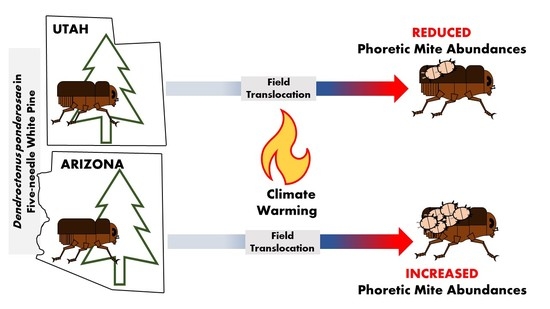
Field Translocation of Mountain Pine Beetles Suggests Phoretic Mite Communities Are Locally Adapted, and Mite Populations Respond Variably to Climate Warming
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
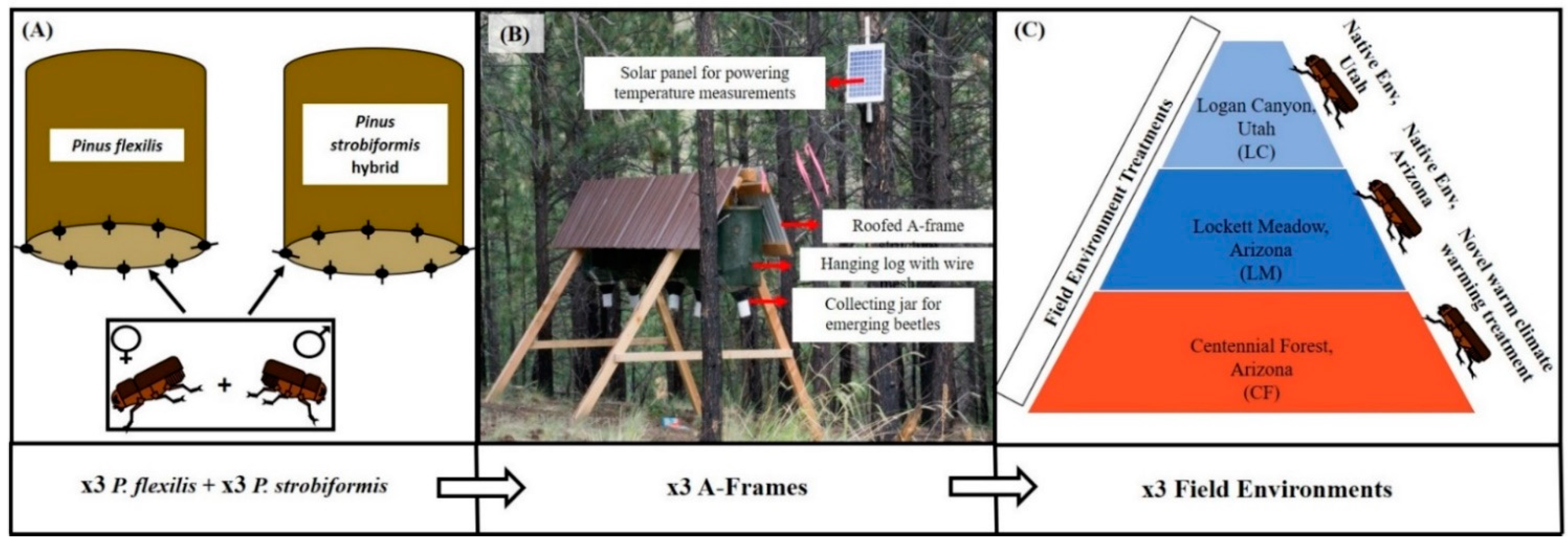
2.1. Parent Beetle Collection and Host Tree Environment
2.2. Translocation Preparation
2.3. Reciprocal Translocation and Field Environmental Profiles
2.4. Mite Collection and Identification
2.5. Data Analysis
3. Results
3.1. Beetle Emergence Patterns
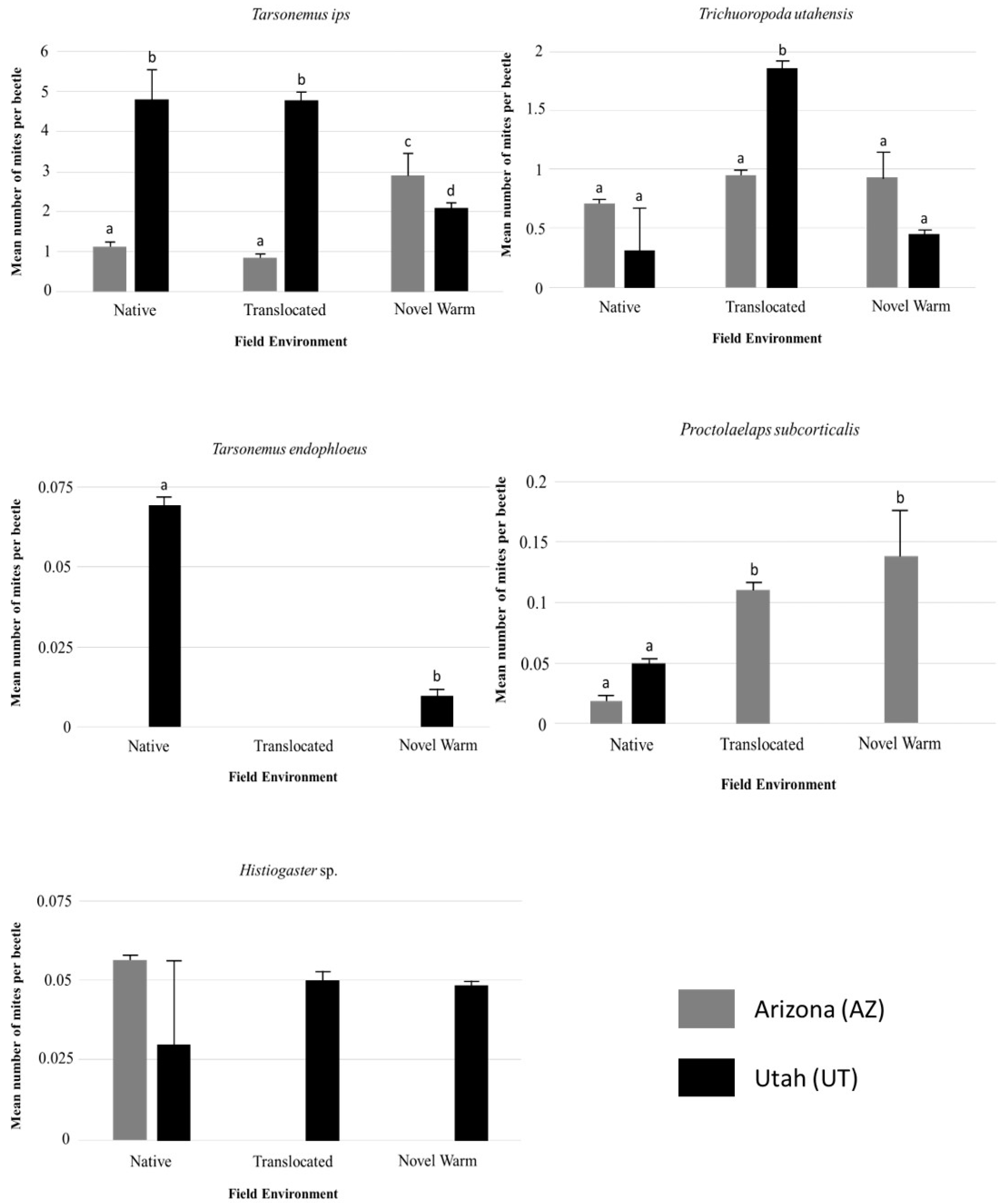
3.2. Overall Mite Community Differences
3.3. Individual Species Differences within and Across Populations
4. Discussion
4.1. Temperature, Beetle Emergence, and Mite Abundance
4.2. Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, J.V.; Stanford, J.A. Thermal responses in the evolutionary ecology of aquatic insects. Ann. Rev. Entomol. 1982, 27, 97–117. [Google Scholar] [CrossRef]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob. Chang. Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.E.; Holzapfel, M.H. Genetic response to rapid climate change: It’s seasonal timing that matters. Mol. Ecol. 2007, 17, 157–166. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, J.C.; Hansen, M.E.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the Western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 601–613. [Google Scholar] [CrossRef]
- Raffa, K.F.; Powell, E.N.; Townsend, P.A. Temperature-driven range expansion of an eruptive insect heightened by weakly co-evolved plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 2193–2198. [Google Scholar] [CrossRef]
- Cudmore, T.J.; Bjo¨rklund, N.; Carroll, A.L.; Lindgren, B.S. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in naïve host tree populations. J. Appl. Ecol. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Bentz, B.; Campbell, E.; Gibson, K.; Kegley, S.; Loan, J.; Six, D. Mountain pine beetle in high-elevation five needle white pine ecosystems. In Proceedings of the High Five Symposium, Missoula, MT, USA, 28–30 June 2010; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2010; Volume 63, pp. 78–84. [Google Scholar]
- Weed, A.S.; Ayres, M.P.; Bentz, B.J. Population Dynamics of Bark Beetles. In Bark Beetle; Elsevier: Amsterdam, The Netherlands, 2015; pp. 157–176. [Google Scholar]
- Six, D. Ecological and evolutionary determinants of bark beetle-fungus symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef]
- Biedermann, P.; Gregoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbach, A.; Hofstetter, R.; Kandasamy, J.; Kolarik, M.; Kostovcik, M.; Krokene, P.; et al. Bark beetle population dynamics in the anthropocene: Challenges and solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef]
- Bentz, B.J.; Schen-Lagenheim, G.; Boone, C.; Raffa, K. Mountain Pine Beetle Outbreak Dynamics in High Elevation Forests: Influence of Climate Change and Tree Chemistry. 2009. Utah State University, Digital Commons. Available online: https://www.fs.usda.gov/treesearch/pubs/43479 (accessed on 24 August 2018).
- Mitton, J.B.; Ferrenberg, S.M. Mountain pine beetle develops an unprecedented generation in response to climate warming. Am. Nat. 2012, 179, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Vandygriff, J.C.; Jensen, C.; Coleman, T.; Maloney, P.; Smith, S.; Grady, A.; Schen-Langenheim, G. Mountain pine beetle voltinism and life history characteristics across latitudinal and elevational gradients in the western United States. For. Sci. 2014, 60, 434–449. [Google Scholar] [CrossRef]
- Carroll, A.L.; Taylor, S.W.; Régnière, J.; Safranyik, L. Effect of climate change on range expansion by the mountain pine beetle in British Columbia. In The Bark Beetles, Fuels, and Fire Bibliography; Utah State University: Logan, UT, USA, 2003; p. 195. [Google Scholar]
- Safranyik, L.; Carroll, A.L. The Biology and Epidemiology of the Mountain Pine Beetle in Lodgepole Pine Forests; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2006. [Google Scholar]
- de la Giroday, H.C.; Carroll, A.L.; Aukema, B.H. breach of the northern Rocky Mountain geoclimatic barrier: Initiation of range expansion by the mountain pine beetle. J. Biogeogr. 2012, 39, 1112–1123. [Google Scholar] [CrossRef]
- Sambaraju, K.; Carrol, A.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukeman, B.H. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Carroll, A.L.; Aukema, B.H. Multiyear weather anomalies associated with range shifts by the mountain pine beetle preceding large epidemics. For. Ecol. Manag. 2019, 438, 86–95. [Google Scholar] [CrossRef]
- Patriquin, M.N.; Wellstead, A.M.; White, W.A. Beetles, trees, and people: Regional economic impact sensitivity and policy considerations related to the mountain pine beetle infestation in British Columbia, Canada. For. Pol. Econ. 2007, 9, 938–946. [Google Scholar] [CrossRef]
- Price, J.I.; McCollum, D.W.; Berrens, R.P. Insect infestation and residential property values: A hedonic analysis of the mountain pine beetle epidemic. For. Pol. Econ. 2010, 12, 415–422. [Google Scholar] [CrossRef]
- Hicke, J.A.; Meddens, J.H.A.; Kolden, C.A. Recent tree mortality in the western United States from bark beetles and forest fires. For. Sci. 2015, 62, 141–153. [Google Scholar] [CrossRef]
- Long, J.A.; Lawrence, R.L. Mapping Percent Tree Mortality Due to Mountain Pine Beetle Damage. For. Sci. 2016, 62, 392–402. [Google Scholar] [CrossRef]
- Taylor, S.W.; Carroll, A.L.; Alfaro, R.I.; Safranyik, L. Forest, climate and mountain pine beetle outbreak dynamics in western Canada. In The Mountain Pine Beetle—A Synthesis of Biology, Management, and Impacts in Lodgepole Pine; Canadian Forest Service: Victoria, BC, Canada, 2006; pp. 67–94. [Google Scholar]
- Soderberg, D.N.; Mock, K.F.; Hofstetter, R.W.; Bentz, B.J. Translocation experiment reveals capacity for mountain pine beetle persistence under climate warming. Ecol. Mongr. 2020, e01437. [Google Scholar] [CrossRef]
- Mock, K.E.; Bentz, B.J.; O’Neill, E.M.; Chong, J.O.; Pfrender, M.E. Landscape-scale genetic variation in a forest outbreak species, the mountain pine beetle (Dendroctonus ponderosae). Mol. Ecol. 2007, 16, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Bleiker, K.P.; Six, D.L. Competition and coexistence in a multi-partner mutualism: Interactions between two fungal symbionts of the mountain pine beetle in beetle-attached trees. Microb. Ecol. 2009, 57, 191–202. [Google Scholar] [CrossRef]
- Mercado, J.; Ortiz-Santana, B.; Kay, S. Fungal Frequency and Mite Load Trends Interact with a Declining Mountain Pine Beetle Population. Forests 2018, 9, 484. [Google Scholar] [CrossRef]
- Vissa, S.; Hofstetter, R.W. The role of mites in bark and ambrosia beetle–fungal interactions. In Insect Physiology and Ecolog; Intech Press: London, UK, 2017; pp. 135–156. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Dempsey, T.D.; Klepzig, K.D.; Ayres, M.P. Temperature-dependent effects on mutualistic and phoretic associations. Comm. Ecol. 2007, 8, 47–56. [Google Scholar] [CrossRef]
- Moore, M.L.; Six, D.L. Effects of temperature on growth, sporulation, and competition of mountain pine beetle fungal symbionts. Microb. Ecol. 2015, 70, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Mullins, D.E. Ecology of mountain pine beetle (Coleoptera: Scolytidae) cold hardening in the intermountain west. Environ. Entomol. 1999, 28, 577–587. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Smith, G.D.; Humble, L.M. Cold tolerance of mountain pine beetle (Coleoptera: Curculionidae) eggs from the historic and expanded ranges. Environ. Entomol. 2017, 46, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L.; Bentz, B.J. Temperature determines symbiont abundance in a multipartite bark beetle-fungus ectosymbiosis. Micro. Ecol. 2007, 54, 112–118. [Google Scholar] [CrossRef]
- Mercado, J.E.; Hofstetter, R.W.; Reboletti, D.M.; Negron, J.F. Phoretic symbionts of the mountain pine beetle (Dendroctonus ponderosae Hopkins). For. Sci. 2014, 60, 512–526. [Google Scholar] [CrossRef]
- Vissa, S.; Mercado, J.E.; Malesky, D.; Uhey, D.A.; Mori, B.A.; Knee, W.; Evenden, M.L.; Hofstetter, R.W. Patterns of diversity in the symbiotic mite assemblages of the mountain pine beetle, Dendroctonus ponderosae Hopkins. Forests 2020, 110, 1102. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Cronin, J.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 2006, 147, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R. An analysis of dispersal in mites. Am. Nat. 1970, 104, 425–431. [Google Scholar] [CrossRef]
- Lindquist, E.E. Relationships between mites and insects in forest habitats. Can. Entomol. 1970, 102, 978–984. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Dinkins-Bookwalter, J.; Klepzig, K.D.; Davis, T.S. Symbiotic associates of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 209–246. [Google Scholar]
- Moser, J.C.; Roton, L.M. Mites associated with southern pine bark beetles in Allen Parish, Louisiana. Can. Entomol. 1971, 103, 1775–1798. [Google Scholar] [CrossRef]
- Klepzig, K.D.; Moser, J.C.; Lombardero, F.J.; Ayres, M.P.; Hofstetter, R.W.; Walkinshaw, C.J. Mutualism and antagonism: Ecological interactions among bark beetles, mites and fungi. In Biotic Interactions in Plant-Pathogen Associations; CAB International: Wallingford, UK, 2001; pp. 237–267. [Google Scholar]
- Hofstetter, R.W.; Moser, J.C.; Blomquist, S. Mites associated with bark beetles and their hypophoretic Ophiostomatoid fungi. In The Ophiostomatoid Fungi: Expanding Frontiers; CBS Biodiversity Series 12; KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2014; pp. 165–176. [Google Scholar]
- Wegensteiner, R.; Wermelinger, B.; Herrmann, M. Natural enemies of bark beetles: Predators, parasitoids, pathogens, and nematodes. In Bark Beetles; Academic Press: Cambridge, MA, USA, 2015; pp. 247–304. [Google Scholar]
- Doberski, J.W. Comparative laboratory studies on three fungal pathogens of the elm bark beetle Scolytus scolytus: Effect of temperature and humidity on infection by Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J. Invert. Pathol. 1981, 37, 195–200. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Mahfouz, J.B.; Klepzig, K.D.; Ayres, M.P. Effects of tree phytochemistry on the interactions among endophloedic fungi associated with the southern pine beetle. J. Chem. Ecol. 2005, 31, 539–560. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Ayres, M.P.; Hofstetter, R.W.; Moser, J.C.; Klepzig, K.D. Strong indirect interaction of Tarsonemus mites (Acarina: Tarsonemidae) and Dendroctonus frontalis (Coleoptera: Scolytidae). Oikos 2003, 102, 243–252. [Google Scholar] [CrossRef]
- Menon, M.; Bagley, J.C.; Friedline, C.J.; Whipple, A.V.; Schoettle, A.W.; Leal-Saenz, A.; Wehenkel, C.; Molina-Friener, F.; Flores-Renteria, L.; Gonzalez-Elizondo, M.S.; et al. The role of hybridization during ecological divergence of southwestern white pine (Pinus strobiformis) and limber pine (P. flexilis). Mol. Ecol. 2018, 27, 1245–1260. [Google Scholar] [CrossRef]
- Amman, G.D.; McGregor, M.D.; Dolph, R.E. Mountain pine beetle. USDA Forest Service. For. Insect Dis. Leafl. 1990, 2, 1–12. [Google Scholar]
- Steinhoff, R.J.; Andresen, J.W. Geographic variation in Pinus flexilis and Pinus strobiformis and its bearing on their taxonomic status. Silvae Genet. 1971, 20, 159–167. [Google Scholar]
- Benkman, C.W.; Balda, R.P.; Smith, C.C. Adaptations for seed dispersal and the compromises due to seed predation in limber pine. Ecology 1984, 65, 632–642. [Google Scholar] [CrossRef]
- Lyon, R.L. A useful secondary sex character in Dendroctonus bark beetles. Can. Entomol. 1958, 3, 582–584. [Google Scholar] [CrossRef]
- Rosenberger, D.W.; Venette, R.C.; Aukema, B.H. Sexing live mountain pine beetles Dendroctonus ponderosae: Refinement of a behavioral method for Dendroctonus spp. Entomol. Exp. Appl. 2016, 160, 195–199. [Google Scholar] [CrossRef]
- West, D.R. Mountain Pine Beetle-Caused Mortality, Host Selection, and Preformed Tree Defenses in Lodgepole and Ponderosa Pine of the Front Range, Colorado. Ph.D. Thesis, 2000–2019-CSU Theses and Dissertations. Colorado State University, Fort Collins, CO, USA, 2013. [Google Scholar]
- Mercado, J.E. Mite and Fungal Associates on Mountain Pine Beetles Attacking Three Pine Species in Northern Colorado. Ph.D. Thesis, Colorado State University Libraries, Fort Collins, CO, USA, 2019. [Google Scholar]
- Lindquist, E.E. New species of Tarsonemus (Acarina: Tarsonemidae) associated with bark beetles. Can. Entomol. 1969, 101, 1219–1314. [Google Scholar] [CrossRef]
- Magowski, W.L.; Moser, J.C. Redescription of Tarsonemus minimax and definition of its species-group in the genus Tarsonemus (Acari: Tarsonemidae) with descriptions of two new species. Ann. Entomol. Soc. Am. 2003, 96, 345–368. [Google Scholar] [CrossRef]
- Ripley, B.; Venebles, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. Package ‘mass’. Cran r 2013, 538, 113–120. [Google Scholar]
- Wang, Y.I.; Naumann, U.; Wright, S.T.; Warton, D.I. mvabund–An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Warton, D.I.; Thibaut, L.; Wang, Y.A. The PIT-trap-A “model-free” bootstrap procedure for inference about regression models with discrete, multivariate responses. PLoS ONE 2017, 12, e0181790. [Google Scholar] [CrossRef]
- Norby, R.J.; Luo, Y. Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytol. 2004, 162, 281–293. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 4, 62. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of species responses to climate change. Sci. Adv. 2017, 3, e1603055. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Wilson, R.J. Intra-and interspecific variation in the responses of insect phenology to climate. J. Anim. Ecol. 2020, 90, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.R. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Insect temperature–body size trends common to laboratory, latitudinal and seasonal gradients are not found across altitudes. Func. Ecol. 2018, 32, 948–957. [Google Scholar] [CrossRef]
- Gibson, K.; Skov, K.; Kegley, S.; Jorgensen, C.; Smith, S.; Witcosky, J. Mountain Pine Beetle Impacts in High-Elevation Five-Needle Pines: Current Trends and Challenges; R1-08-020; USDA Forest Service: Missoula, MT, USA, 2008; pp. 1–32.
- Logan, J.A.; Macfarlane, W.W.; Willcox, L. Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the Greater Yellowstone Ecosystem. Ecol. Appl. 2010, 20, 895–902. [Google Scholar] [CrossRef]
- Mori, B.A.; Proctor, H.C.; Walter, D.E.; Evenden, M.L. Phoretic mite associates of mountain pine beetle at the leading edge of an infestation in northwestern Alberta, Canada. Can. Entomol. 2011, 143, 44–55. [Google Scholar] [CrossRef]
- Evans, L.M.; Hofstetter, R.W.; Ayres, M.P.; Klepzig, K.D. Temperature alters the relative abundance and population growth rates of species within the Dendroctonus frontalis (Coleoptera: Curculionidae) community. Environ. Entomol. 2011, 40, 824–834. [Google Scholar] [CrossRef]
- Hunt, D.W.A.; Borden, J.H.; Rahe, J.E.; Whitney, H.S. Nutrient-mediated germination of Beauveria bassiana conidia on the integument of the bark beetle Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Invertebr. Pathol. 1984, 44, 304–314. [Google Scholar] [CrossRef]
- Rice, A.; Thormann, M.; Langor, D. The mountain pine beetle: A synthesis of biology, management and impacts on lodgepole pine, chapter. In The Biology and Epidemiology of the Mountain Pine Beetle in Lodgepole Pine Forests; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2008. [Google Scholar]
- Dysthe, J.C.; Bracewell, R.; Six, D.L. Temperature effects on growth of funal symbionts of the western pine beetle, Dendroctonus brevicomis. Fung. Ecol. 2015, 17, 62–68. [Google Scholar] [CrossRef]
- Addison, A.L.; Powell, J.A.; Six, D.L.; Moore, M.; Bentz, B.J. The role of temperature variability in stabilizing the mountain pine beetle-fungus mutualism. J. Theor. Biol. 2013, 335, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pfammatter, J.; Raffa, K. Do phoretic mites influence the reproductive success of Ips grandicollis (Coleoptera: Curculionidae)? Environ. Entomol. 2015, 44, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L.; Bracewell, R. Chapter 8—Dendroctonus. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 305–350. [Google Scholar]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Func. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Moser, J.C.; Konrad, H.; Blomquist, S.; Kirisits, T. Do mites phoretic on elm bark beetles contribute to the transmission of Dutch elm disease? Naturwissenschaften 2010, 97, 219–227. [Google Scholar] [CrossRef]
- Six, D.L.; Paine, T.D. Effects of mycangial fungi and host tree species on progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae). Environ. Entomol. 1998, 27, 1393–1401. [Google Scholar] [CrossRef]
- Bracewell, R.; Six, D. Experimental evidence of bark beetle adaptation to a fungal symbiont. Ecol. Evol. 2015, 21, 5109–5119. [Google Scholar] [CrossRef]
- Bentz, B.J.; Six, D.L. Ergosterol content of fungi associated with Dendroctonus ponderosae and Dendroctonus rufipennis (Coleoptera: Curculionidae, Scolytinae). Ann. Entomol. Soc. Am. 2006, 99, 189–194. [Google Scholar] [CrossRef]
- Vissa, S. The Role of Mites in Bark Beetle-Fungal Associations: A Network of Symbioses. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2017. [Google Scholar]
- Six, D.L. Niche construction theory can link bark beetle-fungus symbiosis type and colonization behavior to large scale causal chain-effects. Curr. Opin. Insect Sci. 2020, 39, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.J.; Breuil, C. Leptographium longiclavatum sp. nov., a new species associated with the mountain pine beetle, Dendroctonus ponderosae. Myc. Res. 2005, 109, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L. A comparison of mycangial and phoretic fungi of individual mountain pine beetles. Can. J. For. Res. 2003, 33, 1331–1334. [Google Scholar] [CrossRef]
- Moser, J. Use of sporothecae by phoretic Tarsonemus mites to transport ascospores of coniferous bluestain fungi. Trans. Brit. Mycol. Soc. 1985, 84, 750–753. [Google Scholar] [CrossRef]
- Moser, J.; Macías-Sámano, J. Tarsonemid mite associates of Dendroctonus frontalis (Coleoptera: Scolytidae): Implication for the historical biogeography of D. frontalis. Can. Entomol. 2000, 132, 765–771. [Google Scholar] [CrossRef]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interaction among scolytid bark beetles, their associated fungi, and live host conifers. Ann. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef]
- Reboletti, D.M. A Multi-Partite Mutualism: Bark Beetles, Fungi, and Mites. Ph.D. Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2008. [Google Scholar]
- Chang, R.; Duong, T.A.; Taerum, S.J.; Wingfield, M.J.; Zhou, X.; De Beer, Z.W. Ophiostomatoid fungi associated with conifer-infesting beetles and their phoretic mites in Yunnan, China. MycoKeys 2017, 28, 19. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Moser, J.C.; Klepzig, K.D.; Raffa, K.F. Multipartite symbioses among fungi, mite, nematodes, and the spruce beetle, Dendroctonus rufipennis. Environ. Entomol. 2008, 37, 956–963. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Biology, demography and community interactions of Tarsonemus (Acarina: Tarsonemidae) mites phoretic on Dendroctonus frontalis (Coleoptera: Scolytinae). Agric. For. Entomol. 2000, 2, 193–202. [Google Scholar] [CrossRef]
- Vissa, S.; Hofstetter, R.W.; Bonifácio, L.; Khaustov, A.; Knee, W.; Uhey, D.A. Phoretic mite communities associated with bark beetles in the maritime and stone pine forests of Setúbal, Portugal. Exp. Appl. Acarol. 2019, 77, 117–131. [Google Scholar] [CrossRef]
- Post, D.M.; Palkovacs, E.P. Eco-evolutionary feedbacks in community and ecosystem ecology: Interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B. 2009, 364, 1629–1640. [Google Scholar] [CrossRef]
- Six, D.L. The bark beetle holobiont: Why microbes matter. J. Chem. Ecol. 2013, 39, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Odling-Smee, J.; Erwin, D.H.; Palkovacs, E.P.; Feldman, M.W.; Laland, K.N. Niche construction theory: A practical guide for ecologists. Q. Rev. Biol. 2013, 88, 3–28. [Google Scholar] [CrossRef] [PubMed]
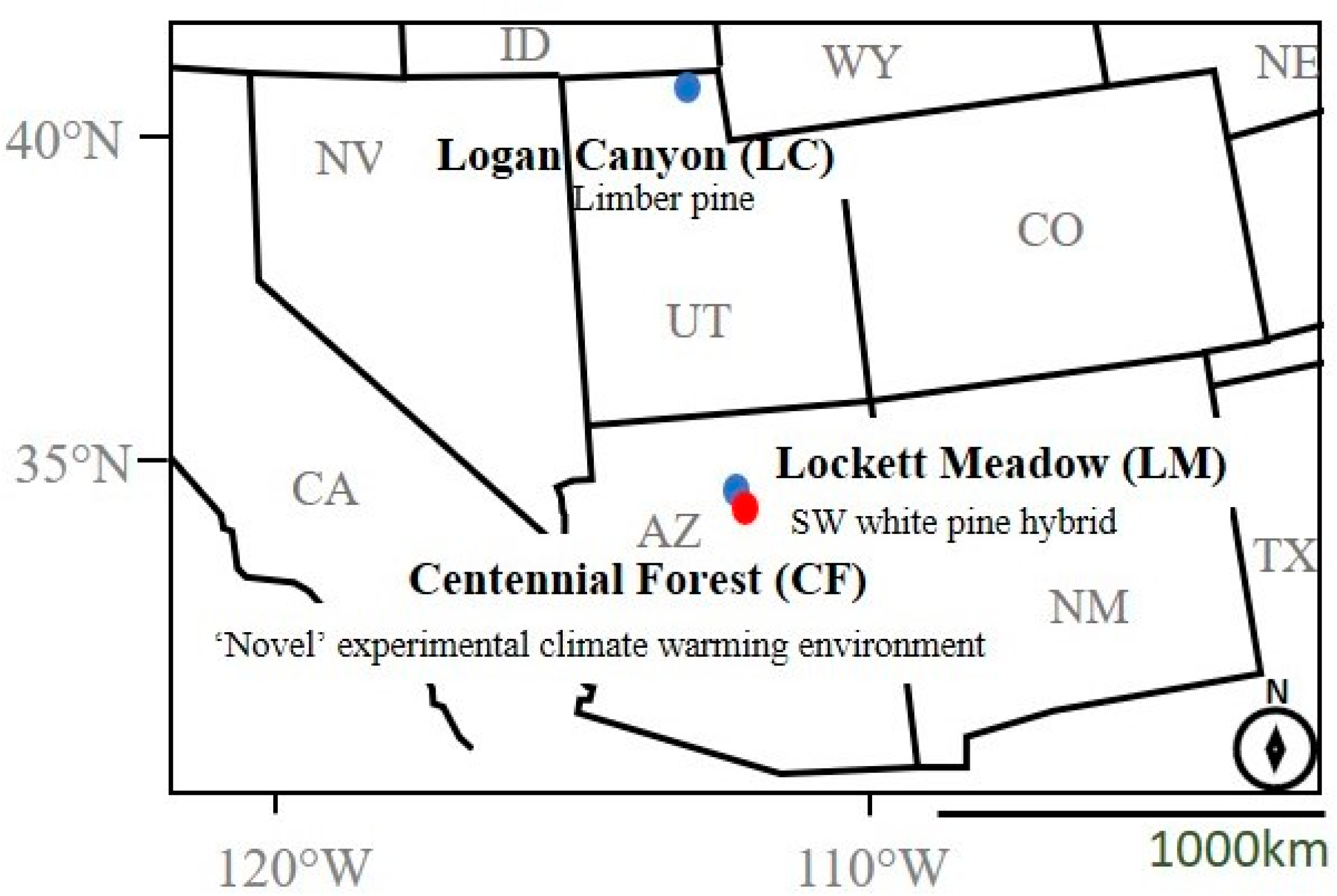

| Field Location | Relevance to Populations | Host Tree (Pinus sp.) | Latitude (N) | Longitude (W) | Elevation (m.) | Total Mean Temp (°C) | Average Min Temp (°C) | Average Max Temp (°C) |
|---|---|---|---|---|---|---|---|---|
| Logan Canyon (LC), UT | Native (UT) | P. flexilis | 41.93 | 111.44 | 2204 | 4.7 | −28.5 | 30.4 |
| Lockett Meadow (LM), AZ | Native (AZ) | P.strobiformis hybrid | 35.35 | −111.62 | 2604 | 9.2 | −14.2 | 28.6 |
| Centennial Forest (CF), AZ | Novel Warming | (P.ponderosa habitat) | 35.14 | −111.71 | 2106 | 10.2 | −14.2 | 32.8 |
| Population | Field Environment | Total No. Beetles Examined | % Beetles with Mites | Estimated Mites per Gallery | List of Species Associated with Populations |
|---|---|---|---|---|---|
| Arizona | Native (LM) | 107 | 59.8% | 56.7 | Tarsonemus ips Lindq. |
| Trichouropoda utahensis Wis. & Hirsch. | |||||
| Translocated (LC) | 100 | 66% | 59.5 | Proctolaelaps subcorticalis Berlese | |
| Histiogaster sp. | |||||
| Novel Warm (CF) | 101 | 63.4% | 131.3 | ||
| Utah | Native (LC) | 101 | 72.3% | 149.5 | Tarsonemus ips Lindq. |
| Tarsonemus endophloeus Lindq. | |||||
| Translocated (LM) | 100 | 80% | 167.9 | Trichouropoda utahensis Wis. & Hirsch. | |
| Proctolaelaps subcorticalis Berlese | |||||
| Novel Warm (CF) | 103 | 68% | 57.5 | Histiogaster sp. |
| Variable | DF | Dev | Pr (>Dev) |
|---|---|---|---|
| Tarsonemus ips Lindq. | |||
| Population | 610 | 29.08 | 0.001 ** |
| Field Environment | 608 | 1.6 | 0.441 |
| Population: Field Environment | 606 | 32.35 | 0.001 ** |
| Trichouropoda utahensis Wis. & Hirsch. | |||
| Population | 610 | 0.013 | 0.911 |
| Field Environment | 608 | 13.767 | 0.01 * |
| Population:Field Environment | 606 | 22.893 | 0.001 ** |
| Proctolaelaps subcorticalis Berlese | |||
| Population | 610 | 11.052 | 0.001 ** |
| Field Environment | 608 | 9.115 | 0.03 * |
| Population: Field Environment | 606 | 5.352 | 0.135 |
| Tarsonemus endophloeus Lindq. | |||
| Population | 610 | 3.407 | 0.02 * |
| Field Environment | 608 | 4.588 | 0.03 * |
| Population: Field Environment | 606 | 0.001 | 0.784 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vissa, S.; Soderberg, D.N.; Hofstetter, R.W. Field Translocation of Mountain Pine Beetles Suggests Phoretic Mite Communities Are Locally Adapted, and Mite Populations Respond Variably to Climate Warming. Insects 2021, 12, 131. https://doi.org/10.3390/insects12020131
Vissa S, Soderberg DN, Hofstetter RW. Field Translocation of Mountain Pine Beetles Suggests Phoretic Mite Communities Are Locally Adapted, and Mite Populations Respond Variably to Climate Warming. Insects. 2021; 12(2):131. https://doi.org/10.3390/insects12020131
Chicago/Turabian StyleVissa, Sneha, David N. Soderberg, and Richard W. Hofstetter. 2021. "Field Translocation of Mountain Pine Beetles Suggests Phoretic Mite Communities Are Locally Adapted, and Mite Populations Respond Variably to Climate Warming" Insects 12, no. 2: 131. https://doi.org/10.3390/insects12020131
APA StyleVissa, S., Soderberg, D. N., & Hofstetter, R. W. (2021). Field Translocation of Mountain Pine Beetles Suggests Phoretic Mite Communities Are Locally Adapted, and Mite Populations Respond Variably to Climate Warming. Insects, 12(2), 131. https://doi.org/10.3390/insects12020131