Simple Summary
In California, grape cultivation for wine, juice, fresh and raisin markets is one of the most profitable agricultural sectors. Pathogens, weeds and insect pests cause millions in crop loss and result in additional costs through the use of pesticides and other control tools. Here, we report on the emergence of a new California grape pest, the carpentermoth Givira ethela (Neumoegen and Dyar, 1893). This paper also aimed at the moth’s identification through molecular and morphological features and updates the distribution of G. ethela in California. Furthermore, the presence of G. ethela larval galleries appears to facilitate the mealybug pest Planococcus ficus Signoret, 1875, providing better access to vine sap and protection from natural enemies, environmental stresses, and pesticide treatments. We propose that management practices against G. ethela should include the correct identification of the pest and of its damage, but also the investigation of monitoring methods, economic thresholds, biological controls, and a better understanding of the relationship between G. ethela and P. ficus or other mealybug pest species.
Abstract
Grape cultivation is a billion-dollar agricultural sector in California, where invasive or novel pest species can disrupt management practices. We report herein on a new pest associated with California vineyards, the carpentermoth Givira ethela (Neumoegen and Dyar, 1893). Rather than an invasive species, G. ethela appears to be a newly recognized wood-boring pest of Vitis vinifera (L.) in regions of California’s Central Valley, where its initial occurrence has been dated back to, at least, the beginning of the 2000s. The habitus of adult, genitalia and pupa is illustrated. Givira ethela distribution in California is updated including published records and new data. Carpentermoth galleries seem to facilitate the access of Planococcus ficus Signoret, 1875 to vine sap and protection from natural enemies, environmental stresses, and pesticide treatments. Notes on pest status, life history, monitoring practices, natural enemies, and management options on grapes are also discussed. Tools for the Integrated Pest Management of G. ethela should include the correct identification of the insect and its damage, a full understanding of its biology and ecology, the application of monitoring methods, and the identification of economic thresholds and injury levels.
Keywords:
carpenter millers; carpentermoth; grape; moth; viticulture; vineyard; wood borer; new pest; mealybug 1. Introduction
Wood boring beetles and moths can damage cultivated crops, particularly fruit and ornamental trees [1]. Among moths, species in the Cossidae, Hepialidae, Sesiidae and Xyloryctidae are widely recognized as having larvae that are wood boring pests on tree crops, where they feed upon both the vascular and/or structural tissues of the plant, e.g., [2,3,4]. The trophic activity of these larvae can cause a reduction in structural stability of the host plant, and direct plant-stress, and may promote the establishment of phytopathogens that further damage plant growth or crop yield, e.g., [1,5,6,7,8,9].
Among the abovementioned families, there are at least five cossid and two sesiid species that are reported to cause damage in vineyards worldwide [5,7,10,11]. Reports of carpentermoth larvae damage to grapevines most commonly refer to the cossid Cossus cossus (Linnaeus, 1758) [12], which has a long history as a polyphagous pest in southern Europe vineyards and orchards and often requires the application of cultural or chemical management practices [1,13]. Other known wood boring cossids include Paropta paradoxus (Herrich-Schäffer, 1851) that was recognized as grapevine pest in Israel and, more recently, in Turkey [14,15] and Polyphagozerra coffeae (Nietner, 1861), a polyphagous pest, feeding on more than 50 plant species, and reported to cause severe damage to grapevines in Taiwan when high population densities were left unchecked [16]. In the 1980s, the cossid Coryphodema tristis (Drury, 1782) was documented to feed on and damage mature (>1-yr-old) grapevine wood in South Africa and was also associated with rot fungi that further damaged the vine [8]. More recently, the carpentermoth Dervishiya cadambae (Moore, 1865) (Lepidoptera: Cossidae) was found boring in both the sapwood and heartwood of grapevine trunks in India, reducing vine vitality and productivity [5] and joining the list of grapevine wood boring pests.
The aim of this paper is to report on a new wood boring carpentermoth infestation on grapevines in California’s San Joaquin Valley. Worldwide, grape value exceeds $68 billion (USD) with about 7.1 million ha in production [17]. In California, wine, juice, fresh and dried grapes are a $5 billion enterprise with about 371,500 ha in production and a leading sector in Californian agriculture [18]. Therefore, novel insect pests, weeds and pathogens that require additional management cost and/or pesticide applications are a concern for vineyard managers and hamper the development of sustainable management practices [19,20]. Here, the habitus of adult, genitalia and pupa of a carpentermoth found causing considerable damage to grapevines is illustrated. The carpentermoth populations appear to be confined to regions in the San Joaquin Valley, in the middle of the state, after 20 years since its first detection. Its distribution in California is here updated including published records and new data. We also observed an association between carpentermoth galleries and mealybugs, and discuss the mutualistic association between the cossid larvae and mealybugs. Finally, notes on pest status, life history, monitoring practices, natural enemies, and management options applicable for carpentermoth control on grapevine are discussed.
2. Materials and Methods
2.1. Insect Collections and Rearing
Carpentermoth infestations on grapevines in California were first noted in 2002 on older (>20-yr-old) raisin vineyards in Fresno County, California (USA), and later tentatively identified as a Givira species by Dr. Jerry Powell (University of California, Berkeley, CA, USA). To determine the extent of infestations, surveys were conducted sporadically from 2005 to 2007 in vineyards known to be infested at four sites: (1) Reedley (36.5749, −119.4261, 100 m a.s.l.), (2) Kingsburg (36.5110, −119.5380, 90 m a.s.l.), (3) Parlier (36.5998, −119.5075, 100 m a.s.l.), and (4) Clovis (36.7999, −119.6177, 115 m a.s.l.). Three of these vineyards were cv. Thompson seedless managed for raisin or wine production, and one vineyard was a cv. Scarlet Royal managed for fresh table grapes. All blocks were mature (>15-yr-old) and had a history of mealybug, Planococcus ficus Signoret, 1875 (Hemiptera: Pseudococcidae) infestations that were treated with 1–2 annual applications of pesticides (imidacloprid, buprofezin, or spirotetramat). In these initial samples, vine bark was peeled back, and sections of the trunk and cordon were searched for carpentermoth galleries. The number of vines with active carpentermoth galleries and the number of galleries with P. ficus were recorded.
In 2018, the Parlier and Clovis sites were more systematically sampled, in order to collect specimens for species identification. Samples were taken on 1 and 14 August at the Parlier site, and 20 August and 30, 31 July at the Clovis site. Collections were made by removing the bark on vine trunks and cordons, with each vine searched for 30 min. During each sample date, 10–25 vines were sampled, split between two different rows in each vineyard. Once active carpentermoth larvae were found, the trunk material surrounding the moth larvae were excised using wood chisels to remove the larvae as well as a portion (4 × 4 cm) of wood surrounding the larvae to collect live and undamaged larvae. In the laboratory, collected larvae were placed singly in a vial provisioned vine wood slivers; after which each vial was checked periodically and received honey-water as additional food and moisture. A few drops of honey-water were added twice a week directly on wood slivers in the vial using a plastic pipette. Larvae and pupae were then reared to adults under laboratory conditions (26 ± 2 °C, 65 ± 5% RH, 16:8 L:D), and freshly eclosed adults were killed in jars with ethyl acetate vapors and pinned for species identification.
2.2. Genitalia Preparation
Genitalia dissection and microscopic slide preparation of the abdomen was performed following methodologies illustrated by Timossi and Ruzzier [21], adapted from Clarke [22] and Hardwick [23]. After its detachment, the abdomen was macerated in boiling 5% KOH solution for 15–20 min, cleaned with distilled water with a few drops of glacial acetic acid, and then stained with chlorazol black. After this preparation, genital parts were dissected and cleaned in 50% ethanol, dehydrated in absolute ethanol, and finally embedded in euparal. For microscopic slide imaging, a Nikon Eclipse E100 microscope was used, equipped with a Sony Colour CCD 5.1 Mp TP 5100 micro-camera with X-Entry software.
2.3. DNA Barcoding
DNA extraction and purification were conducted on five adults (one dry leg each) and two larvae (whole insects), following the salting-out procedure [24]. A partial region of the cytochrome c oxidase subunit I (COI) gene was amplified with primers HCO2198 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and LCO1490 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) [25]. PCR products were purified using Exonuclease and Antarctic Phosphatase (GE Healthcare, Wauwatosa, WI, USA) and sequenced at the BMR Genomics Service (Padua, Italy). Sequences were edited and aligned using MEGA X [26], and subsequently translated with Transeq [27] to exclude the presence of stop codons in the coding region. A GenBank BLAST analysis of the sequences obtained was run through the NCBI website [28] and the integrated bioinformatics platform Barcode of Life Data (BOLD) System database [29] was used to assess the identity of the sequences.
2.4. Phylogenetic Analysis
To produce the most complete COI dataset, barcodes obtained were integrated with the complete sequences available in BOLD System ([29], see Table S1). The cossid species Hypopta palmata Barnes and McDunnough, 1910 and C. cossus were included in the analysis as outgroups. Evolutionary analysis was conducted in MEGA X [26] and the phylogeny was inferred by using the Maximum Likelihood (ML) method and Tamura-Nei model [30], with 1000 bootstrap replications. The Neighbor-Joining (NJ) method was also inferred for comparison, with 1000 bootstrap replications. The pairwise genetic distances between sequences were calculated using MEGA X [26], under default settings.
2.5. Statistical Analysis
Results are presented as mean ± SE. The 2018 data were analyzed to test carpentermoth infestation levels using generalized linear mixed model with the MIXED procedure of SAS (ver. 9.4) [31]. Treatment “site” (Parlier vs. Clovis) and the interaction “row*site” were considered as independent variables, while the survey event (two surveys per site) was included as random factor. The effect of treatments on infestation of carpentermoth larvae was tested using an F test (α = 0.05) followed by the Tukey–Kramer test as post hoc for comparisons (α = 0.05). Prior to the analyses data were log (n + 1) transformed to meet ANOVA assumptions.
3. Results
3.1. Molecular Identification
DNA barcoding resulted in four barcodes (three adults and one larva) of 621 bp with an overall distance of 0.2 ± 0.1% (Table S2). Both analyses conducted in GeneBank and BOLD System returned a mean similarity >99% with Givira ethela (Neumoegen and Dyar, 1893) (Lepidoptera: Cossidae). The phylogenetic reconstruction, inclusive of selected representatives of Givira Walker, 1856 (Hypoptinae), Hypopta Hübner, 1818 (Hypoptinae) and Cossus Fabricius, 1793 (Cossinae), resulted in a consensus tree in which G. ethela clusters together with Givira cornelia (Neumoegen and Dyar, 1893) with high bootstrap support (ML 99%; NJ 100%) and a genetic distance of 1.3%. The G. ethela cluster included seven sequences and had medium-high support (ML 77%; NJ 81%), while the G. cornelia grouped six sequences with high support (ML 97%; NJ 99%; Figure 1).
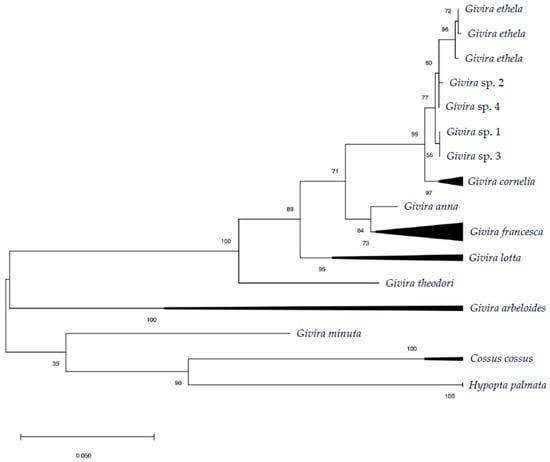
Figure 1.
Maximum likelihood (ML) tree based on COI sequences of selected North American Givira species. Numbers on the nodes refer to ML bootstrap values. Hypopta palmata and Cossus cossus are included as outgroups.
3.2. Morphological Identification and Related Features
The external habitus of the reared specimens fall under G. ethela, following the identification key provided by Barnes and McDunnough [32] and matching the morphological traits reported in Neumoegen and Dyar [33] and Dyar [34], and illustrated in Seitz [35]. Givira ethela is a dark colored moth with grayish–brownish body hairs and scales; adults present a whitish ✓-shaped mark on the forewing (Figure 2). Male and female genitalia are reported in Figure 3 for illustrative purposes only. The pupa shows rows of spine-like processes on abdominal segments (Figure 4).

Figure 2.
Givira ethela adult. (a) On the vine bark; (b) Head and prothorax magnification.
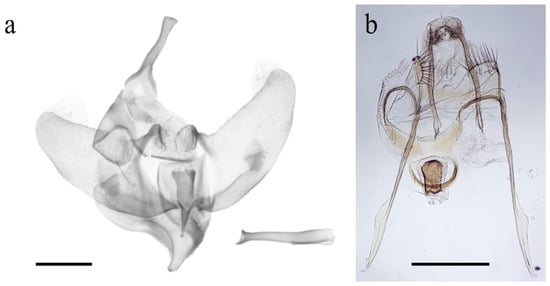
Figure 3.
Genitalia of Givira ethela. (a) Complete male genitalia and aedeagus; (b) Female genitalia. Scale bar = 1.0 mm.

Figure 4.
Pupa of Givira ethela. (a,c) Frontal view, at different magnifications; (b) Back side; (d) Right side. Scale bar: (a,b) = 2.0 mm; (c,d) = 1.5 mm.
3.3. Distribution and Damage Assessment
Including previous published records and present data, the distribution of G. ethela in California is here updated, and it is recorded in nine different counties (Figure 5).
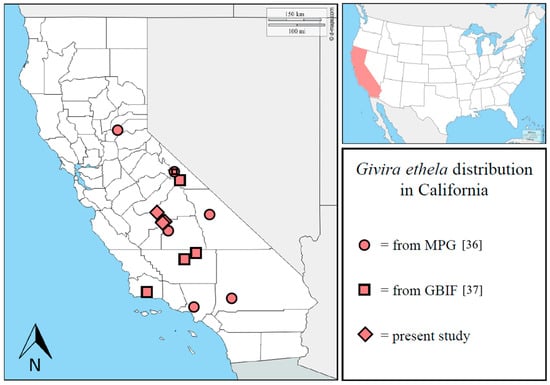
Figure 5.
Updated distribution of Givira ethela in California. Adapted from www.d-maps.com. Data from MPG [36] and GBIF [37] are included.
A monitoring program for G. ethela is not currently developed; however, without peeling bark, signs of an infestation are evident through the presence of emerging exuviae on trunks, and frass or silk at the entrance of galleries or on the bark of highly infested vines. Still, the primary damage is under the bark where the larvae create feeding galleries in the cambium layer (Figure 6).
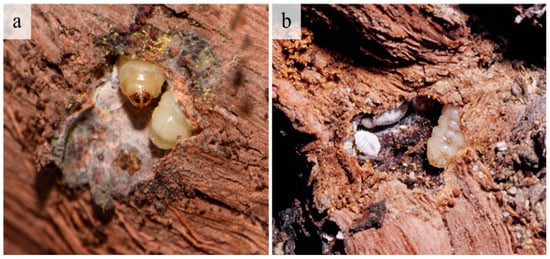
Figure 6.
Galleries and Givira ethela larvae in grapevine trunks. (a) A mature larva, with silk inside the gallery; (b) Larva with different stages of the mealybug Planococcus ficus.
From the 2018 field surveys, 361 larvae were collected, 172 in Parlier and 189 in Clovis. Infestation levels did not differ between the two sites (F1, 80 = 0.50, p = 0.4814), while significant differences were seldom observed among rows of the same field in the “row*site” interaction (F4, 80 = 2.76, p = 0.0334). Infestation level ranged between 0 to 37 larvae per vine. Seven living pupae were collected on 20 and 30 July, and on 1 August 2018. Some of the larvae collected in 2018 pupated soon after being moved to the laboratory, where they eclosed in late August–September.
Often associated with the G. ethela galleries were different stages of the mealybug P. ficus, frequently aggregated in tunnels developing just under the grapevine bark (Figure 6). Several Digonogastra sp. (Hymenoptera: Braconidae) were reared from G. ethela, and in mid to late summer adult Digonogastra were commonly observed searching for and parasitizing carpentermoth in the infested vineyard (Figure 7).

Figure 7.
A Digonogastra sp. ovipositing through the bark of a Givira ethela infested vine (Clovis site, August 2018).
4. Discussion
The carpentermoth G. ethela is here reported as pest on mature grapevines in California’s San Joaquin Valley. Pest occurrence is estimated to the beginning of 2000s, when attacks by a carpentermoth were first observed, and almost as an oddity the carpentermoth remained unidentified until this work. In our preliminary surveys of vineyards, pest presence appears to be scattered rather than clustered throughout the monitored vineyards. We note that our surveys were conducted in vineyards known to be infested by G. ethela for many years and there is no information on how widespread this pest is throughout this region or in other California grape growing areas. Vasquez et al. [38] noted moth larvae feeding on vines in Fresno County in 2010 and are probably referring to this pest. From our limited survey, G. ethela attacked table, raisin and juice grapes, each with some level of insecticide applications targeting P. ficus. The infested vineyards were mature (>20-yr-old) and we suspect this age preference may be associated with the greater size of the trunks, richer in phloem and better capable to support the development of multiple larvae for several years. Indeed, Givira larvae are known to feed on the phloem of their host [39], and other carpentermoth species larvae usually prefer to growth in old grapevine trunks [14].
Givira ethela adults are dark colored and have grayish-brownish body hairs and scales. Quoting Metzler [40], most (11 out of 15) of the North American Givira species are dark colored, including G. ethela, while G. carla Dyar, 1923, G. cornelia, G. durangona (Schaus, 1901), and G. delindae Metzler, 2017 are extensively white with few or no dark markings. According to Clench [41], G. ethela has some similarities for external characters with Givira leonera Clench, 1957, a Neotropical moth described from Chile.
In the phylogenetic reconstruction, G. ethela clusters with G. cornelia with an overall mean distance of 1.3%. Givira cornelia occurs in sympatry with G. ethela for most of its range [32,42,43], and further morphological and barcoding data are required to shed light about the relation between the two species. Although G. ethela and G. cornelia cluster separately in our analyzes, the low genetic diversity and the robustness of their clade (ML 99%; NJ 100%) would seem to indicate their belonging to a single variable taxon. However, in anticipation of a possible taxonomic rearrangement, our specimens remain attributable to the current interpretation of G. ethela.
Givira includes species distributed in the Nearctic and most in the Neotropics [44]. At least 15 species occur in the United States, mostly distributed in the southwestern part of the country [32,45], including pest species as G. lotta Barnes and McDunnough, 1911, a wood boring carpentermoth attacking ponderosa pines [46]. Givira ethela is present in Colorado (type locality), California, Nevada, and Utah [32,34,36,37]. The biology of this species is almost unknown, with only Antelope Bitterbrush, Purshia tridentata (Pursh) DC. (Rosales: Rosaceae), previously indicated as a host plant [34].
The few data available suggest that G. ethela flight is between the end of June and August [36,37]; this observation is supported by the emergence of adults in laboratory conditions (August and September). Carpentermoths usually are nocturnal, and they lay their eggs in plant crevices or under bark, where the newly hatched larva starts to mine the wood, completing the development in one to four years. However, larval biology is unknown for most of the Givira species [45]. Before pupation, the carpentermoth larva prepares a way out by chewing an exit hole up to the surface of the trunk. At adult emergence, the pupal exuvia remains on the gallery exit, protruding from the trunk. This behavior is present in G. ethela (this paper) as well as in other Givira species [39].
In this study, we also observed the association between P. ficus and G. ethela galleries. Planococcus ficus infestation was particularly prevalent in table grape trunks, especially at the Clovis site. Mealybugs are pests found in most of the grapevine production areas in the world and in high densities can reduce fruit quality and vine vigor, and their excreted honeydew can foul fresh marked grapes and promotes the development of molds, thus reducing fruit marketability [47,48]. Moreover, mealybug pest status is increased because most tested species are vectors of grape leafroll virus, which decreases vine vigor and crop size, as well as the quality of the produced wine [49,50].
Carpentermoth larvae are often involved in community ecology; for example, in forest ecosystems they have been reported to make shelters for other insects and promote access to plant sap that is involved in the attraction of other arthropods [39,51,52]. Here, we suggest a novel mutualistic association as G. ethela galleries may favor vineyard mealybugs by facilitating their access to the vine sap, protecting them from predators and parasitoids and offering shelter from environmental stresses and pesticide treatments; in turn, we note that the galleries were always free of mealybug honeydew that we assume the cossid feeds on. Similarly, Crematogaster ashmeadi Wheeler, 1932 (Hymenoptera: Formicidae) does not mine its own galleries but is often found inhabiting abandoned galleries of Givira francesca (Dyar, 1909) in North America and of other xylophagous insects [39]. In Japan, carpentermoth larvae are known to induce and maintain plant sap exudation from trunks through their wood boring activity, attracting other insects that feed on the plant sap [51,52].
Among practices applicable to develop an integrated pest management (IPM) approach is the proper identification of pest species, in this case G. ethela, and the development of monitoring tools to help determine pest control actions [19]. In other ecosystems, adult Givira species were successfully collected using blacklights, mercury vapor lights, and ultraviolet (UV) light traps, e.g., [40,53,54]. Traps baited with sex pheromones are another possible tool that could be developed, as mating disruption with sex pheromones has been used against other carpentermoth species [55,56,57]. Mass trapping has also been tested for the cossids Zeuzera pyrina (Linnaeus, 1761) and C. cossus [58,59,60]. Another case in the use of sex pheromones is for the cossid Coryphodema tristis (Drury, 1782) on Eucalyptus nitens (H. Deane and Maiden) Maiden in South Africa, where the large-scale mass trapping suppressed this pest [61]. Notably, the use of sticky UV-light traps in combination with sex pheromone traps successfully attracted Z. pyrina adults [58], where the light is attractive for both males and females. However, UV-light traps are a wide-spectrum attractants in respect to moths [62], and they may have considerable effects on non-target insects and even vertebrates, e.g., [63,64,65]. For these reasons, monitoring methods should include highly specific traps in order to minimize their impact on non-target species.
Finally, natural enemies could be another variable to take into account when considering carpentermoth control in vineyards [5]. In the present study, a Digonogastra species commonly emerged from G. ethela larvae; this group of braconids is known to parasitize lepidopteran larvae, e.g., [66,67,68] but further efforts are needed to understand Digonogastra’s biology and impact on G. ethela populations in vineyards.
5. Conclusions
This work reports G. ethela as a new wood boring pest of grapevines in California, and updates its distribution in the area. Pest identification comprising morphological and molecular features is a key tool for proper pest management. We report not only on this novel pest association but provide molecular coding data that may more rapidly provide identification of this pest should its geographic range expand. In a view of the IPM framework, tools against G. ethela should include the best applicable solutions, including all possible tasks such as the study of natural enemies or the application of pheromone traps for its control. To develop sustainable IPM programs, the geographic range and pest incidence must be better understood. For this to properly occur, methods to economically monitor G. ethela should be developed as well as a better understanding of its economic injury to the vine and relationship with P. ficus, which is perhaps the most important vineyard pest in California. The possibility to include more sustainable controls would include investigations of the pest’s sex pheromones for monitoring and control, as well as a better understanding of its natural enemies [19,20].
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/12/3/239/s1, Table S1: Barcodes complete sequences of Givira species available in BOLD System [29] used in the maximum likelihood tree, Table S2: Givira ethela barcode details obtained from the analyzed samples.
Author Contributions
Conceptualization, D.S. and K.M.D.; field methodology, D.S. and K.M.D.; identification, D.S. and E.R., formal analysis, D.S., E.R. and K.M.D.; resources, D.S., E.R. and K.M.D.; data curation, D.S., E.R. and K.M.D.; writing—original draft preparation, D.S. and E.R.; writing—review and editing, D.S., E.R. and K.M.D.; project administration and funding acquisition, K.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the California Table Grape Commission, which include the study of black widow spiders under vine bark.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and research guidelines of the University of California.
Data Availability Statement
Barcodes sequences are available in Supplementary Materials.
Acknowledgments
The authors would like to thank Giovanni Timossi (World Biodiversity Association, Italy) for genitalia extraction; Isabel Martinez-Sañudo (University of Padova, Italy) for help with the DNA sequencing; DeAnna Molinar (University of California Berkeley, USA) for earlier surveys, May Yang and Sunny Yang (University of California Berkeley, USA) during the 2018 field collections; the Smeds, Choolgian, Kearney, and Tufenkjian vineyards for access to their vines. We thank Geoff Williams (Australian Museum, Sydney) for the revision of the text. A special thanks to Jean-François Landry (Canadian National Collection of Insects, Arachnids and Nematodes, Canada) for allowing us to use the five barcodes of G. cornelia in his possession.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lieutier, F.; Day, K.R.; Battisti, A.; Grégoire, J.-C.; Evans, H.F. Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 1–569. [Google Scholar]
- Klem, C.C.; Zaspel, J. Pest injury guilds, Lepidoptera, and placing fruit-piercing moths in context: A review. Ann. Entomol. Soc. Am. 2019, 112, 421–432. [Google Scholar] [CrossRef]
- Grehan, J.R. Evolution of arboreal tunnelling by larvae of Aenetus (Lepidoptera: Hepialidae). N. Z. J. Zool. 1987, 14, 441–462. [Google Scholar] [CrossRef][Green Version]
- Lawson, S.A.; Debuse, V.J. Native Phloem and Wood Borers in Australian Mediterranean Forest Trees. In Insects and Diseases of Mediterranean Forest Systems; Paine, T., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 455–473. [Google Scholar]
- Yadav, D.S.; Mhaske, S.H.; Ranade, Y.H.; Ghule, S.B.; Shashank, P.R.; Yakovlev, R.V. First record of occurrence of Dervishiya cadambae on grapevine, Vitis vinifera, along with its morphological and molecular identification and pathogenicity evaluation potential of Metarhizium brunneum as its biocontrol agent. Bull. Insectol. 2020, 73, 137–148. [Google Scholar]
- Mathew, G. Biology, seasonal population trends and impact of the teak carpenterworm Alcterogystia cadambae (Moore) (Lepidoptera: Cossidae). Ann. Entomol. 1991, 92, 39–46. [Google Scholar]
- Dutcher, J.D.; All, J.N. Damage impact of larval feeding of the grape root borer in a commercial Concord grape vineyard. J. Econ. Entomol. 1979, 72, 159–161. [Google Scholar] [CrossRef]
- Höppner, G.F.J.; Ferreira, J.H.S. Fungi associated with the quince borer, Coryphodema tristis (Drury) (Lepidoptera: Cossidae), in grapevines. S. Afr. J. Enol. Vitic. 1990, 11, 67–69. [Google Scholar]
- Guario, A.; Nigro, F.; Boscia, D.; Saponari, M. Rapid drying of olive tree, causes and containment measures. L’Informatore Agrar. 2013, 69, 51–54. [Google Scholar]
- Arita, Y. The clearwing moths of Japan (Lepidoptera: Sesiidae). Holarct. Lepid. 1994, 1, 69–81. [Google Scholar]
- Bournier, A. Grape insects. Annu. Rev. Entomol. 1976, 22, 355–376. [Google Scholar] [CrossRef]
- Feron, J.; Audemard, H.; Balachowsky, A. Super-Famille des Cossoidea. In Entomologie Appliquée à l’Agriculture; Balachowsky, A., Ed.; Masson et Cie: Paris, France, 1966; Volume II-1, pp. 39–50. [Google Scholar]
- Yakovlev, R.V. Trophic relations of Old World carpenter-moths (Lepidoptera, Cossidae). Euroasian Entomol. J. 2012, 11, 189–194. [Google Scholar]
- Plaut, H.N. On the biology of Paropta paradoxus (H.-S.) (Lep., Cossidae) on grapevine in Israel. Bull. Entomol. Res. 1973, 63, 237–245. [Google Scholar] [CrossRef]
- Atay, E.; Sertkaya, E.; Tatlı, M. The first record of Paropta paradoxus (Herrich-Schäffer, 1851) (Lepidoptera: Cossidae) in Hatay Province of Turkey and its external and genital morphology. J. Anatol. Environ. Anim. Sci. 2019, 4, 525–531. [Google Scholar] [CrossRef]
- Tavares, W.D.S.; Kkadan, S.K.; Hendrik, A.M.; Tarigan, M.; Asfa, R.; Yakovlev, R.V.; Tachi, T.; Duran, A.; Wong, C.Y.; Sharma, M. Notes on the biology and natural enemies of Polyphagozerra coffeae (Nietner, 1861) infesting Eucalyptus pellita F. Muell. (Myrtaceae) trees in Riau, Indonesia (Lepidoptera: Cossidae, Zeuzerinae). SHILAP Rev. Lepid. 2020, 48, 333–349. [Google Scholar]
- Alston, J.M.; Sambucci, O. Grapes in the World Economy. In The Grape Genome; Cantu, D., Walker, M.A., Eds.; Springer Nature: Cham, Switzerland, 2019; p. 24. [Google Scholar]
- Alston, J.M.; Lapsley, J.T.; Sambucci, O. Grape and Wine Production in California. In California Agriculture: Dimensions and Issues; Goodhue, R., Martin, P., Wright, B., Eds.; Giannini Foundation of Agricultural Economics: Berkeley, CA, USA, 2018; pp. 1–28. [Google Scholar]
- Daane, K.M.; Vincent, C.; Isaacs, R.; Ioriatti, C. Entomological opportunities and challenges for sustainable viticulture in a global market. Annu. Rev. Entomol. 2018, 63, 193–214. [Google Scholar] [CrossRef]
- Wilson, H.; Daane, K.M. Review of ecologically-based pest management in California vineyards. Insects 2017, 8, 108. [Google Scholar] [CrossRef]
- Timossi, G.; Ruzzier, E. Description of female of Sattleria sophiae Timossi, 2014 (Lepidoptera: Gelechiidae). Zootaxa 2020, 4722, 491–494. [Google Scholar] [CrossRef]
- Clarke, J.F.G. The preparation of slides of the genitalia of Lepidoptera. Bull. Brooklyn Entomol. Soc. 1941, 36, 149–161. [Google Scholar]
- Hardwick, D.F. Preparation of slide mounts of lepidopterous genitalia. Can. Entomol. 1950, 82, 231–235. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- EMBOSS Programs. Available online: http://www.ebi.ac.uk/Tools/emboss/transeq/index.html (accessed on 15 January 2021).
- NCBI. Available online: http://www.ncbi.nlm.nih.gov (accessed on 15 January 2021).
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- SAS Institute. PROC User’s Manual, 6th ed.; SAS Institute: Cary, NC, USA, 2016; Available online: http://support.sas.com/documentation/cdl/en/indbug/68442/PDF/default/indbug.pdf (accessed on 6 November 2020).
- Barnes, W.M.; McDunnough, J.H. Revision of the Cossidae of North America—Contributions to the Natural History of the Lepidoptera of North America; The Review Press: Decatur, IL, USA, 1911; Volume 1, pp. 1–35. [Google Scholar]
- Neumoegen, B.; Dyar, H.G. New species and varieties of Bombyces. J. N. Y. Entomol. Soc. 1893, 1, 29–35. [Google Scholar]
- Dyar, H.G. Family: Cossidae. In The Macrolepidoptera of the American Region; The American Bombyces and Sphinges; Seitz, A., Ed.; Alfred Kernen Verlag: Stuttgart, Germany, 1913; Volume 6, pp. 1263–1287. [Google Scholar]
- Seitz, A. The American Bombyces and Sphinges; Alfred Kernen Publisher: Stuttgart, Germany, 1940. [Google Scholar]
- Moth Photographers Group. 640015.00–2667–Givira ethela–(Neumögen & Dyar, 1893), 2020. Available online: http://mothphotographersgroup.msstate.edu/species.php?hodges=2667 (accessed on 22 November 2020).
- GBIF. Givira ethela (Neumoegen & Dyar, 1893) in GBIF Secretariat (2019). GBIF Backbone Taxonomy, 2019. Checklist Dataset. Available online: https://www.gbif.org/species/10414608 (accessed on 28 November 2020).
- Vasquez, S.; Bentley, W.; Fidelibus, M. Unusual wood-boring worms. Vine Lines 2010, 2, 8. [Google Scholar]
- Tschinkel, W.R. The natural history of the arboreal ant, Crematogaster ashmeadi. J. Insect Sci. 2002, 2, 1–15. [Google Scholar] [CrossRef]
- Metzler, E.H. The Lepidoptera of White Sands National Monument, Otero County, New Mexico, USA 9. A new species of Givira Walker (Cossidae, Hypoptinae) dedicated to Delinda Mix, including a list of species of Cossidae recorded from the Monument. Zoo Keys 2007, 655, 141–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clench, H.K. Cossidae from Chile (Lepidoptera). Mitt. Münchner Entomol. Ges. 1957, 47, 122–142. [Google Scholar]
- GBIF. Givira cornelia (Neumoegen & Dyar, 1893) GBIF Backbone Taxonomy, 2019. Checklist Dataset. Available online: https://www.gbif.org/species/10112396 (accessed on 28 November 2020).
- Moth Photographers Group. 640013.00–2665–Givira cornelia–(Neumögen & Dyar, 1893), 2020. Available online: http://mothphotographersgroup.msstate.edu/species.php?hodges=2665 (accessed on 22 November 2020).
- Schoorl, J.W.J. A phylogenetic study on Cossidae (Lepidoptera: Ditrysia) based on external morphology. Zool. Verh. 1990, 263, 1–295. [Google Scholar]
- Powell, J.A.; Opler, P.A. Moths of Western North America; University of California Press: Berkeley, CA, USA, 2009; p. 369. [Google Scholar]
- Furniss, R.L.; Carolin, V.M. Western Forest Insects; USDA Forest Service Miscellaneous Publication No. 1339; USDA Forest Service: Washington, DC, USA, 1977; pp. 1–654.
- Daane, K.M.; Bentley, W.J.; Smith, R.J.; Haviland, D.R.; Weber, E.; Gispert, C.; Battany, M.C.; Millar, J.G. Planococcus Mealybugs (Vine Mealybug). In Grape Pest Management Publication 3343, 3rd ed.; Bettiga, L.J., Ed.; University of California, Division of Agriculture and Natural Resources: Oakland, CA, USA, 2013; pp. 246–260. [Google Scholar]
- Daane, K.M.; Almeida, R.P.P.; Bell, V.A.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M.; Zaveizo, T. Biology and Management of Mealybugs in Vineyards. In Arthropod Management in Vineyards; Bostanian, N.J., Isaacs, R., Vincent, C., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 271–308. [Google Scholar] [CrossRef]
- Cocco, A.; da Silva, V.C.P.; Benelli, G.; Botton, M.; Lucchi, A.; Lentini, A. Sustainable management of the vine mealybug in organic vineyards. J. Pest. Sci. 2021, 94, 1–33. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Daane, K.M.; Bell, V.A.; Blaisdell, G.A.; Cooper, M.L.; Herrbach, E.; Pietersen, G. Ecology and management of grapevine leafroll disease. Front. Microbiol. 2013, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, J.; Nishida, T. Boring effect of carpenterworms (Lepidoptera: Cossidae) on sap exudation of the oak, Quercus acutissima. Appl. Entomol. Zool. 2007, 42, 403–410. [Google Scholar] [CrossRef][Green Version]
- Yoshimoto, J.; Nishida, T. Plant-mediated indirect effects of carpenterworms on the insect communities attracted to fermented tree sap. Popul. Ecol. 2008, 50, 25–34. [Google Scholar] [CrossRef]
- Zack, R.; Landolt, P.J.; Strenge, D. Goat moths (Lepidoptera: Cossidae) of the Hanford Site and Hanford National Monument, Washington State. Pan Pac. Entomol. 2009, 85, 182–186. [Google Scholar] [CrossRef]
- Landau, D.; Prowell, D. A partial checklist of moths from Longleaf Pine Savannas in Louisiana (Insecta: Lepidoptera). Am. Entomol. Soc. 1999, 125, 127–138. [Google Scholar]
- Hezagi, E.M.; Khafagi, W.E.; Konstantopoulou, M.; Schlyter, F.; Raptopoulos, D.; Shweil, S.; Abd El-Rahman, S.; Atwa, A.; Ali, S.E.; Tawfik, H. Suppression of leopard moth (Lepidoptera: Cossidae) populations in olive trees in Egypt through mating disruption. J. Econ. Entomol. 2010, 103, 1621–1627. [Google Scholar] [CrossRef]
- Nakanishi, T.; Nakamuta, K.; Mochizuki, F.; Fukumoto, T. Mating disruption of the carpenter moth, Cossus insularis (Staudinger) (Lepidoptera: Cossidae) with synthetic sex pheromone in Japanese pear orchards. J. Asia Pac. Entomol. 2013, 16, 251–255. [Google Scholar] [CrossRef]
- Hoshi, H.; Takabe, M.; Nakamuta, K. Mating disruption of a carpenter moth, Cossus insularis (Lepidoptera: Cossidae) in apple orchards with synthetic sex pheromone, and registration of the pheromone as an agrochemical. J. Chem. Ecol. 2016, 42, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, E.M.; Khafagi, W.E.; Konstantopoulou, M.A.; Herz, A.; Raptopoulos, D.; Tawfic, H.; Abd El-Aziz, G.M.; Showiel, S.; Abdel-Rahman, S.M.; Atwa, A.; et al. Efficient mass-trapping method as an alternative tactic for suppressing populations of the leopard moth (Lepidoptera: Cossidae). Ann. Entomol. Soc. Am. 2009, 102, 809–818. [Google Scholar] [CrossRef]
- Maini, S.; Ferrari, R.; Pozzati, M.; Rama, F. Zeuzera pyrina (L.) e Cossus cossus (L.) confronto fra trappole a feromone sessuale per catture simultanee dei maschi. Boll. dell’Istituto Entomol. “Guido Grandi” dell’Università Studi Bologna 2000, 54, 165–173. [Google Scholar]
- Pasqualini, E.; Natale, D.; Witzgall, P.; El-Sayed, A.M. Zeuzera pyrina and Cossus cossus (Lepidoptera; Cossidae) control by pheromones: Four years advances in Italy. IOBC WPRS Bull. 1999, 22, 115–124. [Google Scholar]
- Noeth, K.P.; Verleur, P.M.; Bouwer, M.C.; Crous, J.W.; Roux, J.; Hurley, B.P.; Slippers, B. Mass trapping of Coryphodema tristis (Lepidoptera: Cossidae) using a sex pheromone in Eucalyptus nitens compartments in Mpumalanga, South Africa. South. For. 2020, 82, 271–279. [Google Scholar] [CrossRef]
- Infusino, M.; Brehm, G.; Di Marco, C.; Scalercio, S. Assessing the efficiency of UV LEDs as light sources for sampling the diversity of macro-moths (Lepidoptera). Eur. J. Entomol. 2017, 114, 25–33. [Google Scholar] [CrossRef]
- Li, C.-X.; Smith, M.L.; Fulcher, A.; Kaufman, P.E.; Zhao, T.-Y.; Xue, R.-D. Field evaluation of three new mosquito light traps against two standard light traps to collect mosquitoes (Diptera: Culicidae) and non-target insects in northeast Florida. Fla. Entomol. 2015, 98, 114–117. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Chang. Biol. 2012, 18, 2458–2465. [Google Scholar] [CrossRef]
- Longcore, T.; Rodríguez, A.; Witherington, B.; Penniman, J.F.; Herf, L.; Herf, M. Rapid assessment of lamp spectrum to quantify ecological effects of light at night. J. Exp. Zool. 2018, 329, 511–521. [Google Scholar] [CrossRef]
- Correa-Móndez, A.; Osorio-Osorio, R.; Figueroa-de-la-Rosa, J.I.; Hernández-Hernández, L.U.; de la Cruz-Lázaro, E.; Márquez-Quiroz, C. Digonogastra kimballi Kirkland as a parasitoid of Diatraea lineolata (Walker): New record at Tabasco, México. Southwest. Entomol. 2020, 44, 491–495. [Google Scholar] [CrossRef]
- Kirkland, R.L. Biology of Iphiaulax kimballi (Hym.: Braconidae), a parasite of Diatraea grandiosella (Lep.: Pyralidae). Entomophaga 1982, 27, 129–134. [Google Scholar] [CrossRef]
- Overholt, W.A.; Smith, J.W. Colonization of six exotic parasites (Hymenoptera) against Diatraea grandiosella (Lepidoptera: Pyralidae) in Corn. Environ. Entomol. 1990, 19, 1889–1902. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).