Comparing Light—Emitting—Diodes Light Traps for Catching Anopheles Mosquitoes in a Forest Setting, Western Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Light Traps
2.3. Mosquito Collection
2.4. Morphological Species Identification
2.5. DNA Extraction
2.6. Molecular Species Identification
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer Science & Business Media: Waldsee, Germany, 2010. [Google Scholar]
- Killeen, G.F.; Marshall, J.M.; Kiware, S.S.; South, A.B.; Tusting, L.S.; Chaki, P.P.; Govella, N.J. Measuring, manipulating and exploiting behaviours of adult mosquitoes to optimise malaria vector control impact. BMJ Glob. Health 2017, 2, e000212. [Google Scholar] [CrossRef]
- Morgan, K.; Somboon, P.; Walton, C. Understanding Anopheles diversity in Southeast Asia and its applications for malaria control. Anopheles Mosq. New Insights Malar. Vectors 2013, 327, 355. [Google Scholar]
- Cano, J.; Berzosa, P.; Roche, J.; Rubio, J.; Moyano, E.; Guerra-Neira, A.; Brochero, H.; Mico, M.; Edu, M.; Benito, A. Malaria vectors in the Bioko Island (Equatorial Guinea): Estimation of vector dynamics and transmission intensities. J. Med. Entomol. 2004, 41, 158–161. [Google Scholar] [CrossRef]
- White, M.T.; Griffin, J.T.; Churcher, T.S.; Ferguson, N.M.; Basáñez, M.-G.; Ghani, A.C. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasites Vectors 2011, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Duo-Quan, W.; Lin-Hua, T.; Zhen-Cheng, G.; Xiang, Z.; Man-Ni, Y.; Wei-Kang, J. Comparative evaluation of light-trap catches, electric motor mosquito catches and human biting catches of Anopheles in the Three Gorges Reservoir. PLoS ONE 2012, 7, e28988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dusfour, I.; Carinci, R.; Gaborit, P.; Issaly, J.; Girod, R. Evaluation of four methods for collecting malaria vectors in French Guiana. J. Econ. Entomol. 2010, 103, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Tisgratog, R.; Tananchai, C.; Juntarajumnong, W.; Tuntakom, S.; Bangs, M.J.; Corbel, V.; Chareonviriyaphap, T. Host feeding patterns and preference of Anopheles minimus (Diptera: Culicidae) in a malaria endemic area of western Thailand: Baseline site description. Parasites Vectors. 2012, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Hoel, D.F.; Marika, J.A.; Dunford, J.C.; Irish, S.R.; Geier, M.; Obermayr, U.; Wirtz, R.A. Optimizing collection of Anopheles gambiae ss (Diptera: Culicidae) in biogents sentinel traps. J. Med Entomol. 2014, 51, 1268–1275. [Google Scholar] [CrossRef]
- Ponlawat, A.; Khongtak, P.; Jaichapor, B.; Pongsiri, A.; Evans, B.P. Field evaluation of two commercial mosquito traps baited with different attractants and colored lights for malaria vector surveillance in Thailand. Parasites Vectors 2017, 10, 378. [Google Scholar] [CrossRef]
- Artsob, H.; Spence, L.; Surgeoner, G.; Th’ng, C.; Lampotang, V.; Grant, L.; McCreadie, J. Studies on a focus of California group virus activity in southern Ontario. Mosq. News 1983, 43, 449–455. [Google Scholar]
- Pezzin, A.; Veronesi, R.; Carrieri, M.; Maccagnani, B.; Bellini, R. Comparative study on the effectiveness of different mosquito traps in arbovirus surveillance with a focus on WNV detection. Acta Trop. 2016, 153, 93–100. [Google Scholar] [CrossRef]
- Silver, J.B. Mosquito Ecology: Field Sampling Methods; Springer Science & Business Media: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Silver, J.B. Sampling adults with light-traps. In Mosquito Ecology: Field Sampling Methods; Springer: Berlin/Heidelberg, Germany, 2008; pp. 845–946. [Google Scholar]
- Saeung, M.; Jhaiaun, P.; Bangs, M.J.; Ngoen-Klan, R.; Chareonviriyaphap, T. Transmitted light as attractant with mechanical traps for collecting nocturnal mosquitoes in urban Bangkok, Thailand. J. Am. Mosq. Control Assoc. 2021, 37, 132–142. [Google Scholar] [CrossRef]
- Hoel, D.F.; Butler, J.F.; Fawaz, E.; Watany, N.; El-Hossary, S.; Villinski, J. Response of phlebotomine sand flies to light-emitting diode-modified light traps in southern Egypt. J. Vector Ecol. 2007, 32, 302–309. [Google Scholar] [CrossRef]
- González, M.; Alarcón-Elbal, P.M.; Valle-Mora, J.; Goldarazena, A. Comparison of different light sources for trapping Culicoides biting midges, mosquitoes and other dipterans. Vet. Parasitol. 2016, 226, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Viswanath, N.S.; Unithrattil, S.; Kim, H.J.; Im, W.B. Phosphor plates for high-power LED applications: Challenges and opportunities toward perfect lighting. ECS J. Solid. State. Sci. Technol. 2017, 7, R3134. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, Y.; Wu, W.; Fu, Y. Field evaluation of different wavelengths light-emitting diodes as attractants for adult Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae). Neotrop. Entomol. 2014, 43, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.M.; Bennett, G.F. Response of mosquitoes (Diptera: Culicidae) to visual stimuli. J. Med. Entomol. 1981, 18, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Cohnstaedt, L.; Gillen, J.I.; Munstermann, L.E. Light-emitting diode technology improves insect trapping. J. Am. Mosq. Control Assoc. 2008, 24, 331. [Google Scholar] [CrossRef]
- Costa-Neta, B.M.; Lima-Neto, A.R.; da Silva, A.A.; Brito, J.M.; Aguiar, J.V.C.; Ponte, I.S.; Silva, F.S. Centers for Disease Control-type light traps equipped with high-intensity light-emitting diodes as light sources for monitoring Anopheles mosquitoes. Acta Trop. 2018, 183, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.B.; Johnson, B.J.; Fall, K.; Buhagiar, T.S.; Townsend, M.; Ritchie, S.A. Development, optimization, and field evaluation of the novel collapsible passive trap for collection of mosquitoes. J. Med. Entomol. 2018, 55, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Oriyomi, M.; Babalola, P. Comparison of mosquitoes response to different diodes wavelengths. Int. J. Sci. Res. 2020, 9, 218–223. [Google Scholar]
- Silva, F.S.; Costa-Neta, B.M.; de Almeida, M.d.S.; de Araújo, E.C.; Aguiar, J.V.C. Field performance of a low cost, simple-to-build, non-motorized light-emitting diode (LED) trap for capturing adult Anopheles mosquitoes (Diptera: Culicidae). Acta Trop. 2019, 190, 9–12. [Google Scholar] [CrossRef]
- Sriwichai, P.; Karl, S.; Samung, Y.; Sumruayphol, S.; Kiattibutr, K.; Payakkapol, A.; Mueller, I.; Yan, G.; Cui, L.; Sattabongkot, J. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai-Myanmar border. Parasites Vectors 2015, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Tainchum, K.; Kongmee, M.; Manguin, S.; Bangs, M.J.; Chareonviriyaphap, T. Anopheles species diversity and distribution of the malaria vectors of Thailand. Trends Parasitol. 2015, 31, 109–119. [Google Scholar] [CrossRef]
- Wilton, D.; Fay, R. Air flow direction and velocity in light trap design. Entomol. Exp. Appl. 1972, 15, 377–386. [Google Scholar] [CrossRef]
- Burkett, D.A.; Butler, J.F.; Kline, D.L. Field evaluation of colored light-emitting diodes as attractants for woodland mosquitoes and other Diptera in north central Florida. J. Am. Mosq. Control Assoc.-Mosq. News 1998, 14, 186–195. [Google Scholar]
- Rattanarithikul, R.; Harrison, B.A.; Panthusiri, P.; Peyton, E.L.; Coleman, R.E. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J. Trop. Med. Public Health 2006, 37, 1–85. [Google Scholar] [PubMed]
- Panthusiri, P. Illustrated keys to the mosquitoes of Thailand VI. Tribe Aedini. Southeast Asian J. Trop. Med. Public Health. 2010, 41, 1–225. [Google Scholar]
- Panthusiri, P. Illustrated keys to the mosquitoes of Thailand IV. Anopheles. Southeast Asian J. Trop. Med. Public Health. 2006, 37, 1–128. [Google Scholar]
- Rattanarithikul, R.; Harbach, R.E.; Harrison, B.A.; Panthusiri, P.; Coleman, R.E. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J. Trop. Med. Public Health. 2007, 38, 1–65. [Google Scholar]
- Rattanarithikul, R.; Harrison, B.A.; Panthusiri, P.; Coleman, R.E. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. Southeast Asian J. Trop. Med. Public Health. 2005, 36, 1. [Google Scholar] [PubMed]
- Rattanarithikul, R.; Panthusiri, P. Illustrated keys to the medically important mosquitos of Thailand. Southeast Asian J. Trop. Med. Public Health. 1994, 25, 1–66. [Google Scholar]
- Linton, Y.M.; Harbach, R.E.; Seng, C.M.; Anthony, T.G.; Matusop, A. Morphological and molecular identity of Anopheles (Cellia) sundaicus (Diptera: Culicidae), the nominotypical member of a malaria vector species complex in Southeast Asia. Syst. Entomol. 2001, 26, 357–366. [Google Scholar] [CrossRef]
- Garros, C.; Koekemoer, L.L.; Coetzee, M.; Coosemans, M.; Manguin, S. A single multiplex assay to identify major malaria vectors within the African Anopheles funestus and the Oriental An. minimus groups. Am. J. Trop. Med. Hyg. 2004, 70, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; Somboon, P.; O’Loughlin, S.; Zhang, S.; Harbach, R.; Linton, Y.-M.; Chen, B.; Nolan, K.; Duong, S.; Fong, M.-Y. Genetic diversity and molecular identification of mosquito species in the Anopheles maculatus group using the ITS2 region of rDNA. Infect. Genet. Evol. 2007, 7, 93–102. [Google Scholar] [CrossRef]
- Walton, C.; Handley, J.; Kuvangkadilok, C.; Collins, F.; Harbach, R.; Baimai, V.; Butlin, R. Identification of five species of the Anopheles dirus complex from Thailand, using allele-specific polymerase chain reaction. Med. Vet. Entomol. 1999, 13, 24–32. [Google Scholar] [CrossRef]
- Lee, H.I.; Seo, B.Y.; Shin, E.-H.; Burkett, D.A.; Lee, J.-K.; Shin, Y.H. Efficiency evaluation of Nozawa-style black light trap for control of anopheline mosquitoes. Korean J. Parasitol. 2009, 47, 159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Su, X.; Zhou, G.; Zhang, H.; Puthiyakunnon, S.; Shuai, S.; Cai, S.; Gu, J.; Zhou, X.; Yan, G. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasites Vectors 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Breyev, K. The effect of various light sources on the numbers and species of blood-sucking mosquitoes (Diptera: Culicidae) collected in light traps. Entomol. Rev. 1963, 42, 155–168. [Google Scholar]
- DeLong, D. Fundamental studies on behaviour of larval and adult mosquitoes and evaluation of mosquito repellents. Ohio Engin. Exp. State News 1954, 26, 51–55. [Google Scholar]
- Bentley, M.T.; Kaufman, P.E.; Kline, D.L.; Hogsette, J.A. Response of adult mosquitoes to light-emitting diodes placed in resting boxes and in the field. J. Am. Mosq. Control Assoc. 2009, 25, 285–291. [Google Scholar] [CrossRef][Green Version]
- Silva, J.d.S.; Souto Couri, M.; de Leão Giupponi, A.P.; Alencar, J. Mosquito fauna of the Guapiaçu Ecological Reserve, Cachoeiras de Macacu, Rio de Janeiro, Brazil, collected under the influence of different color CDC light traps. J. Vector Ecol. 2014, 39, 384–394. [Google Scholar] [CrossRef][Green Version]
- Roeder, K.D. Insect Physiology; Wiley: New York, NY, USA, 1953; Volume 76. [Google Scholar]
- Tchouassi, D.P.; Sang, R.; Sole, C.L.; Bastos, A.D.; Cohnstaedt, L.W.; Torto, B. Trapping of Rift Valley Fever (RVF) vectors using Light Emitting Diode (LED) CDC traps in two arboviral disease hot spots in Kenya. Parasites Vectors 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Bernáth, B.; Anstett, V.; Guerin, P.M. Anopheles gambiae females readily learn to associate complex visual cues with the quality of sugar sources. J. Insect Physiol. 2016, 95, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Takemura, S.-Y.; Arikawa, K.; Takagi, M. Comparative study on nocturnal behavior of Aedes aegypti and Aedes albopictus. J. Med. Entomol. 2007, 42, 312–318. [Google Scholar] [CrossRef]
- Muir, L.E.; Thorne, M.J.; Kay, B.H. Aedes aegypti (Diptera: Culicidae) vision: Spectral sensitivity and other perceptual parameters of the female eye. J. Med. Entomol. 1992, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D. Biting behavior of Malaysian mosquitoes, Aedes albopictus Skuse, Armigeres kesseli Ramalingam, Culex quinquefasciatus Say, and Culex vishnui Theobald obtained from urban residential areas in Kuala Lumpur. Asian Biomed 2014, 8, 315–321. [Google Scholar] [CrossRef]
- Tananchai, C.; Tisgratog, R.; Juntarajumnong, W.; Grieco, J.P.; Manguin, S.; Prabaripai, A.; Chareonviriyaphap, T. Species diversity and biting activity of Anopheles dirus and Anopheles baimaii (Diptera: Culicidae) in a malaria prone area of western Thailand. Parasites Vectors 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Harbach, R.E.; Gingrich, J.B.; Pang, L.W. Some entomological observations on malaria transmission in a remote village in northwestern Thailand. J. Am. Mosq. Control Assoc. 1987, 3, 296–301. [Google Scholar] [PubMed]
- Rattanarithikul, R.; Konishi, E.; Linthicum, K.J. Detection of Plasmodium vivax and Plasmodium falciparum circumsporozoite antigen in anopheline mosquitoes collected in southern Thailand. Am. J. Trop. Med. 1996, 54, 114–121. [Google Scholar] [CrossRef]
- Tainchum, K.; Ritthison, W.; Chuaycharoensuk, T.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T. Diversity of Anopheles species and trophic behavior of putative malaria vectors in two malaria endemic areas of northwestern Thailand. J. Vector Ecol. 2014, 39, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.M.; Carrara, V.I.; Pukrittayakamee, S.; McGready, R.; Nosten, F.H. Malaria ecology along the Thailand–Myanmar border. Malar. J. 2015, 14, 1–12. [Google Scholar] [CrossRef]
- Walsh, A.S.; Glass, G.E.; Lesser, C.R.; Curriero, F.C. Predicting seasonal abundance of mosquitoes based on off-season meteorological conditions. Environ. Ecol. Stat. 2008, 15, 279–291. [Google Scholar] [CrossRef]


| Species | Primer Name | Sequence (5′ to 3′) |
|---|---|---|
| Universal forward primer | ITS2A | TGT GAA CTG CAG GAC ACA |
| Anopheles aconitus | ACO | ACA GCG TGT ACG TCC AGT |
| Anopheles harrisoni | MIC | GTT CAT TCA GCA ACA TCA GT |
| Anopheles varuna | VAR | TTG ACC ACT TTC GAC GCA |
| Anopheles minimus | MIA | CCC GTG CGA CTT GAC GA |
| Universal forward primer | 5.8F | ATC ACT CGG CTC GTG GAT CG |
| Anopheles maculatus | MAC | GAC GGT CAG TCT GGT AAA GT |
| Anopheles pseudowillmoei | PSEU | GCC CCC GGG TGT CAA ACA G |
| Anopheles sawadwongporni | SAW | ACGGTC CCG CAT CAG GTG C |
| Anopheles dravidicus | PDRAV | GCC TAC TTT GAG CGA GAC CA |
| Form K | K | TTC ATC GCT CGC CCT TAC AA |
| Universal forward primer | ITS2A | TGT GAA CTG CAG GAC ACA T |
| Anopheles dirus | D-U | GCG CGG GGC CGA GGT GG |
| Anopheles scanloni | D-AC | CAC AGC GAC TCC ACA CG |
| Anopheles cracens | D-B | CGG GAT ATG GGT CGG CC |
| Anopheles baimaii | D-D | GCG CGG GAC CGT CCG TT |
| Anopheles nemophilous | D-F | AAC GGC GGT CCC CTT TG |
| Mosquitoes | Light Sources | Total No. (%) | |||||
|---|---|---|---|---|---|---|---|
| UV LED | Green LED | Blue LED | Red LED | White Fluorescent | UV Fluorescent | ||
| Aedes genus | 23 | 31 | 40 | 11 | 19 | 43 | 167 (19.13) |
| Ae. aegypti | 1 | 0 | 1 | 0 | 0 | 0 | 2 (0.23) |
| Ae. albopictus | 15 | 13 | 28 | 0 | 8 | 27 | 91 (10.42) |
| Ae. albotaeniata | 0 | 0 | 0 | 1 | 0 | 0 | 1 (0.11) |
| Ae. albolateralis | 1 | 0 | 1 | 0 | 1 | 0 | 3 (0.34) |
| Ae. chysolineata | 0 | 2 | 0 | 0 | 1 | 0 | 3 (0.34) |
| Ae. flaripennis | 1 | 3 | 1 | 2 | 3 | 5 | 15 (1.72) |
| Ae. poicilia | 3 | 0 | 0 | 0 | 2 | 0 | 5 (0.57) |
| Ae. vexans | 0 | 0 | 0 | 0 | 0 | 1 | 1 (0.11) |
| Ae. saxicola | 0 | 0 | 1 | 0 | 0 | 1 | 2 (.023) |
| Ae. prominens | 0 | 0 | 0 | 0 | 1 | 1 | 2 (0.23) |
| Ae. khazani | 0 | 0 | 0 | 0 | 1 | 0 | 1 (0.11) |
| Ae. trilineata | 1 | 10 | 6 | 7 | 1 | 2 | 27 (3.09) |
| Ae. mikrokopion | 0 | 0 | 1 | 1 | 0 | 0 | 2 (0.23) |
| Ae. lineatopennis | 0 | 0 | 0 | 0 | 0 | 1 | 1 (0.11) |
| Ae. pipersalatus | 0 | 0 | 0 | 0 | 0 | 1 | 1 (0.11) |
| Aedes spp. * | 1 | 3 | 1 | 0 | 1 | 4 | 10 (1.15) |
| Anopheles genus | 115 | 16 | 54 | 11 | 34 | 284 | 514 (58.88) |
| An. sawadwongporni | 0 | 0 | 1 | 1 | 0 | 6 | 8 (0.92) |
| An. minimus | 1 | 0 | 0 | 1 | 0 | 3 | 5 (0.57) |
| An. harrisoni | 105 | 15 | 51 | 8 | 33 | 266 | 478 (54.75) |
| An. aconitus | 0 | 0 | 1 | 0 | 0 | 0 | 1 (0.11) |
| An. varuna | 3 | 0 | 0 | 0 | 0 | 2 | 5 (0.57) |
| An. dirus | 2 | 0 | 0 | 1 | 0 | 1 | 4 (0.46) |
| An. barbirostris | 1 | 0 | 0 | 0 | 0 | 3 | 4 (0.46) |
| Anopheles spp. * | 3 | 1 | 1 | 0 | 1 | 3 | 9 (1.03) |
| Armigeres genus | 10 | 4 | 12 | 6 | 3 | 9 | 44 (5.04) |
| Ar. (Lei.) longipalpis | 5 | 2 | 7 | 5 | 3 | 9 | 31 (3.55) |
| Ar. subalbatus | 0 | 1 | 2 | 0 | 0 | 0 | 3 (0.34) |
| Ar. achaetae | 1 | 0 | 0 | 0 | 0 | 0 | 1 (0.11) |
| Ar. theobaldi | 0 | 0 | 0 | 1 | 0 | 0 | 1 (0.11) |
| Ar. leicester | 2 | 0 | 0 | 0 | 0 | 0 | 2 (0.23) |
| Armigeres spp. * | 2 | 1 | 3 | 0 | 0 | 0 | 6 (0.69) |
| Culex genus | 29 | 22 | 27 | 7 | 17 | 46 | 148 (16.95) |
| Cx. brevipalpis | 17 | 13 | 11 | 3 | 6 | 23 | 73 (8.36) |
| Cx. tritaeniorhynchus | 7 | 3 | 2 | 3 | 0 | 7 | 22 (2.52) |
| Cx. malayi | 1 | 0 | 1 | 0 | 0 | 4 | 6 (0.69) |
| Cx. nitropunctatus | 3 | 1 | 11 | 1 | 8 | 11 | 35 (4.01) |
| Culex spp. * | 1 | 5 | 2 | 0 | 3 | 1 | 12 (1.37) |
| Total (%) | 177 (20.27) | 73 (8.36) | 133 (15.23) | 35 (4.01) | 73 (8.36) | 382 (43.76) | 873 (100) |
| Mosquitoes | Mean ± SD (Mean Rank) | |||||
|---|---|---|---|---|---|---|
| UV LED | Green LED | White Fluorescent | UV Fluorescent | Blue LED | Red LED | |
| Aedes genus | 0.64 ± 0.99 a | 0.86 ± 1.91 a | 0.52 ± 0.01 a | 1.20 ± 1.94 a | 1.11 ± 1.95 a | 0.31 ± 0.95 a |
| (110.89) | (108.58) | (103.39) | (122.92) | (119.03) | (86.19) | |
| Anopheles genus | 3.19 ± 9.09 a | 0.44 ± 1.11 b | 0.94 ± 4.16 b | 7.89 ± 17.09 a | 1.50 ± 4.74 ab | 0.31 ± 0.52 b |
| (127.82) * | (92.44) | (90.36) | (144.56) * | (103.13) | (92.69) | |
| Armigeres genus | 0.81 ± 1.39 a | 0.61 ± 1.02 a | 0.47 ± 0.91 a | 1.28 ± 2.11 a | 0.75 ± 0.99 a | 0.19 ± 0.47 a |
| (116.86) | (99.28) | (98.79) | (116.38) | (114.56) | (105.14) | |
| Culex genus | 0.28 ± 0.51 a | 0.11 ± 0.39 ac | 0.08 ± 0.28 ac | 0.25 ± 0.44 a | 0.33 ± 0.76 a | 0.17 ± 0.45 bc |
| (114.57) * | (106.17) | (99.01) | (127.14) * | (118.94) * | (85.17) | |
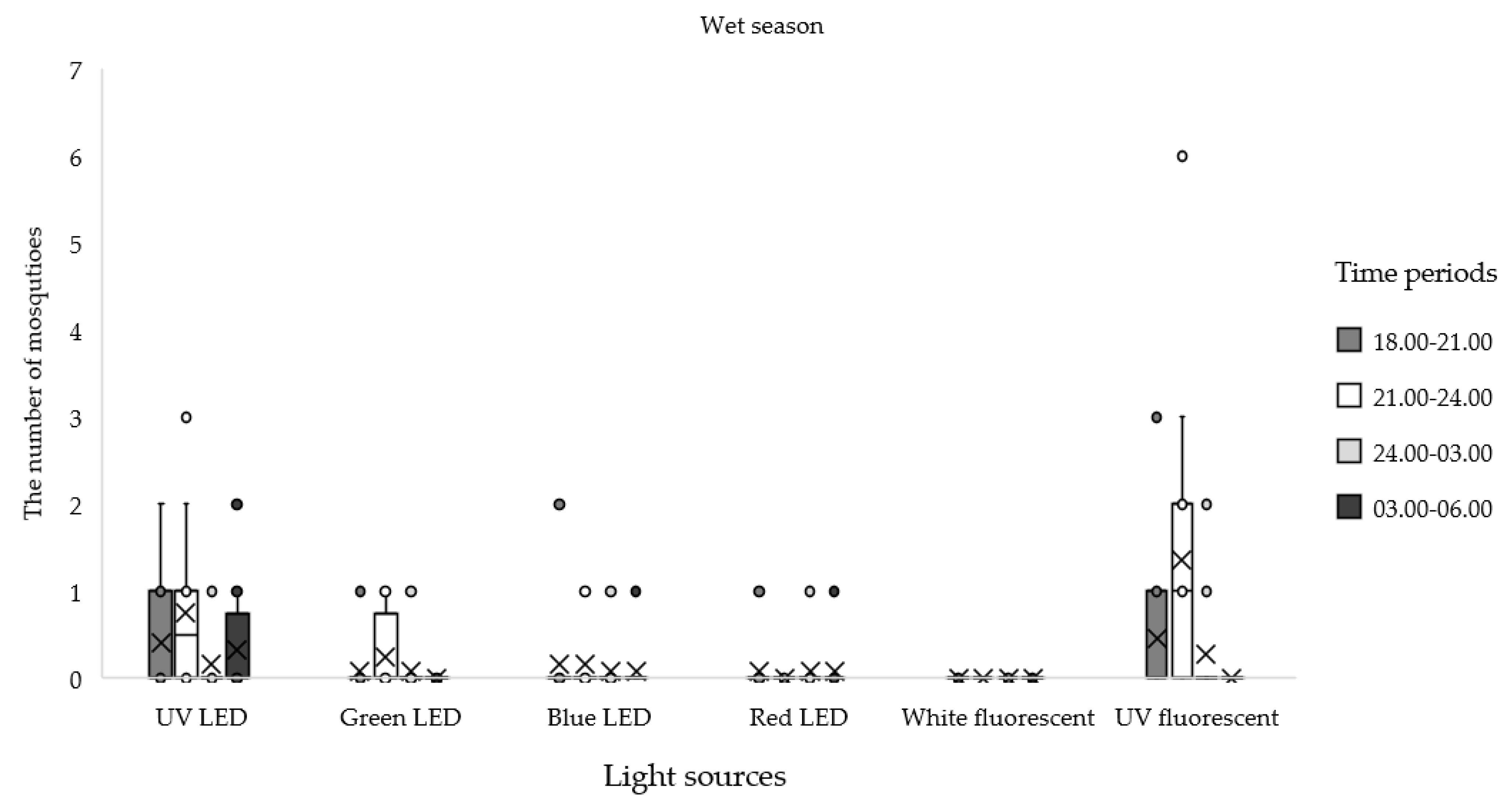
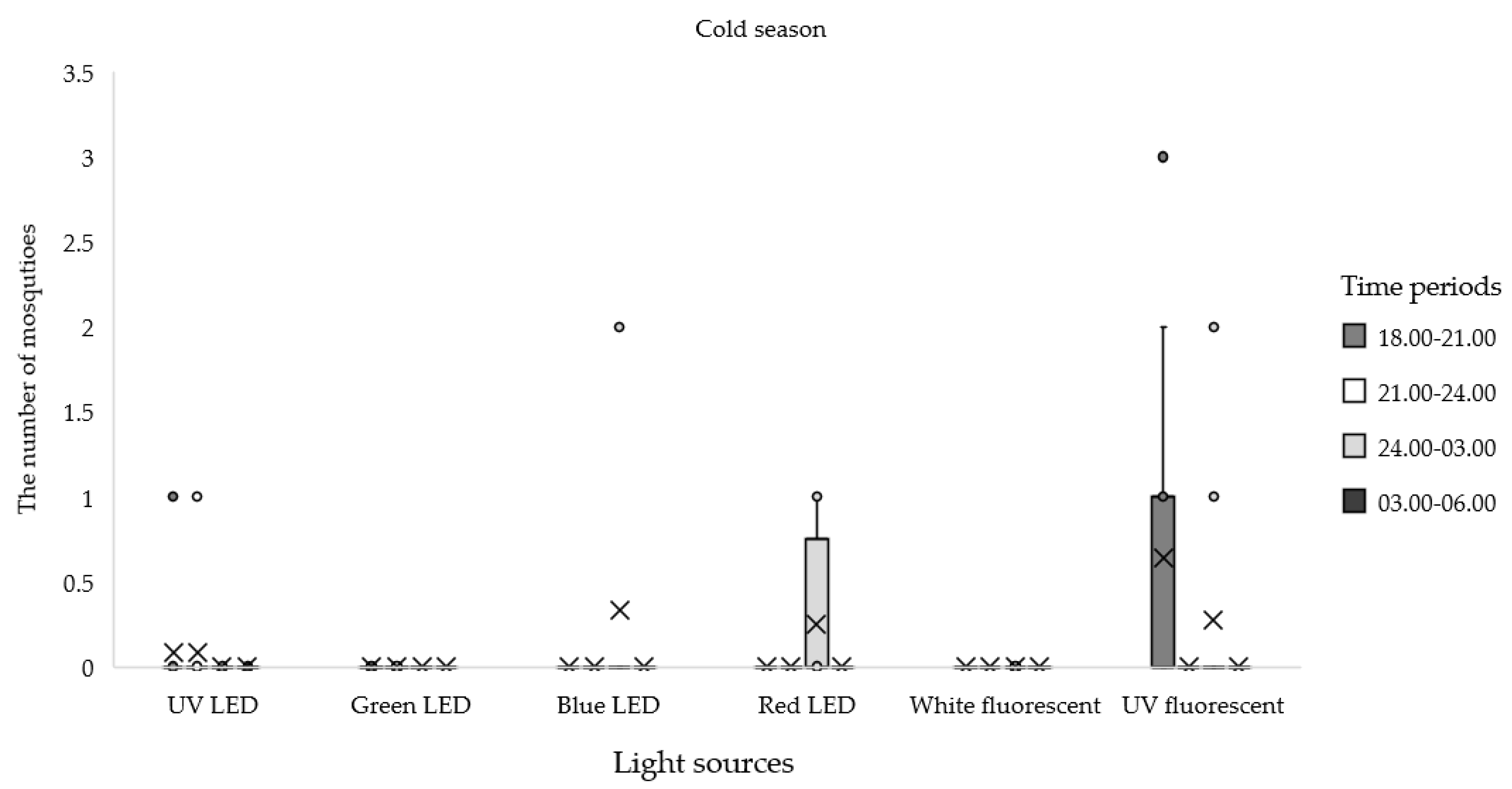
| Seasonal | Light Sources | Total No. (%) | Time Periods | |||
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| 18:00–21:00 h | 21:00–24:00 h | 24:00–03:00 h | 03:00–06:00 h | |||
| Dry (12 nights) | UV LED | 93 (18.09) | 3.67 ± 10.88 | 1.83 ± 3.51 | 2.00 ± 2.26 | 0.25 ± 0.62 |
| Green LED | 11 (2.14) | 0.17 ± 0.58 | 0.25 ± 0.45 | 0.33 ± 0.65 | 0.17 ± 0.58 | |
| Blue LED | 44 (8.56) | 0.17 ± 0.39 | 1.25 ± 2.22 | 2.00 ± 6.02 | 0.25 ± 0.62 | |
| Red LED | 5 (0.97) | 0.00 ± 0.00 | 0.17 ± 0.39 | 0.17 ± 0.39 | 0.08 ± 0.29 | |
| White Fluorescent | 33 (6.42) | 0.75 ± 1.42 | 0.92 ± 2.57 | 0.17 ± 0.39 | 0.92 ± 3.18 | |
| UV Fluorescent | 249 (48.44) | 2.75 ± 4.63 | 7.67 ± 12.21 | 6.67 ± 12.60 | 3.67 ± 4.27 | |
| Wet (12 nights) | UV LED | 20 (3.89) | 0.42 ± 0.67 | 0.75 ± 0.97 | 0.17 ± 0.39 | 0.33 ± 0.65 |
| Green LED | 5 (0.97) | 0.08 ± 0.29 | 0.25 ± 0.45 | 0.08 ± 0.29 | 0.00 ± 0.00 | |
| Blue LED | 6 (1.17) | 0.17 ± 0.58 | 0.17 ± 0.39 | 0.08 ± 0.29 | 0.08 ± 0.29 | |
| Red LED | 3 (0.58) | 0.08 ± 0.29 | 0.00 ± 0.00 | 0.08 ± 0.29 | 0.08 ± 0.29 | |
| White Fluorescent | 1 (0.19) | 0.08 ± 0.29 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| UV Fluorescent | 25 (4.86) | 0.58 ± 1.00 | 1.25 ± 1.82 | 0.25 ± 0.62 | 0.00 ± 0.00 | |
| Cold (12 nights) | UV LED | 2 (0.39) | 0.08 ± 0.29 | 0.08 ± 0.29 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Green LED | 0 (0) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Blue LED | 4 (0.78) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.33 ± 0.78 | 0.00 ± 0.00 | |
| Red LED | 3 (0.58) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.25 ± 0.45 | 0.00 ± 0.00 | |
| White Fluorescent | 0 (0) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| UV Fluorescent | 10 (1.95) | 0.58 ± 1.00 | 0.00 ± 0.00 | 0.25 ± 0.62 | 0.00 ± 0.00 | |
| Parameter | B | SE | 95% Wald Confidence Interval | Hypothesis Test | IRR | 95% Wald Confidence Interval for Exp(B) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Wald Chi-Square | df | Sig | Lower | Upper | ||||
| (Intercept) | −2.199 | 0.3026 | −2.792 | −1.606 | 52.812 | 1 | 0.000 | 0.111 | 0.061 | 0.201 |
| Red LED | −3.111 | 0.3507 | −3.798 | −2.423 | 78.660 | 1 | 0.000 | 0.045 | 0.022 | 0.089 |
| Green LED | −2.772 | 0.3062 | −3.372 | −2.171 | 81.914 | 1 | 0.000 | 0.063 | 0.034 | 0.114 |
| Blue LED | −1.601 | 0.2203 | −2.033 | −1.169 | 52.849 | 1 | 0.000 | 0.202 | 0.131 | 0.311 |
| UV LED | −0.829 | 0.1928 | −1.207 | −0.451 | 18.468 | 1 | 0.000 | 0.437 | 0.299 | 0.637 |
| White Fluorescent | −2.065 | 0.2436 | −2.543 | −1.588 | 71.902 | 1 | 0.000 | 0.127 | 0.079 | 0.204 |
| UV Fluorescent (Standard) | 0 2 | 1 | ||||||||
| Dry Season | 3.075 | 0.2629 | 2.560 | 3.590 | 136.833 | 1 | 0.000 | 21.649 | 12.932 | 36.240 |
| Wet Season | 1.162 | 0.2909 | 0.592 | 1.732 | 15.955 | 1 | 0.000 | 3.196 | 1.807 | 5.651 |
| Cold Season (standard) | 0 2 | 1 | ||||||||
| 18:00–21:00 h | 0.657 | 0.2284 | 0.209 | 1.104 | 8.269 | 1 | 0.004 | 1.928 | 1.233 | 3.017 |
| 21:00–24:00 h | 0.933 | 0.2188 | 0.504 | 1.362 | 18.196 | 1 | 0.000 | 2.543 | 1.656 | 3.904 |
| 24:00–03:00 h | 0.811 | 0.2228 | 0.374 | 1.247 | 13.244 | 1 | 0.000 | 2.249 | 1.454 | 3.481 |
| 03:00–06:00 h (Standard) | 0 2 | 1 | ||||||||
| (Scale) | 1 3 | |||||||||
| (Negative binomial) | 1 3 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhaiaun, P.; Panthawong, A.; Saeung, M.; Sumarnrote, A.; Kongmee, M.; Ngoen-Klan, R.; Chareonviriyaphap, T. Comparing Light—Emitting—Diodes Light Traps for Catching Anopheles Mosquitoes in a Forest Setting, Western Thailand. Insects 2021, 12, 1076. https://doi.org/10.3390/insects12121076
Jhaiaun P, Panthawong A, Saeung M, Sumarnrote A, Kongmee M, Ngoen-Klan R, Chareonviriyaphap T. Comparing Light—Emitting—Diodes Light Traps for Catching Anopheles Mosquitoes in a Forest Setting, Western Thailand. Insects. 2021; 12(12):1076. https://doi.org/10.3390/insects12121076
Chicago/Turabian StyleJhaiaun, Pairpailin, Amonrat Panthawong, Manop Saeung, Anchana Sumarnrote, Monthathip Kongmee, Ratchadawan Ngoen-Klan, and Theeraphap Chareonviriyaphap. 2021. "Comparing Light—Emitting—Diodes Light Traps for Catching Anopheles Mosquitoes in a Forest Setting, Western Thailand" Insects 12, no. 12: 1076. https://doi.org/10.3390/insects12121076
APA StyleJhaiaun, P., Panthawong, A., Saeung, M., Sumarnrote, A., Kongmee, M., Ngoen-Klan, R., & Chareonviriyaphap, T. (2021). Comparing Light—Emitting—Diodes Light Traps for Catching Anopheles Mosquitoes in a Forest Setting, Western Thailand. Insects, 12(12), 1076. https://doi.org/10.3390/insects12121076





