Reduction in Egg Fertility of Aedes albopictus Mosquitoes in Greece Following Releases of Imported Sterile Males
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
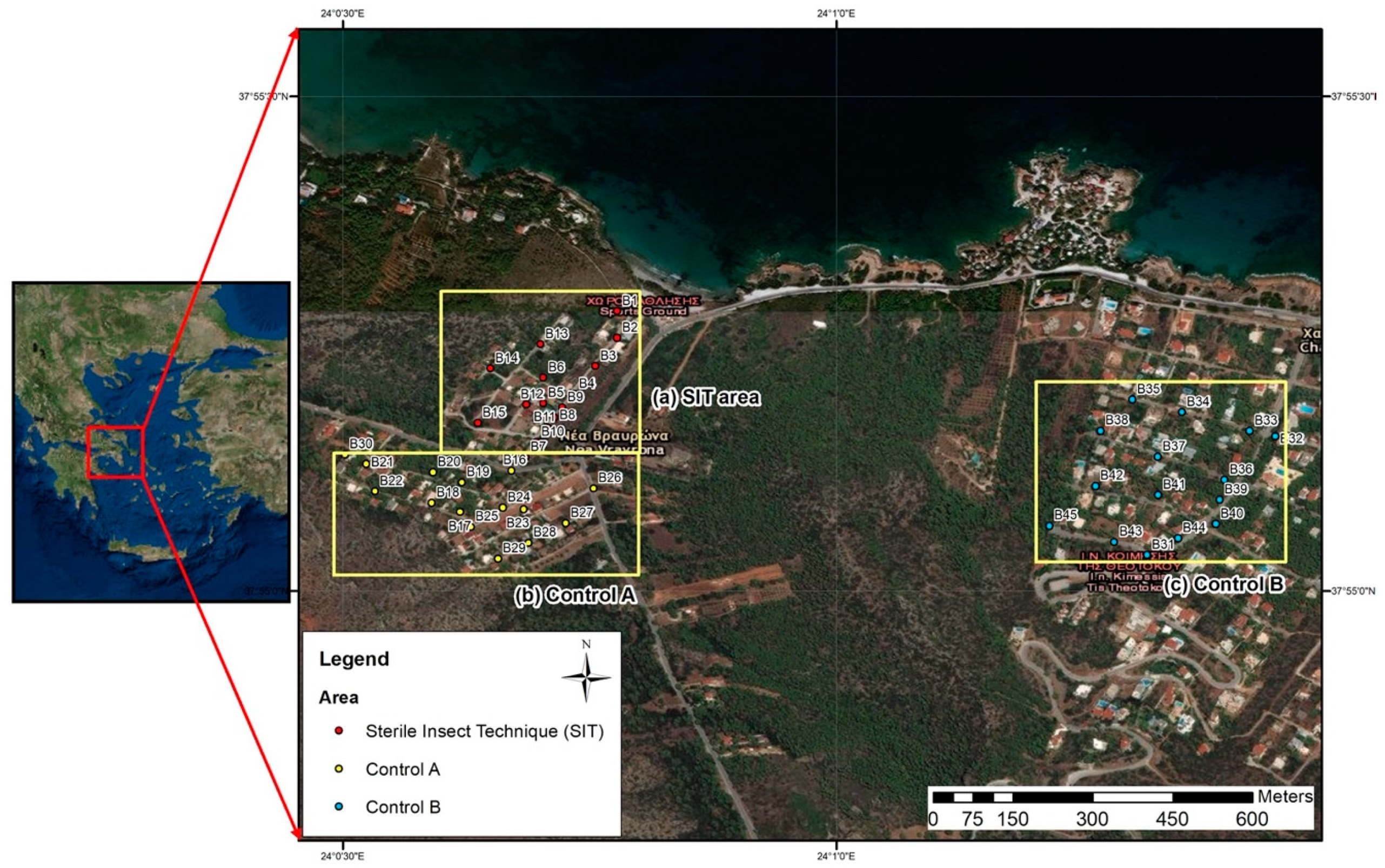
2.1. The Trial Area
2.2. Information Campaign and Public Education
2.3. Mosquito Mass Rearing and Sterilization
2.4. Sterile Mosquito Transport and Releases
2.5. Assessment of Egg Hatch
2.6. Statistical Analysis
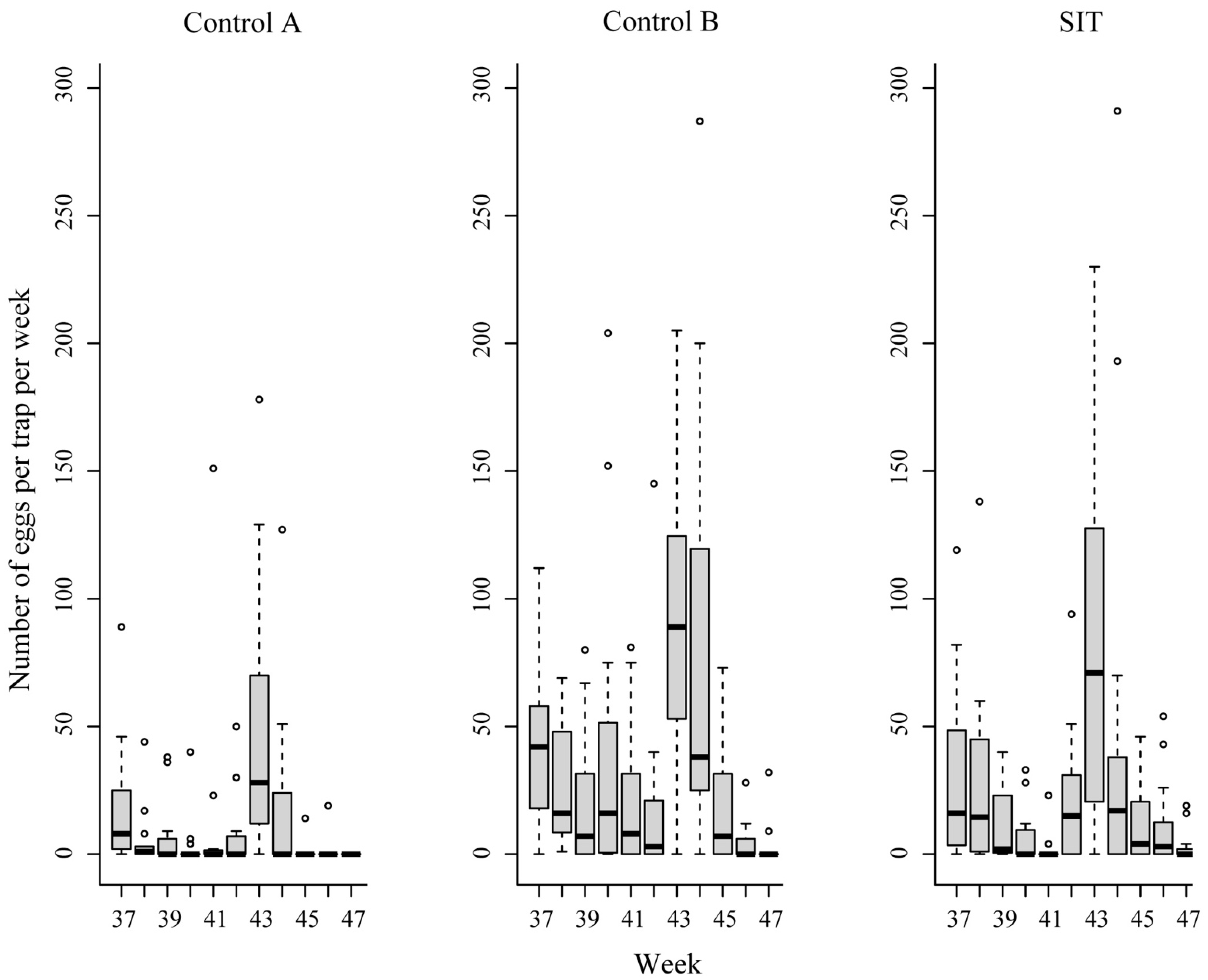
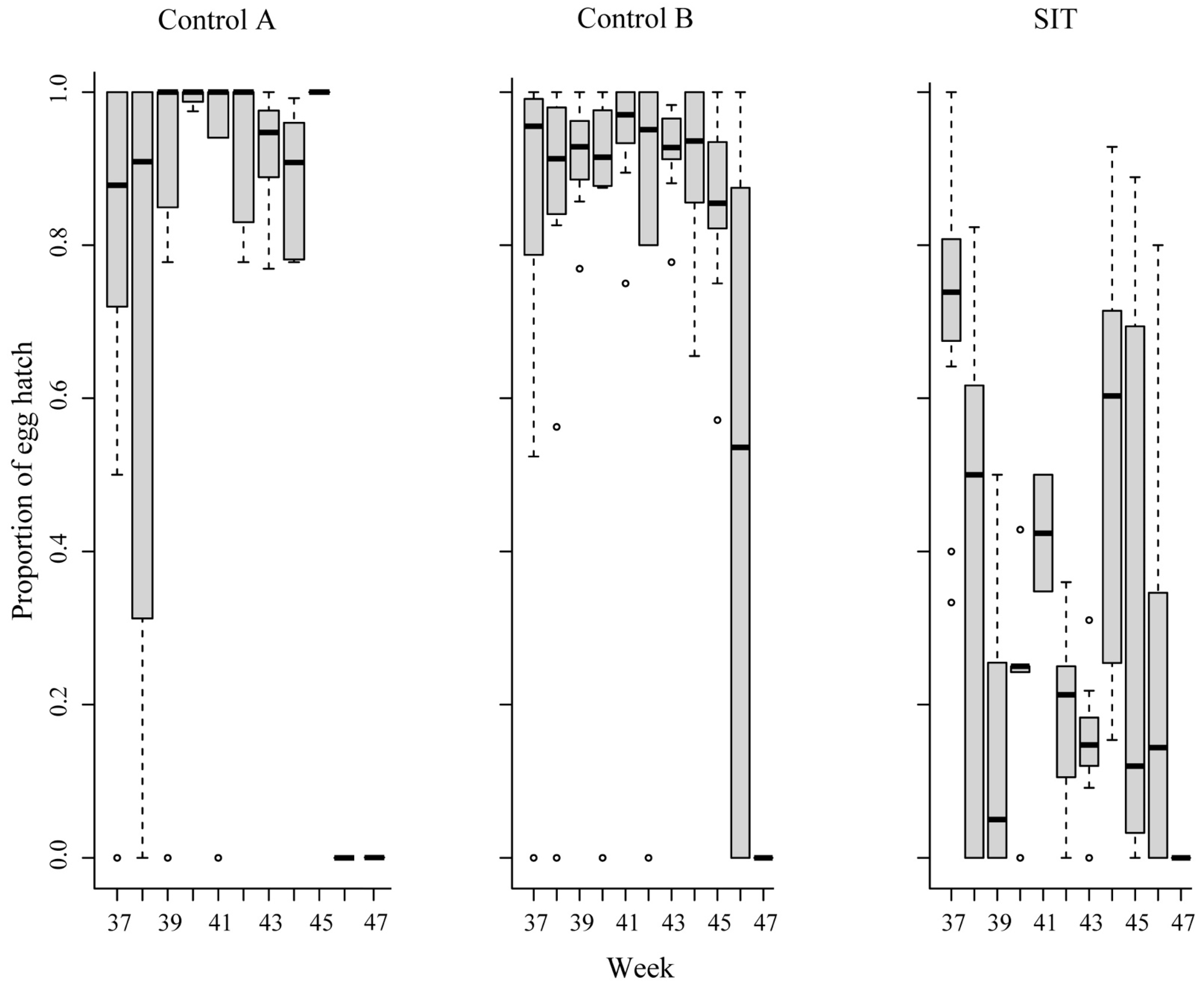
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaffner, F.; Medlock, J.M.; Van Bortel, W.A. Public health significance of invasive mosquitoes in Europe Invasive Mosquito Species in Europe. Clin. Microbiol. Infect. 2013, 19, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.G. Critical review of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Giatropoulos, A.K.; Michaelakis, A.Ν.; Koliopoulos, G.T.; Pontikakos, C.M. Records of Aedes albopictus and Aedes cretinus (Diptera: Culicidae) in Greece from 2009 to 2011. Hell. Plant Prot. J. 2012, 5, 49–56. [Google Scholar]
- Badieritakis, E.; Papachristos, D.; Latinopoulos, D.; Stefopoulou, A.; Kolimenakis, A.; Bithas, K.; Patsoula, E.; Beleri, S.; Maselou, D.; Balatsos, G.; et al. Aedes albopictus (Skuse, 1895) (Diptera: Culicidae) in Greece: 13 years of living with the Asian tiger mosquito. Parasitol. Res. 2017, 117, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stefopoulou, A.; Balatsos, G.; Petraki, A.; Ladeau, S.L.; Papachristos, D.; Michaelakis, A. Reducing Aedes albopictus breeding sites through education: A study in urban area. PLoS ONE 2018, 13, e0202451. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; Della Torre, A.; Montarsi, F.; Rizzoli, A. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Invasive Mosquitoes in Europe; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2012.
- Canali, M.; Rivas-Morales, S.; Beutels, P.; Venturelli, C. The cost of arbovirus disease prevention in Europe: Area-wide integrated control of tiger mosquito, Aedes albopictus, in Emilia-Romagna, Northern Italy. Int. J. Environ. Res. Public Health 2017, 14, 444. [Google Scholar] [CrossRef]
- Tsiodras, S.; Pervanidou, D.; Papadopoulou, E.; Kavatha, D.; Baka, A.; Koliopoulos, G.; Badieritakis, E.; Michaelakis, A.; Gavana, E.; Patsoula, E.; et al. Imported Chikungunya fever case in Greece in June 2014 and public health response. Pathog. Glob. Health 2016, 110, 68–73. [Google Scholar] [CrossRef]
- Kolimenakis, A.; Latinopoulos, D.; Bithas, K.; Richardson, C.; Lagouvardos, K.; Stefopoulou, A.; Papachristos, D.; Michaelakis, A. Exploring public preferences, priorities, and policy perspectives for controlling invasive mosquito species in Greece. Trop. Med. Infect. Dis. 2019, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.; Bonilauri, P.; Puggioli, A.; Lelli, D.; Gaibani, P.; Landini, M.P.; Carrieri, M.; Michaelakis, A.; Papachristos, D.; Giatropoulos, A.; et al. Chikungunya and dengue risk assessment in Greece. Vector Biol. J. 2016, 1, 1–4. [Google Scholar] [CrossRef]
- Bellini, R.; Michaelakis, A.; Schaffner, F.; Alten, B.; Angelini, P.; Aranda, C.; Becker, N.; Carrieri, M.; Di Luca, M.; Falcuta, E.; et al. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Marois, E. Genetic control of vectors. In Pests and Vector-Borne Diseases in the Livestock Industry; Garros, C., Bouyer, J., Takken, W., Smallegange, R.C., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; Volume 5, pp. 435–451. ISBN 978-90-8686-315-0. [Google Scholar]
- Vreysen, M.J.; Robinson, A.S.; Hendrichs, J. Area-Wide Control of Insect Pests: From Research to Field Implementation; Springer Science & Business Media: Berlin, Germany, 2007; ISBN 9781402060588. [Google Scholar]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer Science & Business Media: Berlin, Germany, 2006; ISBN 9781402040504. [Google Scholar]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-insect methods for control of mosquito-borne diseases: An analysis. Vector Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.; Calvitti, M.; Medici, A.; Carrieri, M.; Celli, G.; Maini, S. Use of the sterile insect technique against Aedes albopictus in Italy: First results of a pilot trial. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Springer: Dordrecht, The Netherlands, 2007; pp. 505–515. [Google Scholar]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J.B. Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef]
- Lees, R.; Carvalho, S.D.O.; Bouyer, J. Potential impact of integrating the sterile insect technique into the fight against disease-transmitting mosquitoes. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, A.V., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Vienna, Austria, 2020; pp. 1081–1118. [Google Scholar]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Chansang, C.; Chansang, U.; Mongkalangoon, P.; Kittayapong, P.; Ninphanomchai, S.; Limohpasmanee, W. Combined sterile insect technique and incompatible insect technique: The first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Negl. Trop. Dis. 2019, 13, e0007771. [Google Scholar] [CrossRef]
- Balestrino, F.; Medici, A.; Candini, G.; Carrieri, M.; Maccagnani, B.; Calvitti, M. γ Ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J. Med. Entomol. 2010, 47, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, F.; Puggioli, A.; Gilles, J.; Bellini, R. Validation of a new larval rearing unit for Aedes albopictus (Diptera: Culicidae) mass rearing. PLoS ONE 2014, 9, e91914. [Google Scholar] [CrossRef]
- Medici, A.; Carrieri, M.; Scholte, E.-J.; Maccagnani, B.; Dindo, M.L.; Bellini, R. Studies on Aedes albopictus larval mass-rearing optimization. J. Econ. Entomol. 2011, 104, 266–273. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.-L. Model selection for extended quasi-likelihood models in small samples. Biometrics 1995, 51, 1077–1084. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Fang, Y. Asymptotic equivalence between cross-validations and Akaike information criteria in mixed-effects models. J. Data Sci. 2011, 9, 15–21. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Van Bortel, W. A review of the invasive mosquitoes in europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Tagaris, E.; Sotiropoulou, R.E.P.; Sotiropoulos, A.; Spanos, I. Climate change impact on the establishment of the invasive mosquito species (IMS). In Perspectives on Atmospheric Sciences; Springer: Cham, Switzerland, 2017; pp. 689–694. ISBN 9783319350950. [Google Scholar]
- Sotiropoulou, R.E.P.; Tagaris, E.; Sotiropoulos, A.; Spanos, I.; Milonas, P.; Michaelakis, A. Specific case: Regional estimates of global climate change: A dynamical downscaling approach to southeast Europe. In Energy, Transportation and Global Warming; Springer: Cham, Switzerland, 2016; pp. 99–108. [Google Scholar]
- World Health Organization. The International Atomic Energy Agency. Guidance Framework for Testing the Sterile Insect Technique as a Vector Control Tool against Aedes-Borne Diseases; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789240002371. [Google Scholar]
- World Health Organization. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Bellini, R.; Medici, A.; Puggioli, A.; Balestrino, F. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J. Med. Entomol. 2013, 50, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; L’Ambert, G.; Lacour, G.; Benoît, R.; Demarchi, M.; Cros, M.; Cailly, P.; Aubry-Kientz, M.; Balenghien, T.; Ezanno, P. A rainfall- and temperature-driven abundance model for Aedes albopictus populations. Int. J. Environ. Res. Public Health 2013, 10, 1698–1719. [Google Scholar] [CrossRef] [PubMed]
- Roiz, D.; Rosà, R.; Arnoldi, D.; Rizzoli, A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010, 10, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Albieri, A.; Carrieri, M.; Angelini, P.; Baldacchini, F.; Venturelli, C.; Zeo, S.M.; Bellini, R. Quantitative monitoring of Aedes albopictus in Emilia-Romagna, Northern Italy: Cluster investigation and geostatistical analysis. Bull. Insectol. 2010, 63, 209–216. [Google Scholar]
- Carrieri, M.; Albieri, A.; Angelini, P.; Baldacchini, F.; Venturelli, C.; Zeo, S.M.; Bellini, R. Surveillance of the chikungunya vector Aedes albopictus (Skuse) in Emilia-Romagna (northern Italy): Organizational and technical aspects of a large scale monitoring system. J. Vector Ecol. 2011, 36, 108–116. [Google Scholar] [CrossRef]
- Vavassori, L.; Saddler, A.; Müller, P. Active dispersal of Aedes albopictus: A mark-release-recapture study using self-marking units. Parasit. Vect. 2019, 12, 1–14. [Google Scholar] [CrossRef]
| Date | No of Males Delivered | Mortality during Transport (%). | Estimated Number of Sterile Males Released/Ha/Week |
|---|---|---|---|
| 14 September 2018 | 15,000 | 12.7 | 2619 |
| 28 September 2018 | 18,000 | 16.8 | 2995 |
| 5 October 2018 | 15,000 | 15.1 | 2547 |
| 12 October 2018 | 19,000 | 24.9 | 2853 |
| 19 October 2018 | 12,000 | 5.0 | 2280 |
| 26 October 2018 | 15,000 | 10.4 | 2688 |
| 6 November 2018 | 17,000 | 24.7 | 2560 |
| Plots | Results Related To Eggs | No of Week (Period) | ||
|---|---|---|---|---|
| 34 (24–31 August 2018) | 35 (31 August–7 September 2018) | 36 (7–14 September 2018) | ||
| SIT | Hatching rate (%) | 92.5 | 96.5 | 89.9 |
| Total number eggs/plot/week | 1755 | 1388 | 951 | |
| Average No. of eggs/trap (±SE) | 58.5 (±22.1) | 46.3 (±20.8) | 31.7 (±10.9) | |
| Control A | Hatching rate (%) | 92.1 | 94.4 | 80.0 |
| Total number eggs/plot/week | 784 | 649 | 310 | |
| Average No. of eggs/trap (±SE) | 52.3 (±12.8) | 43.3 (±10.1) | 20.7 (±6.5) | |
| Control B | Hatching rate (%) | 95.5 | 93.4 | 85.9 |
| Total number eggs/plot/week | 1521 | 1131 | 817 | |
| Average No. of eggs/trap (±SE) | 101.4 (±16.6) | 75.4 (±22.2) | 54.5 (±15.4) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balatsos, G.; Puggioli, A.; Karras, V.; Lytra, I.; Mastronikolos, G.; Carrieri, M.; Papachristos, D.P.; Malfacini, M.; Stefopoulou, A.; Ioannou, C.S.; et al. Reduction in Egg Fertility of Aedes albopictus Mosquitoes in Greece Following Releases of Imported Sterile Males. Insects 2021, 12, 110. https://doi.org/10.3390/insects12020110
Balatsos G, Puggioli A, Karras V, Lytra I, Mastronikolos G, Carrieri M, Papachristos DP, Malfacini M, Stefopoulou A, Ioannou CS, et al. Reduction in Egg Fertility of Aedes albopictus Mosquitoes in Greece Following Releases of Imported Sterile Males. Insects. 2021; 12(2):110. https://doi.org/10.3390/insects12020110
Chicago/Turabian StyleBalatsos, Georgios, Arianna Puggioli, Vasileios Karras, Ioanna Lytra, George Mastronikolos, Marco Carrieri, Dimitrios P. Papachristos, Marco Malfacini, Angeliki Stefopoulou, Charalampos S. Ioannou, and et al. 2021. "Reduction in Egg Fertility of Aedes albopictus Mosquitoes in Greece Following Releases of Imported Sterile Males" Insects 12, no. 2: 110. https://doi.org/10.3390/insects12020110
APA StyleBalatsos, G., Puggioli, A., Karras, V., Lytra, I., Mastronikolos, G., Carrieri, M., Papachristos, D. P., Malfacini, M., Stefopoulou, A., Ioannou, C. S., Balestrino, F., Bouyer, J., Petrić, D., Pajović, I., Kapranas, A., Papadopoulos, N. T., Milonas, P. G., Bellini, R., & Michaelakis, A. (2021). Reduction in Egg Fertility of Aedes albopictus Mosquitoes in Greece Following Releases of Imported Sterile Males. Insects, 12(2), 110. https://doi.org/10.3390/insects12020110








