Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Insects
2.3. Bioassay
2.4. Activity Assays of Detoxification Enzymes GST, CarE and P450s
2.5. qRT-PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Volatile Exposure on Larval Susceptibility to the Insecticide
3.2. Effect of Volatile Exposure on Detoxification Enzymes
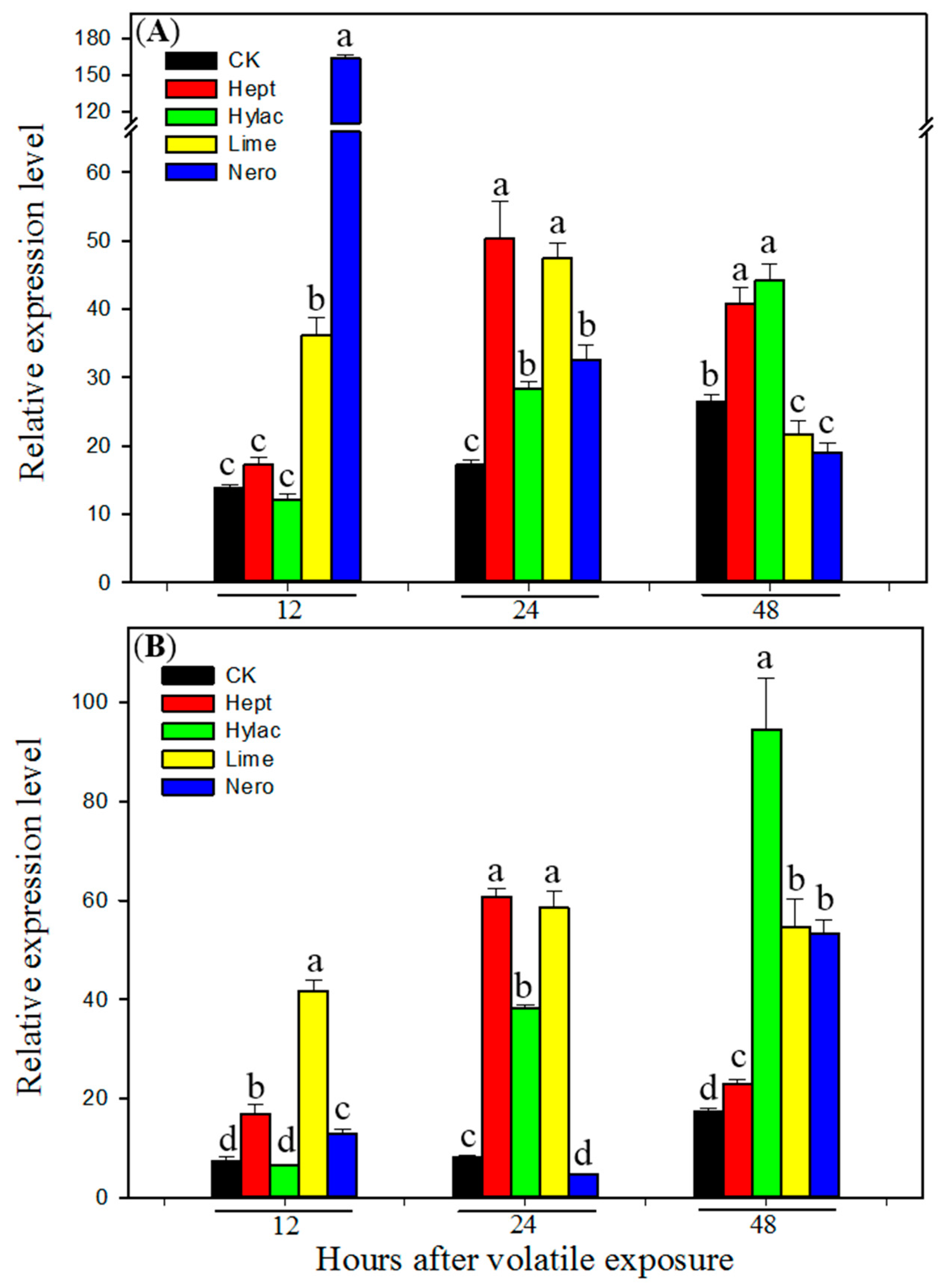
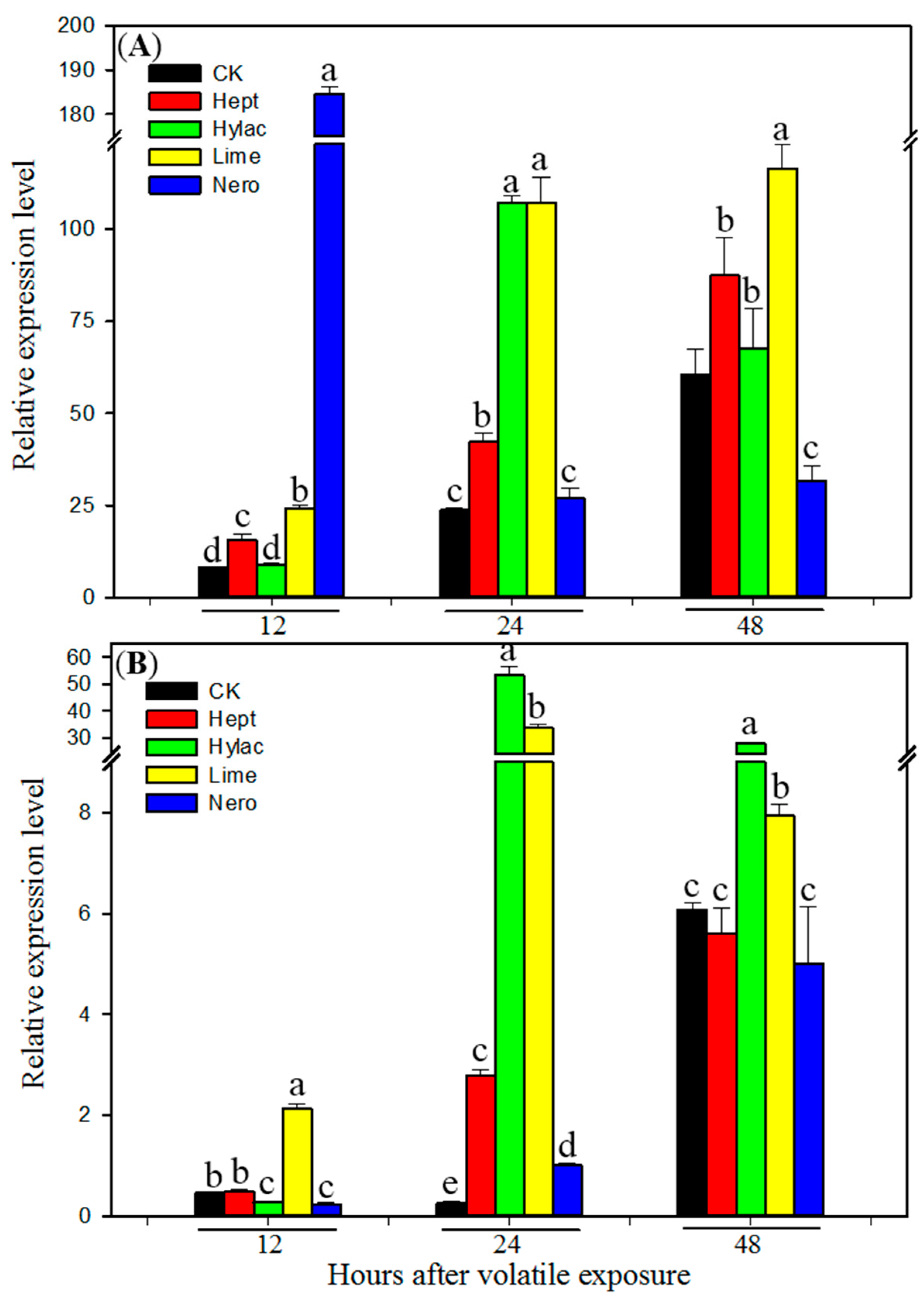
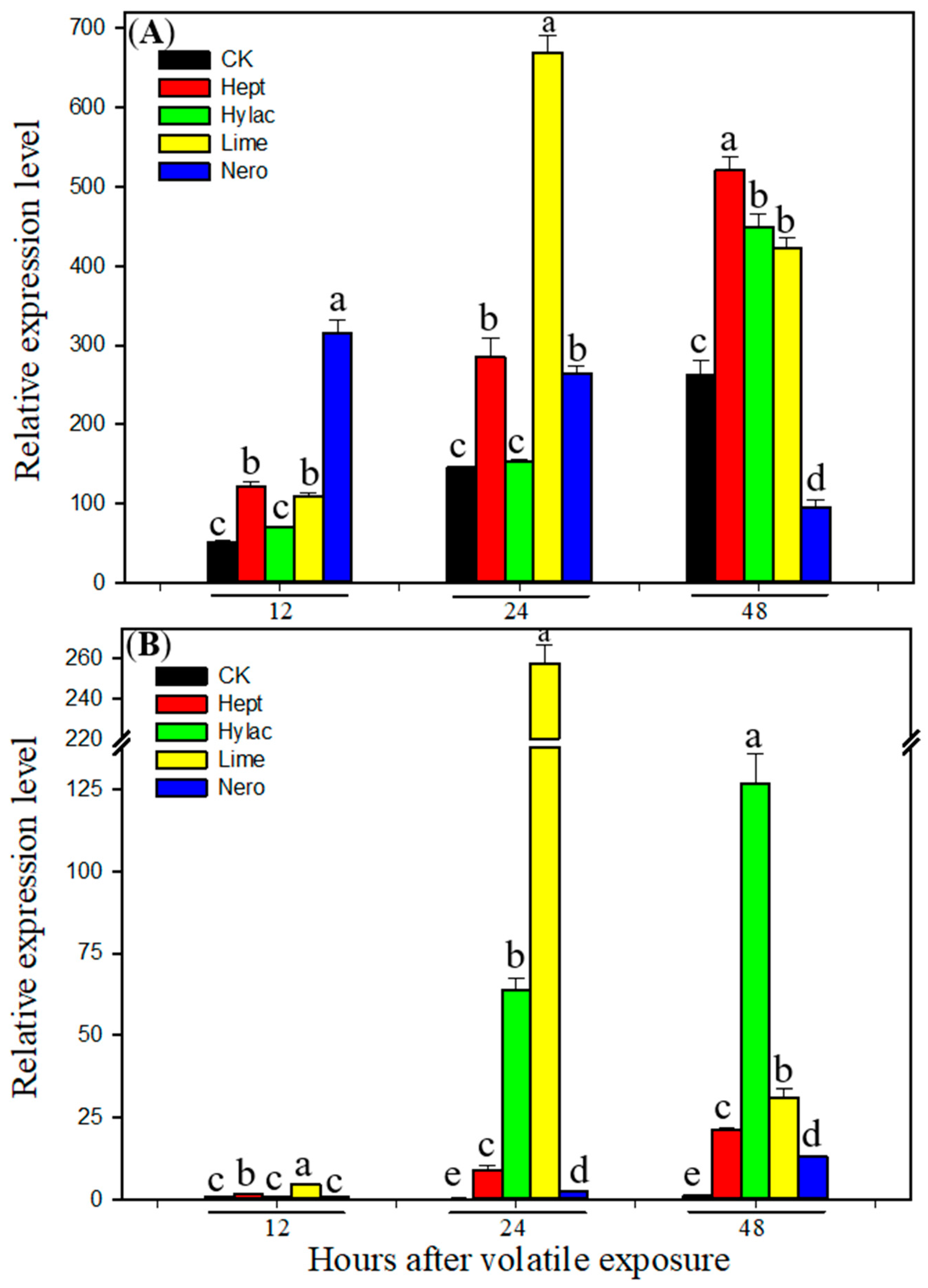
3.3. Effect of Volatile Exposure on Transcript Levels of P450 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Campos-Herrera, R.; Kaplan, F.; Duncan, L.W.; Rodriguez-Saona, C.; Koppenhofer, A.M.; Stelinski, L.L. Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS ONE 2012, 7, e38146. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Zhang, H.; Mallik, A.U.; Zeng, R.S. Control of Panama disease of banana by rotating and intercropping with Chinese chive (Allium tuberosum Rottler): Role of plant volatiles. J. Chem. Ecol. 2013, 39, 243–252. [Google Scholar] [CrossRef]
- Becerra, J.X. The Impact of herbivore-plant coevolution on plant community structure. Proc. Natl. Acad. Sci. USA 2007, 104, 7483–7488. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.J.; Ge, L.; Hu, L.; Lu, J. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef]
- Aartsma, Y.; Bianchi, F.J.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, M.L.; Tcj, T.; Ambrosetti, L.; Bassetti, P.; Dorn, S. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol. Exp. App. 1998, 87, 133–142. [Google Scholar] [CrossRef]
- Veyrat, N.; Robert, C.A.M.; Turlings, T.C.J.; Erb, M.; De Deyn, G. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J. Ecol. 2016, 104, 591–600. [Google Scholar] [CrossRef]
- Gasmi, L.; Martínez-Solís, M.; Frattini, A.; Ye, M.; Carmen Collado, M.; Turlings, T.C.J.; Erb, M.; Herrero, S. Can herbivore-induced volatiles protect plants by increasing the herbivores’ susceptibility to natural pathogens? Appl. Environ. Microb. 2019, 85, e01468-18. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.T.; Seidl-Adams, I.; Tumlinson, J.H. Herbivore-specific plant volatiles prime neighboring plants for non-specific defense responses. Plant Cell Environ. 2020, 43, 787–800. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsui, K.; Iijima, Y.; Akakabe, Y.; Muramoto, S.; Ozawa, R.; Akitake, S. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc. Natl. Acad. Sci. USA 2014, 111, 7144–7149. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Cardé, R.T. Use of habitat odour by host-seeking insects. Biol. Rev. 2017, 92, 1241–1249. [Google Scholar] [CrossRef]
- Beyaert, I.; Hilker, M. Plant odour plumes as mediators of plant–insect interactions. Biol. Rev. 2014, 89, 68–81. [Google Scholar] [CrossRef]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lin, Y.; Wang, R.; Li, Q.; Shi, Q.; Baerson, S.R.; Song, Y. Olfactory perception of herbivore-induced plant volatiles elicits counter-defences in larvae of the tobacco cutworm. Funct. Ecol. 2021, 35, 384–397. [Google Scholar] [CrossRef]
- Avilla, C.; González-Zamora, J.E. Monitoring resistance of Helicoverpa armigera to different insecticides used in cotton in Spain. Crop Prot. 2010, 29, 100–103. [Google Scholar] [CrossRef]
- Bird, L.J. Pyrethroid and carbamate resistance in Australian Helicoverpa armigera (Lepidoptera: Noctuidae) from 2008 to 2015: What has changed since the introduction of Bt cotton? Bull. Entomol. Res. 2018, 108, 781–791. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Wu, S.; Yue, L.; Wu, Y. Constitutive overexpression of multiple cytochrome P450 genes associated with pyrethroid resistance in Helicoverpa armigera. J. Econ. Entomol. 2006, 99, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.K.; Joussen, N.; Tian, K.; McGaughran, A.; Anderson, C.J.; Qiu, X.; Heckel, D.G. Multiple recombination events between two cytochrome P450 loci contribute to global pyrethroid resistance in Helicoverpa armigera. PLoS ONE 2018, 13, e0197760. [Google Scholar] [CrossRef] [PubMed]
- Shiojiri, K.; Kishimoto, K.; Ozawa, R.; Kugimiya, S.; Urashimo, S.; Arimura, G.; Horiuchi, J.; Nishioka, T.; Matsui, K.; Takabayashi, J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Birah, A.; Rani, S. Development of artificial diet for mass rearing of American bollworm, Helicoverpa armigera. Indian J. Agric. Sci. 2004, 74, 548–551. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Su, S.; Wang, K.; Tian, R.; Chen, M. Identification, expression, and regulation of an omega class glutathione S-transferase in Rhopalosiphum padi (L.) (Hemiptera: Aphididae) under insecticide stress. Front. Physiol. 2018, 9, 427. [Google Scholar] [CrossRef]
- Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Chen, S.; Elzaki, M.E.A.; Ding, C.; Li, Z.F.; Wang, J.; Zeng, R.S.; Song, Y.Y. Plant allelochemicals affect tolerance of polyphagous lepidopteran pest Helicoverpa armigera (Hübner) against insecticides. Pestic. Biochem. Physiol. 2019, 154, 32–38. [Google Scholar] [CrossRef]
- Omura, T. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]
- Rose, R.L.; Barbhaiya, L.; Roe, R.M.; Rock, G.C.; Hodgson, E. Cytochrome P450-associated insecticide resistance and the development of biochemical diagnostic assays in Heliothis virescens. Pestic. Biochem. Physiol. 1995, 51, 178–191. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, J.; Shiojiri, K. Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions. Curr. Opin. Insect Sci. 2019, 32, 110–117. [Google Scholar] [CrossRef]
- Sokame, B.M.; Ntiri, E.S.; Ahuya, P.; Torto, B.; Le Ru, B.P.; Kilalo, D.C.; Calatayud, P.A. Caterpillar-induced plant volatiles attract conspecific and heterospecific adults for oviposition within a community of lepidopteran stemborers on maize plant. Chemoecology 2019, 29, 89–101. [Google Scholar] [CrossRef]
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Li, E.; Chen, Q.; Kang, H.; Zhang, S.; Li, K.; Ren, B. A herbivore-induced plant volatile of the host plant acts as a collective foraging signal to the larvae of the meadow moth, Loxostege sticticalis (Lepidoptera: Pyralidae). J. Insect Physiol. 2019, 118, 103941. [Google Scholar] [CrossRef]
- De Lange, E.S.; Laplanche, D.; Guo, H.; Xu, W.; Vlimant, M.; Erb, M.; Ton, J.; Turlings, T.C. Spodoptera frugiperda caterpillars suppress herbivore-induced volatile emissions in maize. J. Chem. Ecol. 2020, 46, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Veyrat, N.; Xu, H.; Hu, L.; Turlings, T.C.J.; Erb, M. An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci. Adv. 2018, 4, eaar4767. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, E.; Venkatesan, R. Plant volatiles modulate immune responses of Spodoptera litura. J. Chem. Ecol. 2019, 45, 715–724. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Salzman, K.; Zeng, R.S. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Després, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trend Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.E.; Rhoades, J.H.; Nelson, D.R.; Kuhar, D.; Lancaster, J.; Lehner, B.; Tholl, D.; Weber, D.C.; Gundersen-Rindal, D.E. A transcriptome survey spanning life stages and sexes of the harlequin bug, Murgantia histrionica. Insects 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.E.; Nelson, D.R.; Haber, A.I.; Weber, D.C.; Harrison, R.L. Transcriptome sequencing of the striped cucumber beetle, Acalymma vittatum (F.), reveals numerous sex-specific transcripts and xenobiotic detoxification genes. BioTech 2020, 9, 21. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Elsevier: San Diego, CA, USA, 2012; pp. 236–316. [Google Scholar]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s—Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef]
- Cheng, T.; Wu, J.; Wu, Y.; Chilukuri, R.V.; Huang, L.; Yamamoto, K.; Feng, L.; Li, W.; Chen, Z.; Guo, H.; et al. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 2017, 1, 1747–1756. [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.; Chen, X. Regulation of insect P450s in response to phytochemicals. Curr. Opin. Insect Sci. 2021, 43, 108–116. [Google Scholar] [CrossRef]
- Danielson, P.B.; MacIntyre, R.J.; Fogleman, J.C. Molecular cloning of a family of xenobiotic-inducible drosophilid cytochrome P450s: Evidence for involvement in host-plant allelochemical resistance. Proc. Natl. Acad. Sci. USA 1997, 94, 10797–10802. [Google Scholar] [CrossRef]
- Zeng, R.S.; Wen, Z.; Niu, G.; Schuler, M.A.; Berenbaum, M.R. Allelochemical induction of cytochrome P450 monooxygenases and amelioration of xenobiotic toxicity in Helicoverpa zea. J. Chem. Ecol. 2007, 33, 449–461. [Google Scholar] [CrossRef]
- Giraudo, M.; Hilliou, F.; Fricaux, T.; Audant, P.; Feyereisen, R.; Le Goff, G. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): Responses to plant allelochemicals and pesticides. Insect Mol. Biol. 2015, 24, 115–128. [Google Scholar] [CrossRef]
- Zhou, X.; Sheng, C.; Li, M.; Wan, H.; Liu, D.; Qiu, X. Expression responses of nine cytochrome P450 genes to xenobiotics in the cotton bollworm Helicoverpa armigera. Pestic. Biochem. Phys. 2010, 97, 209–213. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, Y.; Wu, P.; Zheng, J.; Qiu, L. Effects of three insecticides on the expression of cytochrome P450 CYP6B7 in Helicoverpa armigera. J. App. Entomol. 2021. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Qin, J.; Zhao, W.; Qiu, L. Over-expression of multiple cytochrome P450 genes in fenvalerate-resistant field strains of Helicoverpa armigera from north of China. Pestic. Biochem. Physiol. 2016, 132, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Liu, D.; Yuan, Y.; Li, M.; Qiu, X. CYP6B6 is involved in esfenvalerate detoxification in the polyphagous lepidopteran pest, Helicoverpa armigera. Pestic. Biochem. Physiol. 2017, 138, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.; Campbell, B.; Hobbs, A.A. Over-expression of cytochrome P450 CYP6B7 mRNA and pyrethroid resistance in Australian populations of Helicoverpa armigera (Hübner). Pestic. Sci. 1998, 54, 195–202. [Google Scholar] [CrossRef]

| Volatiles | GST | CarEs | P450s | |||
|---|---|---|---|---|---|---|
| Midgut | Fatbody | Midgut | Fatbody | Midgut | Fatbody | |
| CK | 3.56 ± 0.15 c | 1.78 ± 0.32 b | 28.55 ± 0.29 b | 6.23 ± 0.78 b | 0.26 ± 0.02 c | 0.21 ± 0.01 c |
| Hept | 5.12 ± 0.17 a | 2.75 ± 0.04 a | 37.45 ± 1.01 a | 9.74 ± 0.17 a | 0.52 ± 0.01 a | 0.79 ± 0.03 a |
| Hylac | 2.11 ± 0.19 d | 1.82 ± 0.05 b | 26.22 ± 1.29 b | 5.67 ± 0.15 b | 0.44 ± 0.02 a | 0.33 ± 0.01 b |
| Limo | 4.34 ± 0.24 b | 2.55 ± 0.04 a | 28.78 ± 0.59 b | 10.97 ± 0.64 a | 0.44 ± 0.03 a | 0.38 ± 0.01 b |
| Nero | 3.68 ± 0.12 bc | 1.85 ± 0.25 b | 28.00 ± 0.33 b | 5.36 ± 0.66 b | 0.35 ± 0.02 b | 0.32 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Ding, C.; Chen, S.; Wu, X.; Zhang, L.; Song, Y.; Li, W.; Zeng, R. Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl. Insects 2021, 12, 238. https://doi.org/10.3390/insects12030238
Wu C, Ding C, Chen S, Wu X, Zhang L, Song Y, Li W, Zeng R. Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl. Insects. 2021; 12(3):238. https://doi.org/10.3390/insects12030238
Chicago/Turabian StyleWu, Choufei, Chaohui Ding, Shi Chen, Xiaoying Wu, Liqin Zhang, Yuanyuan Song, Wu Li, and Rensen Zeng. 2021. "Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl" Insects 12, no. 3: 238. https://doi.org/10.3390/insects12030238
APA StyleWu, C., Ding, C., Chen, S., Wu, X., Zhang, L., Song, Y., Li, W., & Zeng, R. (2021). Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl. Insects, 12(3), 238. https://doi.org/10.3390/insects12030238









