Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S.
Abstract
Simple Summary
Abstract
1. Introduction
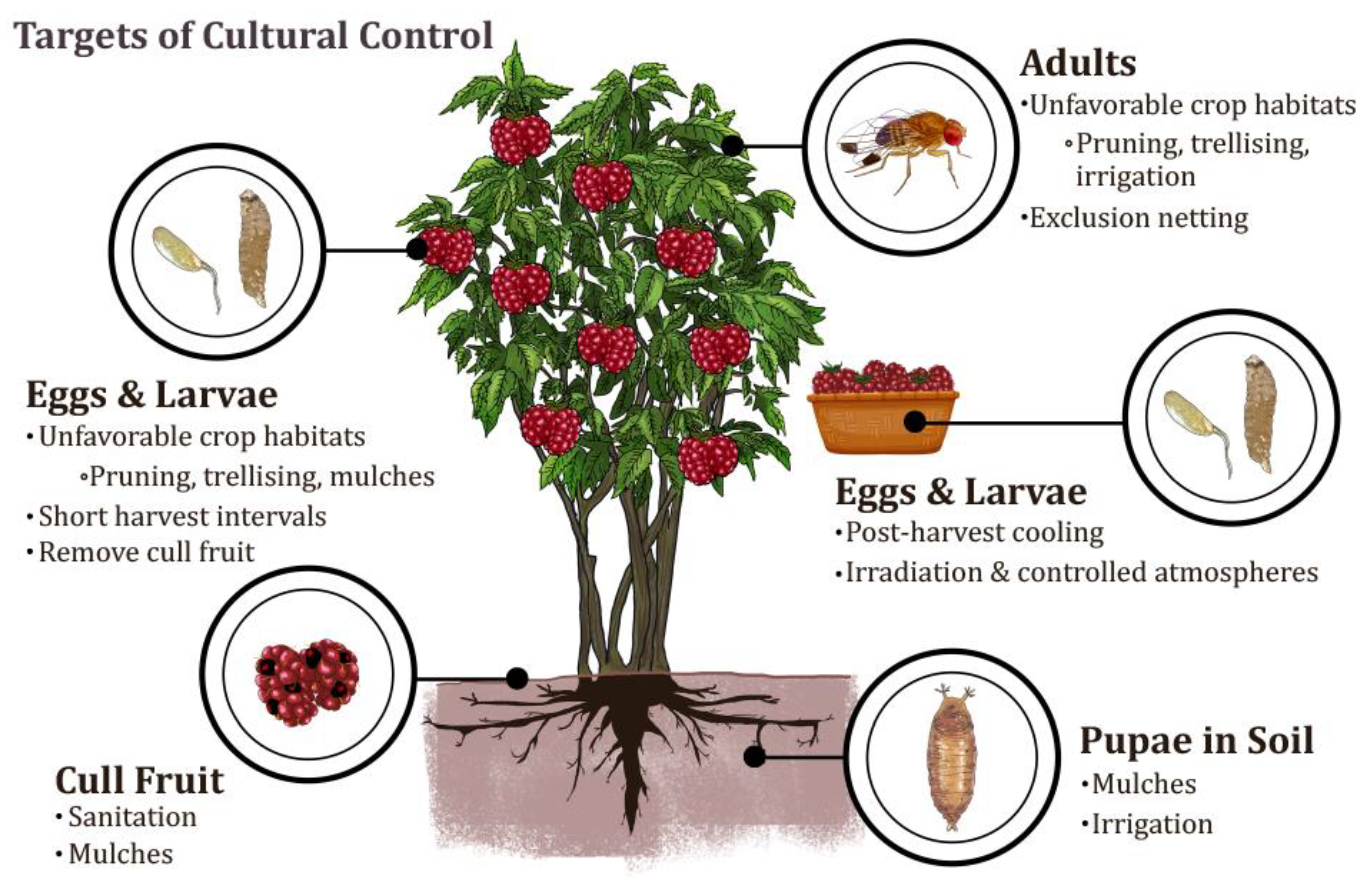
2. Cultural Control Tactics
2.1. Preventative Tactics to Reduce Pressure
2.1.1. Crop and Cultivar Selection
2.1.2. Exclusion
2.2. Manipulating within Crop Microclimate
2.2.1. Pruning and Trellising
2.2.2. Mulching and Ground Management
2.2.3. Irrigation
2.3. Harvest Management
2.4. Postharvest Sanitation
2.5. Reducing Suitability of Postharvest Fruit
2.5.1. Cooling
2.5.2. Irradiation and Quarantine Management
3. Adoption of Cultural Controls in IPM Programs
3.1. Blueberries—Pacific Northwest U.S.
3.2. Caneberries—Eastern U.S.
4. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Rechcigl, J.E.; Rechcigl, N.A. Insect Pest Management: Techniques for Environmental Protection; CRC Press: Chelsea, MI, USA, 2000. [Google Scholar]
- Halaj, J.; Cady, A.B.; Uetz, G.W. Modular habitat refugia enhance generalist predators and lower plant damage in soybeans. Environ. Entomol. 2000, 29, 383–393. [Google Scholar] [CrossRef]
- Lawton, J. Plant architecture and the diversity of phytophagous insects. Annu. Rev. Entomol. 1983, 28, 23–39. [Google Scholar] [CrossRef]
- Bushway, L.; Pritts, M.; Handley, D. Raspberry and Blackberry Production Guide for the Northeast, Midwest, and Eastern Canada; Natural Resource, Agriculture, and Engineering Service (NRAES): Ithaca, NY, USA, 2008; Volume 35. [Google Scholar]
- Krugner, R.; Groves, R.L.; Johnson, M.W.; Flores, A.P.; Hagler, J.R.; Morse, J.G. Seasonal population dynamics of Homalodisca vitripennis (Hemiptera: Cicadellidae) in sweet orange trees maintained under continuous deficit irrigation. J. Econ. Entomol. 2009, 102, 960–973. [Google Scholar] [CrossRef]
- Kührt, U.; Samietz, J.; Dorn, S. Effect of plant architecture and hail nets on temperature of codling moth habitats in apple orchards. Entomol. Exp. Appl. 2006, 118, 245–259. [Google Scholar] [CrossRef]
- Simon, S.; Lauri, P.-E.; Brun, L.; Defrance, H.; Sauphanor, B. Does manipulation of fruit-tree architecture affect the development of pests and pathogens? A case study in an organic apple orchard. J. Hortic. Sci. Biotechnol. 2006, 81, 765–773. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Kienzle, R.; Groß, L.B.; Caughman, S.; Rohlfs, M. Resource use by individual Drosophila suzukii reveals a flexible preference for oviposition into healthy fruits. Sci. Rep. 2020, 10, 3132. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Song, Y.; Yi, C.; Zhang, Q.; Xia, H.; Lin, J.; Zhang, H.; Yang, J.; Ji, Q.; Chen, J. Potential host fruits for Drosophila suzukii: Olfactory and oviposition preferences and suitability for development. Entomol. Exp. Appl. 2019, 167, 880–890. [Google Scholar] [CrossRef]
- Olazcuaga, L.; Rode, N.O.; Foucaud, J.; Facon, B.; Ravigné, V.; Ausset, A.; Leménager, N.; Loiseau, A.; Gautier, M.; Estoup, A. Oviposition preference and larval performance of Drosophila suzukii (Diptera: Drosophilidae), spotted-wing Drosophila: Effects of fruit identity and composition. Environ. Entomol. 2019, 48, 867–881. [Google Scholar] [CrossRef]
- Walsh, D.B.; O’Neal, S.D.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Bruck, D.J.; Lee, J.; Walton, V.M.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef]
- DiGiacomo, G.; Hadrich, J.; Hutchison, W.D.; Peterson, H.; Rogers, M. Economic impact of spotted wing drosophila (Diptera: Drosophilidae) yield loss on Minnesota raspberry farms: A grower survey. J. Integr. Pest Manag. 2019, 10, 11. [Google Scholar] [CrossRef]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.P.; Lopes-da-Silva, M.; Santos, R.S.S.d. Potential spread and economic impact of invasive Drosophila suzukii in Brazil. Pesq. Agropec. Bras. 2016, 51, 571–578. [Google Scholar] [CrossRef]
- De Ros, G.; Conci, S.; Pantezzi, T.; Savini, G. The economic impact of invasive pest Drosophila suzukii on berry production in the Province of Trento, Italy. J. Berry Res. 2015, 5, 89–96. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef]
- Jones, B.A. Invasive Species Control, Agricultural Pesticide Use, and Infant Health Outcomes. Land Econ. 2020, 96, 149–170. [Google Scholar] [CrossRef]
- Van Timmeren, S.; Isaacs, R. Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
- USDA-NASS. 2017 Census of Agriculture: U.S. Summary and State Data Volume 1, Geographic Area Series, Part 51; United States Department of Agriculture: Washington, DC, USA, 2019.
- Demchak, K. Small fruit production in high tunnels. HortTechnology 2009, 19, 44–49. [Google Scholar] [CrossRef]
- Hall, H.K.; Funt, R.C. Blackberries and their Hybrids. Crop Production Science in Horticulture; CABI: Wallingford, UK, 2017; Volume 27. [Google Scholar]
- Strik, B.C. A review of optimal systems for organic production of blueberry and blackberry for fresh and processed markets in the northwestern United States. Sci. Hortic. 2016, 208, 92–103. [Google Scholar] [CrossRef]
- Finn, C.; Strik, B. Blackberry production in the Pacific northwestern US: A long history and a bright future. In Proceedings of the XI International Rubus and Ribes Symposium, Asheville, NC, USA, 21–24 June 2015; pp. 35–44. [Google Scholar]
- Olmstead, J.W.; Finn, C.E. Breeding highbush blueberry cultivars adapted to machine harvest for the fresh market. HortTechnology 2014, 24, 290–294. [Google Scholar] [CrossRef]
- Joshi, N.; Butler, B.; Demchak, K.; Biddinger, D. Seasonal occurrence of spotted wing drosophila in various small fruits and berries in Pennsylvania and Maryland. J. Appl. Entomol. 2017, 141, 156–160. [Google Scholar] [CrossRef]
- Wang, X.-G.; Stewart, T.J.; Biondi, A.; Chavez, B.A.; Ingels, C.; Caprile, J.; Grant, J.A.; Walton, V.M.; Daane, K.M. Population dynamics and ecology of Drosophila suzukii in Central California. J. Pest Sci. 2016, 89, 701–712. [Google Scholar] [CrossRef]
- Hamby, K.; Bolda, M.; Sheehan, M.; Zalom, F. Seasonal monitoring for Drosophila suzukii (Diptera: Drosophilidae) in California commercial raspberries. Environ. Entomol. 2014, 43, 1008–1018. [Google Scholar] [CrossRef]
- Ryan, G.D.; Emiljanowicz, L.; Wilkinson, F.; Kornya, M.; Newman, J.A. Thermal tolerances of the spotted-wing Drosophila Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2016, 109, 746–752. [Google Scholar] [CrossRef]
- Fanning, P.D.; Johnson, A.E.; Luttinen, B.E.; Espeland, E.M.; Jahn, N.T.; Isaacs, R. Behavioral and physiological resistance to desiccation in spotted wing Drosophila (Diptera: Drosophilidae). Environ. Entomol. 2019, 48, 792–798. [Google Scholar] [CrossRef]
- Green, C.K.; Moore, P.J.; Sial, A.A. Impact of heat stress on development and fertility of Drosophila suzukii Matsumura (Diptera: Drosophilidae). J. Insect Physiol. 2019, 114, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, H.; Kunimi, Y.; Nakai, M. Effects of temperature on the reproduction and development of Drosophila suzukii (Diptera: Drosophilidae). Appl. Entomol. Zool. 2014, 49, 297–304. [Google Scholar] [CrossRef]
- Hooper, H.; Grieshop, M.J. Postharvest Burial of Drosophila suzukii (Diptera: Drosophilidae) Infested Fruit Waste Reduces Adult Emergence. Environ. Entomol. 2020, 49, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.; Moses, J.; Hanson, E.; Fanning, P.; Isaacs, R. Rapid harvest schedules and fruit removal as non-chemical approaches for managing spotted wing Drosophila. J. Pest Sci. 2018, 91, 219–226. [Google Scholar] [CrossRef]
- Alnajjar, G.; Collins, J.; Drummond, F.A. Behavioral and preventative management of Drosophila suzukii Matsumura (Diptera: Drosophilidae) in Maine wild blueberry (Vaccinium angustifolium Aiton) through attract and kill trapping and insect exclusion-netting. Int. J. Entomol. Nematol. 2017, 3, 51–61. [Google Scholar]
- Leach, H.; Van Timmeren, S.; Isaacs, R. Exclusion netting delays and reduces Drosophila suzukii (Diptera: Drosophilidae) infestation in raspberries. J. Econ. Entomol. 2016, 109, 2151–2158. [Google Scholar] [CrossRef]
- Kamiyama, M.T.; Guédot, C. Varietal and developmental susceptibility of tart cherry (Rosales: Rosaceae) to Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2019, 112, 1789–1797. [Google Scholar] [CrossRef]
- Poyet, M.; Eslin, P.; Héraude, M.; Le Roux, V.; Prévost, G.; Gibert, P.; Chabrerie, O. Invasive host for invasive pest: When the Asiatic cherry fly (Drosophila suzukii) meets the American black cherry (Prunus serotina) in Europe. Agric. For. Entomol. 2014, 16, 251–259. [Google Scholar] [CrossRef]
- Burrack, H.J.; Fernandez, G.E.; Spivey, T.; Kraus, D.A. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag. Sci. 2013, 69, 1173–1180. [Google Scholar] [CrossRef]
- Wöhner, T.; Pinggera, J.; Fritzsche, E.; Peil, A.; Pinczinger, D.; Hanke, M.V. Insights into the susceptibility of raspberries to Drosophila suzukii oviposition. J. Appl. Entomol. 2020. [Google Scholar] [CrossRef]
- Ehret, D.L.; Frey, B.; Forge, T.; Helmer, T.; Bryla, D.R.; Zebarth, B.J. Effects of nitrogen rate and application method on early production and fruit quality in highbush blueberry. Can. J. Plant Sci. 2014, 94, 1165–1179. [Google Scholar] [CrossRef]
- Ehret, D.L.; Frey, B.; Forge, T.; Helmer, T.; Bryla, D.R. Effects of drip irrigation configuration and rate on yield and fruit quality of young highbush blueberry plants. HortScience 2012, 47, 414–421. [Google Scholar] [CrossRef]
- Gong, X.; Bräcker, L.; Bölke, N.; Plata, C.; Zeitlmayr, S.; Metzler, D.; Olbricht, K.; Gompel, N.; Parniske, M. Strawberry accessions with reduced Drosophila suzukii emergence from fruits. Front. Plant Sci. 2016, 7, 1880. [Google Scholar] [CrossRef]
- Bräcker, L.B.; Gong, X.; Schmid, C.; Dawid, C.; Ulrich, D.; Phung, T.; Leonhard, A.; Ainsworth, J.; Olbricht, K.; Parniske, M. A strawberry accession with elevated methyl anthranilate fruit concentration is naturally resistant to the pest fly Drosophila suzukii. PLoS ONE 2020, 15, e0234040. [Google Scholar] [CrossRef] [PubMed]
- Tonina, L.; Giomi, F.; Sancassani, M.; Ajelli, M.; Mori, N.; Giongo, L. Texture features explain the susceptibility of grapevine cultivars to Drosophila suzukii (Diptera: Drosophilidae) infestation in ripening and drying grapes. Sci. Rep. 2020, 10, 10245. [Google Scholar] [CrossRef]
- Jaffe, B.; Guedot, C. Vertical and temporal distribution of spotted-wing drosophila (Drosophila suzukii) and pollinators within cultivated raspberries. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Drummond, F.; Ballman, E.; Collins, J. Population dynamics of spotted wing Drosophila (Drosophila suzukii (Matsumura)) in Maine wild blueberry (Vaccinium angustifolium Aiton). Insects 2019, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Hampton, E.; Koski, C.; Barsoian, O.; Faubert, H.; Cowles, R.S.; Alm, S.R. Use of early ripening cultivars to avoid infestation and mass trapping to manage Drosophila suzukii (Diptera: Drosophilidae) in Vaccinium corymbosum (Ericales: Ericaceae). J. Econ. Entomol. 2014, 107, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Arsenault-Benoit, A.; Taylor, C.M.; Butler, B.R.; Dalton, D.T.; Walton, V.M.; Petran, A.; Rogers, M.A.; Diepenbrock, L.M.; Burrack, H.J.; et al. Pruning of small fruit crops can affect habitat suitability for Drosophila suzukii. Agric. Ecosyst. Environ. 2020, 294, 106860. [Google Scholar] [CrossRef]
- Leach, H.; Van Timmeren, S.; Wetzel, W.; Isaacs, R. Predicting within-and between-year variation in activity of the invasive spotted wing drosophila (Diptera: Drosophilidae) in a temperate region. Environ. Entomol. 2019, 48, 1223–1233. [Google Scholar] [CrossRef]
- Janke, R.R.; Altamimi, M.E.; Khan, M. The use of high tunnels to produce fruit and vegetable crops in North America. Agric. Sci. 2017, 8, 692–715. [Google Scholar] [CrossRef]
- Stockton, D.G.; Hesler, S.P.; Wallingford, A.K.; Leskey, T.C.; McDermott, L.; Elsensohn, J.E.; Riggs, D.I.M.; Pritts, M.; Loeb, G.M. Factors affecting the implementation of exclusion netting to control Drosophila suzukii on primocane raspberry. Crop Prot. 2020, 105191. [Google Scholar] [CrossRef]
- Rogers, M.A.; Burkness, E.C.; Hutchison, W.D. Evaluation of high tunnels for management of Drosophila suzukii in fall-bearing red raspberries: Potential for reducing insecticide use. J. Pest Sci. 2016, 89, 815–821. [Google Scholar] [CrossRef]
- Cormier, D.; Veilleux, J.; Firlej, A. Exclusion net to control spotted wing Drosophila in blueberry fields. IOBC-WPRS Bull. 2015, 109, 181–184. [Google Scholar]
- McDermott, L.; Nickerson, L. Evaluation of insect exclusion and mass trapping as cultural controls of spotted wing Drosophila in organic blueberry production. N. Y. Fruit. Q. 2014, 22, 25–28. [Google Scholar]
- Kuesel, R.; Scott Hicks, D.; Archer, K.; Sciligo, A.; Bessin, R.; Gonthier, D. Effects of fine-mesh exclusion netting on pests of blackberry. Insects 2019, 10, 249. [Google Scholar] [CrossRef]
- Ebbenga, D.N.; Burkness, E.C.; Hutchison, W.D. Evaluation of exclusion netting for spotted-wing drosophila (Diptera: Drosophilidae) management in Minnesota wine grapes. J. Econ. Entomol. 2019, 112, 2287–2294. [Google Scholar] [CrossRef]
- Tochen, S.; Woltz, J.; Dalton, D.; Lee, J.; Wiman, N.; Walton, V. Humidity affects populations of Drosophila suzukii (Diptera: Drosophilidae) in blueberry. J. Appl. Entomol. 2016, 140, 47–57. [Google Scholar] [CrossRef]
- Haye, T.; Girod, P.; Cuthbertson, A.; Wang, X.; Daane, K.; Hoelmer, K.; Baroffio, C.; Zhang, J.; Desneux, N. Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J. Pest Sci. 2016, 89, 643–651. [Google Scholar] [CrossRef]
- Kraus, C.; Pennington, T.; Herzog, K.; Hecht, A.; Fischer, M.; Voegele, R.T.; Hoffmann, C.; Töpfer, R.; Kicherer, A. Effects of canopy architecture and microclimate on grapevine health in two training systems. Vitis 2018, 57, 53–60. [Google Scholar]
- Diepenbrock, L.; Burrack, H. Variation of within-crop microhabitat use by Drosophila suzukii (Diptera: Drosophilidae) in blackberry. J. Appl. Entomol. 2017, 141, 1–7. [Google Scholar] [CrossRef]
- Heuvel, J.E.V.; Proctor, J.T.; Sullivan, J.A. Trellising system and cane density affect yield and fruit quality of red raspberry. HortScience 2000, 35, 1215–1219. [Google Scholar] [CrossRef]
- Prange, R.K.; DeEll, J.R. Preharvest factors affecting postharvest quality of berry crops. HortScience 1997, 32, 824–830. [Google Scholar] [CrossRef]
- Yeary, W.; Fulcher, A.; Zhu, H.; Klingeman, W.; Grant, J. Spray Penetration and Natural Enemy Survival in Dense and Sparse Plant Canopies Treated with Carbaryl: Implications for Chemical and Biological Control. J. Environ. Hortic. 2018, 36, 21–29. [Google Scholar] [CrossRef]
- Cross, J.; Walklate, P.; Murray, R.; Richardson, G. Spray deposits and losses in different sized apple trees from an axial fan orchard sprayer: 1. Effects of spray liquid flow rate. Crop Prot. 2001, 20, 13–30. [Google Scholar] [CrossRef]
- Ballman, E.S.; Collins, J.A.; Drummond, F.A. Pupation Behavior and Predation on Drosophila suzukii (Diptera: Drosophilidae) Pupae in Maine Wild Blueberry Fields. J. Econ. Entomol. 2017, 110, 2308–2317. [Google Scholar] [CrossRef]
- Woltz, J.M.; Lee, J.C. Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol. Control 2017, 110, 62–69. [Google Scholar] [CrossRef]
- Kader, M.; Senge, M.; Mojid, M.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Tarara, J.M. Microclimate modification with plastic mulch. HortScience 2000, 35, 169–180. [Google Scholar] [CrossRef]
- Xuemei, J.; Qiong, Y.; Ya, W.; Yanmei, L.; Yongqiang, Z.; Rangjin, X.; Shaolan, H.; Lie, D.; Shilai, Y.; Qiang, L. Effects of DuPont Tyvek®® non-woven material mulching on fruit quality and chlorophyll fluorescence in Wanzhou Rose Orange. Sci. Hortic. 2017, 219, 31–36. [Google Scholar] [CrossRef]
- Rendon, D.; Hamby, K.A.; Arsenault-Benoit, A.L.; Taylor, C.M.; Evans, R.K.; Roubos, C.R.; Sial, A.A.; Rogers, M.; Petran, A.; Van Timmeren, S. Mulching as a cultural control strategy for Drosophila suzukii in blueberry. Pest Manag. Sci. 2020, 76, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, L.E.; Liburd, O.E. The effect of border sprays and between-row soil tillage on Drosophila suzukii in organic blackberry production. J. Appl. Entomol. 2017, 141, 19–27. [Google Scholar] [CrossRef]
- Knapp, L.; Mazzi, D.; Finger, R. Management strategies against Drosophila suzukii: Insights into Swiss grape growers choices. Pest Manag. Sci. 2019, 75, 2820–2829. [Google Scholar] [CrossRef]
- Perfect, T. Irrigation as a factor influencing the management of agricultural pests. Philos. Trans. R. Soc. A Math. Phys. Sci. 1986, 316, 347–354. [Google Scholar] [CrossRef]
- Daane, K.; Williams, L. Manipulating vineyard irrigation amounts to reduce insect pest damage. Ecol. Appl. 2003, 13, 1650–1666. [Google Scholar] [CrossRef]
- Rendon, D.; Walton, V.M. Drip and overhead sprinkler irrigation in blueberry as cultural control for Drosophila suzukii (Diptera: Drosophilidae) in Northwestern United States. J. Econ. Entomol. 2019, 112, 745–752. [Google Scholar] [CrossRef]
- Evans, R.K.; Toews, M.D.; Sial, A.A. Diel periodicity of Drosophila suzukii (Diptera: Drosophilidae) under field conditions. PLoS ONE 2017, 12, e0171718. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, S.; Horejsi, L.; Larson, S.; Spink, K.; Fanning, P.; Isaacs, R. Diurnal Activity of Drosophila suzukii (Diptera: Drosophilidae) in Highbush Blueberry and Behavioral Response to Irrigation and Application of Insecticides. Environ. Entomol. 2017, 46, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B.K.; Little, B.A.; Taylor, M.D.; Jacobs, J.L.; Lovett, W.E.; Holland, R.M.; Sial, A.A. Effect of simulated rainfall on the effectiveness of insecticides against spotted wing drosophila in blueberries. Crop Prot. 2016, 81, 122–128. [Google Scholar] [CrossRef]
- Andika, I.P.; Vandervoort, C.; Wise, J.C. Rainfastness of Insecticides Used to Control Spotted-Wing Drosophila in Tart Cherry Production. Insects 2019, 10, 203. [Google Scholar] [CrossRef]
- Mazzetto, F.; Lessio, F.; Giacosa, S.; Rolle, L.; Alma, A. Relationships between Drosophila suzukii and grapevine in North-western Italy: Seasonal presence and cultivar susceptibility. Bull. Insectol. 2020, 73, 29–38. [Google Scholar]
- Stockton, D.G.; Brown, R.; Loeb, G.M. Not berry hungry? Discovering the hidden food sources of a small fruit specialist, Drosophila suzukii. Ecol. Entomol. 2019, 44, 810–822. [Google Scholar] [CrossRef]
- Bal, H.K.; Adams, C.; Grieshop, M. Evaluation of off-season potential breeding sources for spotted wing Drosophila (Drosophila suzukii Matsumura) in Michigan. J. Econ. Entomol. 2017, 110, 2466–2470. [Google Scholar] [CrossRef]
- Kirkpatrick, D.M.; Gut, L.J.; Miller, J.R. Estimating monitoring trap plume reach and trapping area for Drosophila suzukii (Diptera: Drosophilidae) in Michigan tart cherry. J. Econ. Entomol. 2018, 111, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Tait, G.; Grassi, A.; Pfab, F.; Crava, C.M.; Dalton, D.T.; Magarey, R.; Ometto, L.; Vezzulli, S.; Rossi-Stacconi, M.V.; Gottardello, A. Large-scale spatial dynamics of Drosophila suzukii in Trentino, Italy. J. Pest Sci. 2018, 91, 1213–1224. [Google Scholar] [CrossRef]
- Lee, J.C.; Dreves, A.J.; Cave, A.M.; Kawai, S.; Isaacs, R.; Miller, J.C.; Van Timmeren, S.; Bruck, D.J. Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 2015, 108, 117–129. [Google Scholar] [CrossRef]
- Yeh, D.A.; Drummond, F.A.; Gómez, M.I.; Fan, X. The Economic Impacts and Management of Spotted Wing Drosophila (Drosophila Suzukii): The Case of Wild Blueberries in Maine. J. Econ. Entomol. 2020, 113, 1262–1269. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- Hooper, H.; Grieshop, M.J. Composting susceptible fruit wastes reduces Drosophila suzukii (Diptera: Drosophilidae) reproductive habitat. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Interspecific Competition between the House Fly, Musca domestica L. (Diptera: Muscidae) and Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae) When Reared on Poultry Manure. Insects 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C. House fly and lesser fly control utilizing the black soldier fly in manure management systems for caged laying hens. Environ. Entomol. 1983, 12, 1439–1442. [Google Scholar] [CrossRef]
- Mangan, R.L.; Hallman, G.J. Temperature treatments for quarantine security: New approaches for fresh commodities. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Routledge: New York, NY, USA, 1998; pp. 201–234. [Google Scholar]
- Strachan, L.A.; Tarnowski-Garner, H.E.; Marshall, K.E.; Sinclair, B.J. The evolution of cold tolerance in Drosophila larvae. Physiol. Biochem. Zool. 2011, 84, 43–53. [Google Scholar] [CrossRef]
- Rajamohan, A.; Sinclair, B.J. Hardening trumps acclimation in improving cold tolerance of Drosophila melanogaster larvae. Physiol. Entomol. 2009, 34, 217–223. [Google Scholar] [CrossRef]
- Aly, M.F.; Kraus, D.A.; Burrack, H.J. Effects of postharvest cold storage on the development and survival of immature Drosophila suzukii (Diptera: Drosophilidae) in artificial diet and fruit. J. Econ. Entomol. 2017, 110, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kraft, L.J.; Yeh, D.A.; Gómez, M.I.; Burrack, H.J. Determining the Effect of Postharvest Cold Storage Treatment on the Survival of Immature Drosophila suzukii (Diptera: Drosophilidae) in Small Fruits. J. Econ. Entomol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, R.; Gariepy, T.D.; Sinclair, B.J. Adult plasticity of cold tolerance in a continental-temperate population of Drosophila suzukii. J. Insect Physiol. 2015, 79, 1–9. [Google Scholar] [CrossRef]
- Stephens, A.; Asplen, M.; Hutchison, W.D.; Venette, R.C. Cold hardiness of winter-acclimated Drosophila suzukii (Diptera: Drosophilidae) adults. Environ. Entomol. 2015, 44, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Stockton, D.G.; Wallingford, A.K.; Loeb, G.M. Phenotypic plasticity promotes overwintering survival in a globally invasive crop pest, Drosophila suzukii. Insects 2018, 9, 105. [Google Scholar] [CrossRef]
- Stockton, D.G.; Wallingford, A.K.; Brind’amore, G.; Diepenbrock, L.; Burrack, H.; Leach, H.; Isaacs, R.; Iglesias, L.E.; Liburd, O.; Drummond, F. Seasonal polyphenism of spotted-wing Drosophila is affected by variation in local abiotic conditions within its invaded range, likely influencing survival and regional population dynamics. Ecol. Evol. 2020, 10, 7669–7685. [Google Scholar] [CrossRef]
- Stockton, D.; Wallingford, A.; Rendon, D.; Fanning, P.; Green, C.K.; Diepenbrock, L.; Ballman, E.; Walton, V.M.; Isaacs, R.; Leach, H. Interactions between biotic and abiotic factors affect survival in overwintering Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2019, 48, 454–464. [Google Scholar] [CrossRef]
- Jakobs, R.; Ahmadi, B.; Houben, S.; Gariepy, T.D.; Sinclair, B.J. Cold tolerance of third-instar Drosophila suzukii larvae. J. Insect Physiol. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Follett, P.A.; Yang, M.-M.; Lu, K.-H.; Chen, T.-W. Irradiation for postharvest control of quarantine insects. Formos. Entomol. 2007, 27, 1–15. [Google Scholar]
- Tucker, J. Impact of Fumigation and Irradiation Requirements on Organic Imports; United States Department of Agriculture Agricultural Marketing Service National Organic Program: Washington, DC, USA, 2018. [Google Scholar]
- Follett, P.A.; Swedman, A.; Price, D.K. Postharvest irradiation treatment for quarantine control of Drosophila suzukii (Diptera: Drosophilidae) in fresh commodities. J. Econ. Entomol. 2014, 107, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Park, C.G. X-ray radiation and developmental inhibition of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Int. J. Radiat. Biol. 2016, 92, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Lee, Y.J.; Park, C.G. Developmental inhibition of Drosophila suzukii by ionizing radiation. Entomol. Res. 2018, 48, 331–338. [Google Scholar] [CrossRef]
- Follett, P.A.; Neven, L.G. Current trends in quarantine entomology. Annu. Rev. Entomol. 2006, 51, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Swedman, A.; Mackey, B. Effect of low-oxygen conditions created by modified atmosphere packaging on radiation tolerance in Drosophila suzukii (Diptera: Drosophilidae) in sweet cherries. J. Econ. Entomol. 2018, 111, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.S.B.; Park, K.R.; Blood, R.A.; Walton, V.M. Intraspecific competition affects the pupation behavior of spotted-wing drosophila (Drosophila suzukii). Sci. Rep. 2019, 9, 7775. [Google Scholar]
- Da Silva, C.S.B.; Price, B.E.; Walton, V.M. Water-deprived parasitic wasps (Pachycrepoideus vindemmiae) kill more pupae of a pest (Drosophila suzukii) as a water-intake strategy. Sci. Rep. 2019, 9, 3592. [Google Scholar]
- Strik, B.; Buller, G.; Hellman, E. Pruning severity affects yield, berry weight, and hand harvest efficiency of highbush blueberry. HortScience 2003, 38, 196–199. [Google Scholar] [CrossRef]
- Stacconi, R.; Tait, G.; Rendon, D.; Grassi, A.; Boyer, G.; Nieri, R.; Walton, V. Gumming up the Works: Field Tests of a New Food-Grade Gum as Behavioral Disruptor for Drosophila suzukii (Diptera: Drosophilidae). J. Econ Entomol. 2020, 113, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Tait, G.; Kaiser, C.; Stacconi, R.; Dalton, D.T.; Anfora, G.; Walton, V.M. A food-grade gum as a management tool for Drosophila suzukii. Bull. Insectology 2018, 71, 295–307. [Google Scholar]
- Cornell CALS. Netting against Spotted Wing Drosophila in Berries. Available online: https://www.hortidaily.com/article/9245088/netting-against-spotted-wing-drosophila-in-berries/ (accessed on 13 November 2020).
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Scarascia, G.; Picuno, P.; Guarde, D.; Dejean, C. Review, mapping and analysis of the agricultural plastic waste generation and consolidation in Europe. Waste Manag. Res. 2013, 31, 1262–1278. [Google Scholar] [CrossRef]
- Goldberger, J.R.; DeVetter, L.W.; Dentzman, K.E. Polyethylene and biodegradable plastic mulches for strawberry production in the United States: Experiences and opinions of growers in three regions. HortTechnology 2019, 29, 619–628. [Google Scholar] [CrossRef]
- Moore, J.; Wszelaki, A. Plastic Mulch in Fruit and Vegetable Production: Challenges for Disposal; Washington State University Extension Fact Sheet FA-2016-02: Pullman, WA, USA, 2016. [Google Scholar]
- Bonhotal, J.; Bonacquist-Currin, M. Agricultural Plastics Recycling in New York State Case Study; Cornell Waste Management Institute: Ithaca, NY, USA, 2019. [Google Scholar]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
| Crop | Production System | Preventative Tactics | Unfavorable Crop Microclimate▲ | Harvest Management | Post-Harvest Management |
|---|---|---|---|---|---|
Blueberry | Fresh-Market Retail |
|
|
|
|
| Processed |
|
|
|
| |
| You-Pick |
|
|
|
| |
Caneberries | Fresh-Market Retail |
|
|
|
|
| Processed |
|
|
|
| |
| You-Pick |
|
|
|
|
 Amortization, likelihood of in-season weather damage, impact on agritourism, and supplemental pollination should be considered.
Amortization, likelihood of in-season weather damage, impact on agritourism, and supplemental pollination should be considered.  Can contribute to plastic waste and be difficult to recycle and break down. ▲ Most effective in regions that are arid during the growing season. ■ Export quarantine tactic.
Can contribute to plastic waste and be difficult to recycle and break down. ▲ Most effective in regions that are arid during the growing season. ■ Export quarantine tactic.  Not yet tested as a standalone technique.
Not yet tested as a standalone technique.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöneberg, T.; Lewis, M.T.; Burrack, H.J.; Grieshop, M.; Isaacs, R.; Rendon, D.; Rogers, M.; Rothwell, N.; Sial, A.A.; Walton, V.M.; et al. Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S. Insects 2021, 12, 172. https://doi.org/10.3390/insects12020172
Schöneberg T, Lewis MT, Burrack HJ, Grieshop M, Isaacs R, Rendon D, Rogers M, Rothwell N, Sial AA, Walton VM, et al. Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S. Insects. 2021; 12(2):172. https://doi.org/10.3390/insects12020172
Chicago/Turabian StyleSchöneberg, Torsten, Margaret T. Lewis, Hannah J. Burrack, Matthew Grieshop, Rufus Isaacs, Dalila Rendon, Mary Rogers, Nikki Rothwell, Ashfaq A. Sial, Vaughn M. Walton, and et al. 2021. "Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S." Insects 12, no. 2: 172. https://doi.org/10.3390/insects12020172
APA StyleSchöneberg, T., Lewis, M. T., Burrack, H. J., Grieshop, M., Isaacs, R., Rendon, D., Rogers, M., Rothwell, N., Sial, A. A., Walton, V. M., & Hamby, K. A. (2021). Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S. Insects, 12(2), 172. https://doi.org/10.3390/insects12020172