p = 0.003) and I-AB6 (p = 0.002).
According to the Mann-Whitney U test, on day 15 the spore loads were significantly lower in groups treated with fumagillin than in the corresponding A. blazei treated groups (Figure 3).
3.3. Comparison of Oxidative Stress Parameters
Analysis of variance showed significant differences (p < 0.01) between fumagillin-treated groups in all oxidative stress parameters at all time points (day 6, 9 and 15). However, the most significant changes were detected on day 15, when CAT activity was higher in I-F3 and I-F6 compared to all other groups, while GST activity was highest in the I-F6 group and lowest in the F and I-F1 groups. The activity of SOD was highest in the I group but lowest in NI and F groups. MDA concentration was higher in I-F1 than in F, I-F3 and I-F6 groups (Figure 4 and Figure S1).
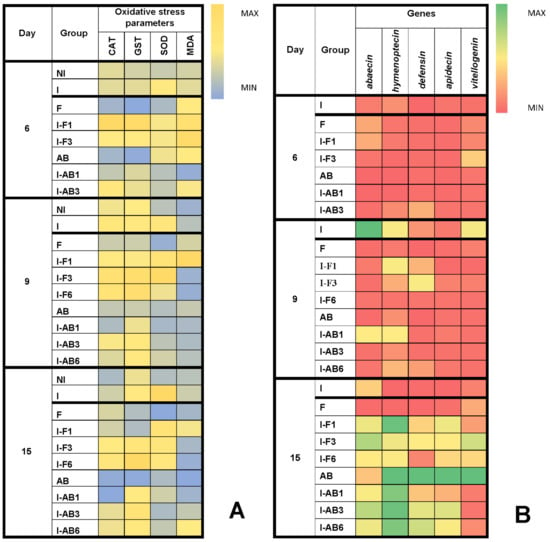
Figure 4.
Heatmaps: (A) mean values for superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) activities and malondialdehyde (MDA) concentration; (B) immune-related genes (medians of Log2 of relative expression ratios for abaecin, hymenoptaecin, defensin, apidaecin and vitellogenin) at different time points in experimental groups. Non-infected control (NI), N. ceranae infected control (I) and groups infected and treated with fumagillin from day 1 (I-F1), day 3 (I-F3) and day 6 (I-F6) or A. blazei extract from day 1 (I-AB1), day 3 (I-AB3) and day 6 (I-AB6). Group names are indicated in Table 1.
Analysis of variance revealed statistically significant differences (p < 0.01) within A. blazei extract treated groups at all the time points (day 6, 9 and 15) and in all parameters, except for MDA concentration on day 15. Again, the most significant changes were detected on day 15, as the Tukey test determined. CAT activity in AB and I-AB1 groups was significantly lower than in I, I-AB3 and I-AB6 (Figure 4 and Figure S1). GST activity was lowest in the AB group compared to all others, and SOD activity was highest in the I group, while, similarly to day 9, there were no significant differences in MDA concentrations (Figure 4 and Figure S1).
3.4. Gene Expression Analyses
Analyzing bees treated with fumagillin and collected on day 6, the Kruskal-Wallis test showed no differences between the groups in the expression of the immune-related genes (p > 0.05). On day 9 the differences between groups were significant for all of the monitored genes (Figure 4): apidaecin, defensin, vitellogenin, abaecin (p < 0.01 for these four) and hymenoptaecin (p < 0.05), according to the Kruskal-Wallis test. The most important changes were obtained on day 15 (Figure 4 and Figure S2), when differences in mRNA levels were significant for apidaecin, defensin, abaecin and hymenoptaecin (p < 0.01) as well as for vitellogenin (p < 0.05). When comparing levels of each gene between two groups with the Mann-Whitney U test, a lower abaecin mRNA level was found in group F compared to I (p = 0.037) and all other groups (p = 0.012). Hymenoptaecin mRNA levels were lower in group I than in all the others (p = 0.012), except for the F group, while they were higher (p < 0.05) in I-F1 than in all groups except I-F3. Defensin mRNA level (Figure 4 and Figure S2) was lower (p = 0.012) in group F than in all other groups treated with fumagillin (I-F1, I-F3 and I-F6). Moreover defensine expression levels were lower in groups I and I-F6 compared to I-F1 and I-F3 (p ≤ 0.037). Apidaecin mRNA levels were lower in groups I and F compared to the other groups (p ≤ 0.022) and in group I-F6 compared to I-F1 (p = 0.012). Vitellogenin mRNA levels were higher in group I-F3 than in all other groups (p ≤ 0.037).
According to the Kruskal-Wallis test, gene expression in bees treated with Agaricus blazei extract collected on day 6 (Figure 4) was significantly different (p < 0.05) only when considering the abaecin gene (p = 0.022). On day 9, the Kruskal-Wallis test revealed significantly different gene expression levels of abaecin (p = 0.003), defensin (p = 0.003) and vitellogenin (p = 0.015). Again, the most important results were obtained on day 15, when expression levels of all genes (Figure 4 and Figure S2 differed significantly between the groups (Kruskal-Wallis Test, p ≤ 0.012). Abaecin gene expression was significantly higher in the I-AB3 group than in AB (p = 0.012) and I-AB1 (p = 0.011). Levels of hymenoptaecin, defensin and apidaecin gene expression were higher (p < 0.05) in the AB group and lower (p < 0.05) in the I group than in all the rest. The mRNA of the vitellogenin gene was also significantly higher (p < 0.05) in the AB group than in all other groups (Figure 4 and Figure S2).
4. Discussion
Higher bee mortality in the infected (I) group compared to the non-infected (NI) group confirmed that N. ceranae was a cause of bee mortality, which is consistent with previous cage experiments [27,36,81,82]. However, the mortality rates were not high, and were below 20% in the infected group within 15 days. Similar bee mortality in the infected (I) and fumagillin-treated (F) groups indicates that fumagillin given to non-infected bees had some impact on their mortality, which was also reported in previous works [28,48,51]. By contrast, the mortality was significantly lower in all N. ceranae infected groups that received fumagillin (I-F1, I-F3 and I-F6) than in the infected control (I), proving better survival of N. ceranae infected bees treated with fumagillin [57]. Better bee survival during the experiment was also detected in groups infected with N. ceranae and treated with Agaricus blazei extract from days 1 (I-AB1) and 3 (I-AB3), while good bee survival in the non-infected group treated with the A. blazei extract (AB) indicates that this extract does not increase bee mortality. This is not surprising, given the recent research of Perish et al. [83], in which diets that contained fungal spores increased bee longevity. Although our previous work showed that this extract increased colony strength parameters [66], this is the first research conducted to test its effect on Nosema infected bees, prompted by the findings that some other polysaccharide-rich extracts (mostly from algae) showed promising effects [59,60].
The absence of Nosema spores in non-infected bee groups (NI, F and AB) confirms that the cage-type experiment used in this study prevents cross-contamination during the research [27]. The presence of Nosema spores in all infected groups proved that the inoculum with the final concentration of 1 × 106 spores/ml succeeded in causing the infection, similar to some previous experiments [24,27,76,77].
On day 6, Nosema spores were not detected in any of the experimental groups. This was expected given that, at that time (3 days post-infection), only a few epithelial cells were infected with Nosema [2] and that an intense development of Nosema was recorded six [24], nine [27,76], ten [2,84] or even twelve days post-infection [49]. On day 9, and at the end of the experiment (on day 15), the highest spore load was in the infected but not treated (I) group as compared to the treated group (Figure 3), indicating the anti-Nosema effect of the applied treatments. For fumagillin, the highest spore number (excluding the I group) was in the group treated from day 6 (I-F6), and the lowest was in the group treated from day 1 (I-F1), which proves the direct relation between fumagillin treatment and the number of Nosema spores, confirming the known anti-Nosema effect of fumagillin [3,28,45,57,85,86,87].
Treatment with A. blazei extract also showed an anti-Nosema effect in all groups on day 9 and 15, with the exception of the bees from the group treated from day 6 (I-AB6) and collected on day 9. The lowest number of spores was detected in the I-AB1 group, which received the extract from the first day of the experiment. Such an effect, more potent in prevention (applied before or at the time of the infection) than in therapy, was also proven for some other plant extracts [88] and dietary supplements [27].
On day 15 the levels of most of the oxidative stress parameters were significantly higher in the infected group (I) compared to group treated with fumagillin (F) (especially SOD and GST) and the AB group (especially SOD and CAT). This confirmed the previously described N. ceranae–induced oxidative stress (reviewed in [19]), detected especially through GST activity [29,40]. By contrast, lower CAT, SOD and GST activity and MDA concentration in the group treated with fumagillin (F) showed the absence of fumagillin-induced oxidative stress, although some other substances applied to honey bees, such as caffeine [89,90] or vitamin C [91], could cause an increase in anti-oxidative activity. Lower oxidative stress detected in group AB (treated with A. blazei extract from the beginning of the experiment) could be explained by its proven anti-oxidative effect [68].
Nosema ceranae–induced suppression of immune-related genes (group I) on day 15 in this experiment is consistent with the results of other similar research [23,24,25,26,27,28]. Moreover, the lower levels of immune-related genes detected in group F, which received fumagillin (without Nosema infection), proved its immune-suppressive effect. This is the first study in which the effects of fumagillin were investigated by monitoring the expression of immune genes and oxidative stress parameters. The obtained immune-suppressive effect of fumagillin is comparable with recent findings of the impact of some other antibiotics on the expression of genes for immune peptides [92]. However, the levels of immune-related genes were significantly higher in all Nosema infected groups that received fumagillin. It may be assumed that this effect was achieved through reducing Nosema levels in fumagillin-treated groups (which is confirmed in Nosema spore load analyses), and the resulting prevention of Nosema-induced immune suppression. Despite the negative effects of fumagillin described previously [47,48,49,50] as well as its genotoxic potential [50,52,53] and risk of leaving residues in bee products [54,55,56,57,58], this antibiotic is still considered to be most effective in the treatment of N. ceranae infection [44,50,57,85,86].
Gene expression levels in bees treated with A. blazei were increased on day 15, showing the immune-stimulating effect in group AB. Gene expression levels for hymenoptaecin, defensin and apidaecin in groups I-AB1, I-AB3 and I-AB6, infected with Nosema and treated with A. blazei extract (higher when compared to group I) indicate the positive effect of the extract in protection from N. ceranae–induced immune suppression. The water extract of A. blazei mushroom used in this experiment is comparable with other polysaccharide-rich extracts that showed a positive effect in Nosema-infected bees [60]. Based on the findings of Hayman et al. [93], Roussel et al. [60] suggested that sulphated polysaccharides could have the potential to prevent microsporidian spore adherence to host cells and their subsequent infection. However, spore loads were lower in the group treated with fumagillin (F) than in the A. blazei extract–treated group (AB). This could be explained by the autoinfection process in the bee midgut [2], which occurs secondarily between neighboring cells and which cannot be inhibited by sulphated polysaccharides [60], as happens in the case of adherence of microsporidia from the gut lumen.
5. Conclusions
Agaricus blazei extract in this study increased the expression of the majority of immune-related genes, irrespective of the presence of Nosema infection. Moreover, fumagillin showed a beneficial effect in terms of reducing some negative effects of Nosema infection (by decreasing Nosema loads and consequently preventing Nosema-induced immune suppression and oxidative stress). Bearing in mind the observed negative effects of fumagillin and the absence of registered fumagillin formulations in Europe [45,46], it is justified to look for a natural alternative for Nosema control. The positive protective effect of completely natural A. blazei extract proven in this research shows potential in combatting Nosema and deserves to be further investigated.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects12040282/s1, Figure S1: Levels of CAT, GST and SOD activities and MDA concentrations in experimental groups on day 15; Figure S2: Expression levels of immune-related genes (abaecin, hymenoptaecin, defensin, apidaecin and vitellogenin) in experimental groups on day 15.
Author Contributions
Conceptualization: Z.S., U.G. and J.S.; design of experiment and methodology: U.G. and Z.S.; laboratory analysis: U.G., M.R. (Marko Ristanic), M.R. (Milan Rajkovic) and D.D.; data curation: U.G. and N.L.; writing, review and editing: U.G., Z.S. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract no. 451-03-68/2020-14/200143, for the project led by Zoran Stanimirovic).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the excessive data size.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef]
- Higes, M.; Garcia-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, 73–79. [Google Scholar] [CrossRef]
- Chen, Y.P.; Evans, J.D.; Murphy, C.; Gutell, R.; Zuker, M.; Gundersen-Rindal, D.; Pettis, J.S. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J. Eukaryot. Microbiol. 2009, 56, 142–147. [Google Scholar] [CrossRef]
- Copley, T.R.; Jabaji, S.H. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. J. Appl. Microbiol. 2012, 112, 15–24. [Google Scholar] [CrossRef]
- Gisder, S.; Hedtke, K.; Möckel, N.; Frielitz, M.C.; Linde, A.; Genersch, E. Five-year cohort study of Nosema spp. in Germany: Does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microb. 2010, 76, 3032–3038. [Google Scholar] [CrossRef]
- Glavinic, U.; Stevanovic, J.; Gajic, B.; Simeunovic, P.; Đuric, S.; Vejnovic, B.; Stanimirovic, Z. Nosema ceranae DNA in honey bee haemolymph and honey bee mite Varroa destructor. Acta Vet. Beograd. 2014, 64, 349–357. [Google Scholar]
- Higes, M.; García-Palencia, P.; Urbieta, A.; Nanetti, A.; Martín-Hernández, R. Nosema apis and Nosema ceranae tissue tropism in worker honey bees (Apis mellifera). Vet. Pathol. 2020, 57, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Chauzat, M.P.; Higes, M.; Martín-Hernández, R.; Meana, A.; Cougoule, N.; Faucon, J.P. Presence of Nosema ceranae in French honeybee colonies. J. Apicult. Res. 2007, 46, 127–128. [Google Scholar] [CrossRef]
- Martín-Hernandez, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microb. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tapaszti, Z.; Forgách, P.; Kővágó, C.; Békési, L.; Bakonyi, T.; Rusvai, M. First detection and dominance of Nosema ceranae in Hungarian honeybee colonies. Acta Vet. Hung. 2009, 57, 383–388. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Genersch, E.; Kovacevic, R.S.; Ljubenkovic, J.; Radakovic, M.; Aleksic, N. Dominance of Nosema ceranae in honey bees in the Balkan countries in the absence of symptoms of colony collapse disorder. Apidologie 2011, 41, 49–58. [Google Scholar] [CrossRef]
- Stevanovic, J.; Simeunovic, P.; Gajic, B.; Lakic, N.; Radovic, D.; Fries, I.; Stanimirovic, Z. Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie 2013, 44, 522–536. [Google Scholar] [CrossRef]
- Stevanovic, J.; Schwarz, R.S.; Vejnovic, B.; Evans, J.D.; Irwin, R.E.; Glavinic, U.; Stanimirovic, Z. Species-specific diagnostics of Apis mellifera trypanosomatids: A nine-year survey (2007–2015) for trypanosomatids and microsporidians in Serbian honey bees. J. Invertebr. Pathol. 2016, 139, 6–11. [Google Scholar] [CrossRef]
- Taric, E.; Glavinic, U.; Stevanovic, J.; Vejnovic, B.; Aleksic, N.; Dimitrijevic, V.; Stanimirovic, Z. Occurrence of honey bee (Apis mellifera L.) pathogens in commercial and traditional hives. J. Apicult. Res. 2019, 58, 433–443. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Garrido-Bailón, E.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; García-Palencia, P.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernandez, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [PubMed]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.; Vejnović, B.; Stevanović, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet. Beograd. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef]
- Higes, M.; Meana, A.; Bartolomé, C.; Botías, C.; Martín-Hernández, R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef]
- Simeunovic, P.; Stevanovic, J.; Cirkovic, D.; Radojicic, S.; Lakic, N.; Stanisic, L.J.; Stanimirovic, Z. Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. J. Apicult. Res. 2014, 53, 545–554. [Google Scholar] [CrossRef]
- Antunez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune-suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J. Insect Physiol. 2012, 58, 1090–1095. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS ONE 2017, 002012, e0173438. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef]
- Glavinic, U. The Effects of Various Antimicrobials and Supplements on the Expression of Immune-Related Genes, Oxidative Stress and Survival of Honey Bee Apis mellifera Infected with microsporidium Nosema ceranae. Ph.D. Thesis, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia, 2019. [Google Scholar]
- Dussaubat, C.; Brunet, J.L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.H.; Martín-Hernández, R.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef] [PubMed]
- Kurze, C.; Le Conte, Y.; Dussaubat, C.; Erler, S.; Kryger, P.; Lewkowski, O.; Müller, T.; Widder, M.; Moritz, R.F. Nosema tolerant honeybees (Apis mellifera) escape parasitic manipulation of apoptosis. PLoS ONE 2015, 10, e0140174. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Higes, M.; Sagastume, S.; Juarranz, Á.; Dias-Almeida, J.; Budge, G.E.; Meana, A.; Boonham, N. Microsporidia infection impacts the host cell’s cycle and reduces host cell apoptosis. PLoS ONE 2017, 12, e0170183. [Google Scholar] [CrossRef]
- Higes, M.; Juarranz, A.; Dias-Almeida, J.; Lucena, S.; Botias, C.; Meana, A.; García-Palencia, P.; Martín-Hernández, R. Apoptosis in the pathogenesis of Nosema ceranae (Microsporidia: Nosematidae) in honey bees (Apis mellifera). Environ. Microbiol. Rep. 2013, 5, 530–536. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Copley, T.; Jabaji, S. Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012, 58, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Vidau, C.; Panek, J.; Texier, C.; Biron, D.G.; Belzunces, L.P.; Le Gall, M.; Broussard, C.; Delbac, F.; El Alaoui, H. Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J. Invertebr. Pathol. 2014, 121, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernandez, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; Alaoui, H.E.; et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef]
- Vejnovic, B. Molecular Genetic Identification of Lotmaria passim Schwarz, 2014 Trypanosome and the Analysis of its Impact on the Health of Bee Colonies and Economic Effects in Apiculture. Ph.D. Thesis, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia, 2019. [Google Scholar]
- Hanson, F.R.; Eble, T.E. An antiphage agent isolated from Aspergillus sp. J. Bacteriol. 1949, 58, 527–529. [Google Scholar] [CrossRef]
- Katznelson, H.; Jamieson, C.A. Control of Nosema disease of honeybees with fumagillin. Science 1952, 115, 70–71. [Google Scholar] [CrossRef]
- Bailey, L. Effect of fumagillin upon Nosema apis (Zander). Nature 1953, 171, 212–213. [Google Scholar] [CrossRef]
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martín-Hernández, R.; Bernal, J.L. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377. [Google Scholar] [CrossRef]
- McCallum, R.; Olmstead, S.; Shaw, J.; Glasgow, K. Evaluating efficacy of Fumagilin-B® against Nosemosis and tracking seasonal srends of Nosema spp. in Nova Scotia Honey bee colonies. J. Apic. Sci. 2020. [Google Scholar] [CrossRef]
- Sarlo, E.G.; Medici, S.K.; Porrini, M.P.; Garrido, P.M.; Floris, I.; Eguaras, M.J. Comparison between different fumagillin dosage and evaluation method in the apiary control of Nosemosis type C. Redia 2011, 94, 39–44. [Google Scholar]
- Liu, T.P. Ultrastructural changes in the secretion granules of the hypopharangeal glands of the honeybee infected by Nosema apis and after treatment with fumagillin. Tissue Cell. 1990, 22, 523–531. [Google Scholar] [CrossRef]
- Rada, V.; Machova, M.; Huk, J.; Marounek, M.; Dušková, D. Microflora in the honeybee digestive tract: Counts, characteristics and sensitivity to veterinary drugs. Apidologie 1997, 28, 357–365. [Google Scholar] [CrossRef][Green Version]
- Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef] [PubMed]
- Van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.A.; Pernal, S.F. Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assays. Apidologie 2016, 47, 617–630. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Otto, S.J.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. The effect of dicyclohexylamine and fumagillin on Nosema ceranae-infected honey bee (Apis mellifera) mortality in cage trial assays. Apidologie 2016, 47, 663–670. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Stevanovic, J.; Bajic, V.; Radovic, I. Evaluation of genotoxic effects of fumagillin (dicyclohexylamine) by citogenetic tests in vivo. Mut. Res. Gen. Tox. En. 2007, 628, 1–10. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Radakovic, M.; Stojic, V. In vitro evaluation of the clastogenicity of fumagillin. Environ. Mol. Mutagen. 2008, 49, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.I.; Pettis, J.S.; Smith, I.B.; Chu, P.S. Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS. J. Agric. Food Chem. 2008, 56, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Nozal, M.J.; Bernal, J.L.; Martin, M.T.; Bernal, J.; Alvaro, A.; Martín, R.; Higes, M. Trace analysis of fumagillin in honey by liquid chromatography-diode array-electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1190, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Sasamoto, T.; Takeba, K.; Hayashi, H.; Kusano, T.; Matsushima, Y.; Nakajima, T.; Kanai, S.; Takano, I. Rapid determination of fumagillin residues in honey by liquid chromatography-tandem mass spectrometry using the QuEChERS method. J. AOAC Int. 2011, 94, 878–885. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agr. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Pernal, S.F. Determination of dicyclohexylamine and fumagillin in honey by LC-MS/MS. Food Anal. Method. 2015, 8, 767–777. [Google Scholar] [CrossRef]
- Charistos, L.; Parashos, N.; Hatjina, F. Long term effects of a food supplement HiveAlive™ on honey bee colony strength and Nosema ceranae spore counts. J. Apicult. Res. 2015, 54, 420–426. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee Nosemosis. Carbohyd. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Nanetti, A.; Rodriguez-García, C.; Meana, A.; Martín-Hernández, R.; Higes, M. Effect of oxalic acid on Nosema ceranae infection. Res. Vet. Sci. 2015, 102, 167–172. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R.; Koziatek, S. Evaluation of the effectiveness of selected treatments of Nosema spp. infection by the hemocytometric method and duplex PCR. Acta Vet. Beograd. 2016, 66, 115–124. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R. Estimation of the influence of selected products on co-infection with N. apis/N. ceranae in Apis mellifera using real-time PCR. Invertebr. Reprod. Dev. 2018, 62, 92–97. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® Treatments against Nosema ceranae Infection in Apis mellifera Investigated by Two qPCR Methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef]
- Stamets, P.E.; Naeger, N.L.; Evans, J.D.; Han, J.O.; Hopkins, B.K.; Lopez, D.; Moershel, H.M.; Nally, R.; Sumerlin, D.; Taylor, A.W.; et al. Extracts of polypore mushroom mycelia reduce viruses in honey bees. Sci. Rep. UK 2018, 8, 13936. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Simeunovic, P.; Lakic, N.; Radovic, I.; Sokovic, M.; Van Griensven, J.L.D. The effect of Agaricus brasiliensis extract supplementation on honey bee colonies. An. Acad. Bras. Cienc. 2018, 90, 219–229. [Google Scholar] [CrossRef] [PubMed]
- OIE–Office International des Epizooties. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2019. Available online: https://www.oie.int/standard-setting/terrestrial-manual/access-online/ (accessed on 15 December 2020).
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; et al. Standard methods for Nosema research. In:Dietemann V, Ellis JD and Neumann P (Eds.) The COLOSS BEEBOOK, Volume II: Standard methods for Apis mellifera research. J. Apicult. Res. 2013, 52, 51. [Google Scholar] [CrossRef]
- Wei, S.; Van Griensven, L. Pro- and antioxidative properties of medicinal mushroom extracts. Int. J. Med. Mushrooms. 2008, 10, 315–324. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Ruthes, A.C.; Van Arkel, J.; Chanput, W.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Polysaccharides from Agaricus bisporus and Agaricus brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complem. Altern. M 2011, 11, 58. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Alquini, G.; Tadra-Sfeir, M.Z.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Agaricus bisporus and Agaricus brasiliensis (1 → 6) β-D-glucans show immunostimulatory activity on human THP-1 derived macrophages. Carbohyd. Polym. 2013, 94, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Niksic, M.; Vrvic, M.M.; Van Griensven, L.J.L.D. Dietary polysaccharide extracts of Agaricus brasiliensis fruiting bodies: Chemical characterization and bioactivities at different levels of purification. Food Res. Int. 2014, 64, 53–64. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard methods for counting Nosema spores. Amer. Bee J. 1970, 110, 222–223. [Google Scholar]
- OIE–Office International Des Epizooties. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 2.2.4. Nosemosis of Honey Bees. 2018. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.02.04_NOSEMOSIS_FINAL.pdf (accessed on 15 December 2020).
- Glavinic, U.; Tesovnik, T.; Stevanovic, J.; Zorc, M.; Cizelj, I.; Stanimirovic, Z.; Narat, M. Response of adult honey bees treated in larval stage with prochloraz to infection with Nosema ceranae. PeerJ. 2019, 7, e6325. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Dubovskiy, I.M.; Martemyanov, V.V.; Vorontsova, Y.L.; Rantala, M.J.; Gryzanova, E.V.; Glupov, V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Phys. C. 2008, 148, 1–5. [Google Scholar]
- Williams, G.R.; Shutler, D.; Burgher-MacLellan, K.L.; Rogers, R.E.L. Infrapopulation and -community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS ONE 2014, 9, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.F.; Solter, L.; Aronstein, K.; Huang, Z. Infectivity and virulence of Nosema ceranae and Nosema apis in commercially available North American honey bees. J. Invertebr. Pathol. 2015, 124, 107–113. [Google Scholar] [CrossRef]
- Parish, J.B.; Scott, E.S.; Hogendoorn, K. Nutritional benefit of fungal spores for honey bee workers. Sci. Rep. UK 2020, 10, 15671. [Google Scholar] [CrossRef]
- Milbrath, M.O.; van Tran, T.; Huang, W.F.; Solter, L.F.; Tarpy, D.R.; Lawrence, F.; Huang, Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invertebr. Pathol. 2015, 125, 9–15. [Google Scholar] [CrossRef]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in Western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Shutler, D.; Little, C.; Burgher-MacLellan, K.; Rogers, R. The microsporidian Nosema ceranae, the antibiotic Fumagilin-B®, and Western honey bee (Apis mellifera) colony strength. Apidologie 2011, 42, 15–22. [Google Scholar] [CrossRef]
- Giacobino, A.; Rivero, R.; Molineri, A.I.; Cagnolo, N.B.; Merke, J.; Orellano, E.; Salto, C.; Signorini, M. Fumagillin control of Nosema ceranae (Microsporidia: Nosematidae) infection in honey bee (Hymenoptera: Apidae) colonies in Argentina. Vet. Ital. 2016, 52, 145–151. [Google Scholar]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apicult. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochemistry 2014, 79, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Balieira, K.V.B.; Mazzo, M.; Bizerra, P.F.V.; Guimarães, A.R.D.J.S.; Nicodemo, D.; Mingatto, F.E. Imidacloprid-induced oxidative stress in honey bees and the antioxidant action of caffeine. Apidologie 2018, 49, 562–572. [Google Scholar] [CrossRef]
- Farjan, M.; Łopieńska-Biernat, E.; Lipiński, Z.; Dmitryjuk, M.; Żółtowska, K. Supplementing with vitamin C the diet of honeybees (Apis mellifera carnica) parasitized with Varroa destructor: Effects on antioxidative status. Parasitology 2014, 141, 770–776. [Google Scholar] [CrossRef]
- Li, J.; Heerman, M.C.; Evans, J.D.; Rose, R.; Li, W.; Rodríguez-García, C.; DeGrandi-Hoffman, G.; Zhao, Y.; Huang, S.; Li, Z.; et al. Pollen reverses decreased lifespan, altered nutritional metabolism, and suppressed immunity in honey bees (Apis mellifera) treated with antibiotics. J. Exp. Biol. 2019, 222, 202077. [Google Scholar] [CrossRef] [PubMed]
- Hayman, J.R.; Southern, T.R.; Nash, T.E. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect. Immun. 2005, 73, 841–848. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).