Functional Characterization of a Trehalose-6-Phosphate Synthase in Diaphorina citri Revealed by RNA Interference and Transcriptome Sequencing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sample Prepartion
2.2. Cloning of DcTPS1 and Bioinformatic Analysis
2.3. dsRNA Synthesis and DcTPS1 RNA Interference Analysis
2.4. qPCR Analysis of DcTPS1
2.5. cDNA Libaray Preparation and Illumina Sequencing
2.6. Transcriptome Analsysis after Silencing of DcTPS1
3. Results
3.1. Analysis of the cDNA and Protein Sequence of DcTPS1
3.2. Tissue Distribution and Developmental Stages Expression Patterns of DcTPS1
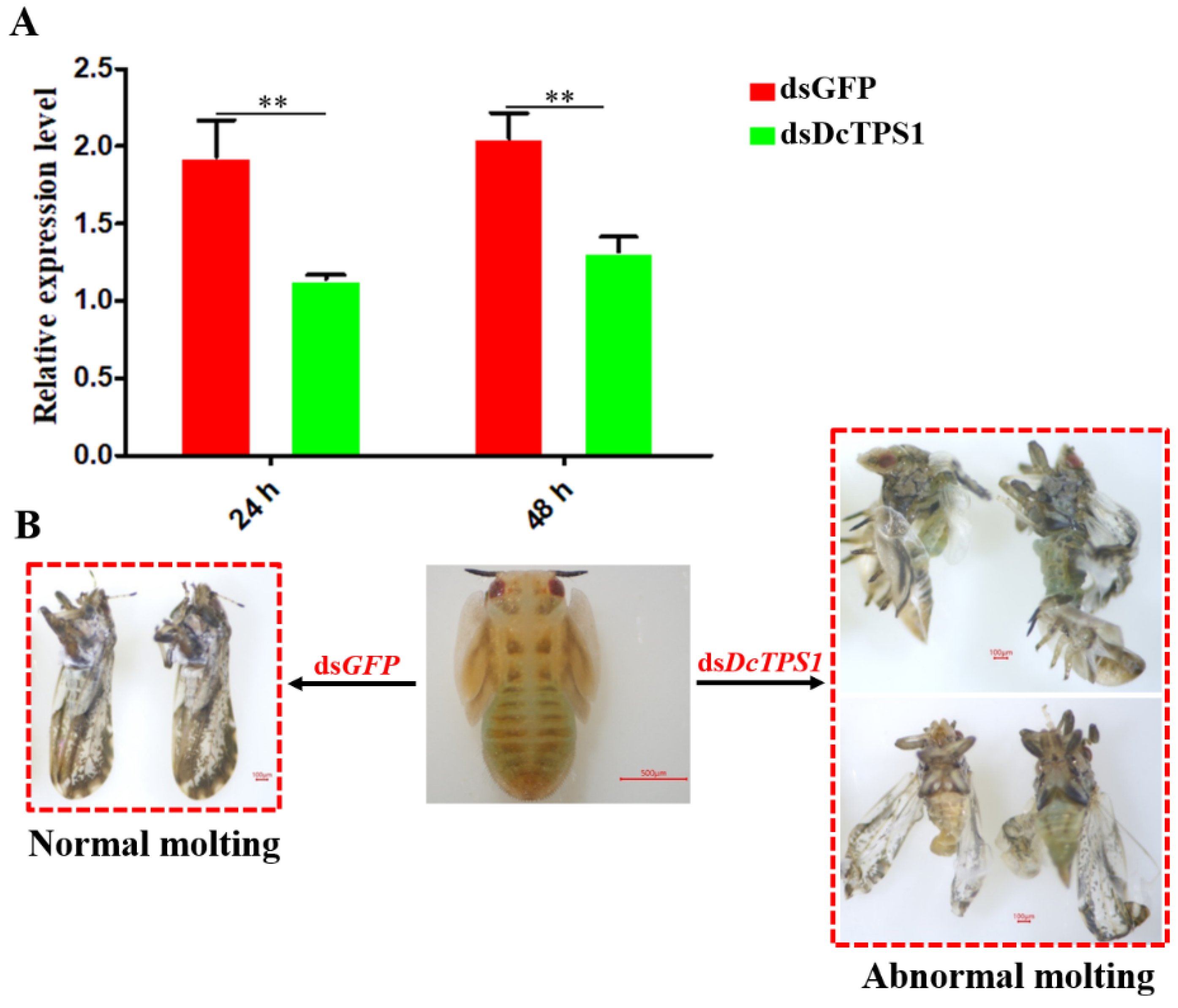
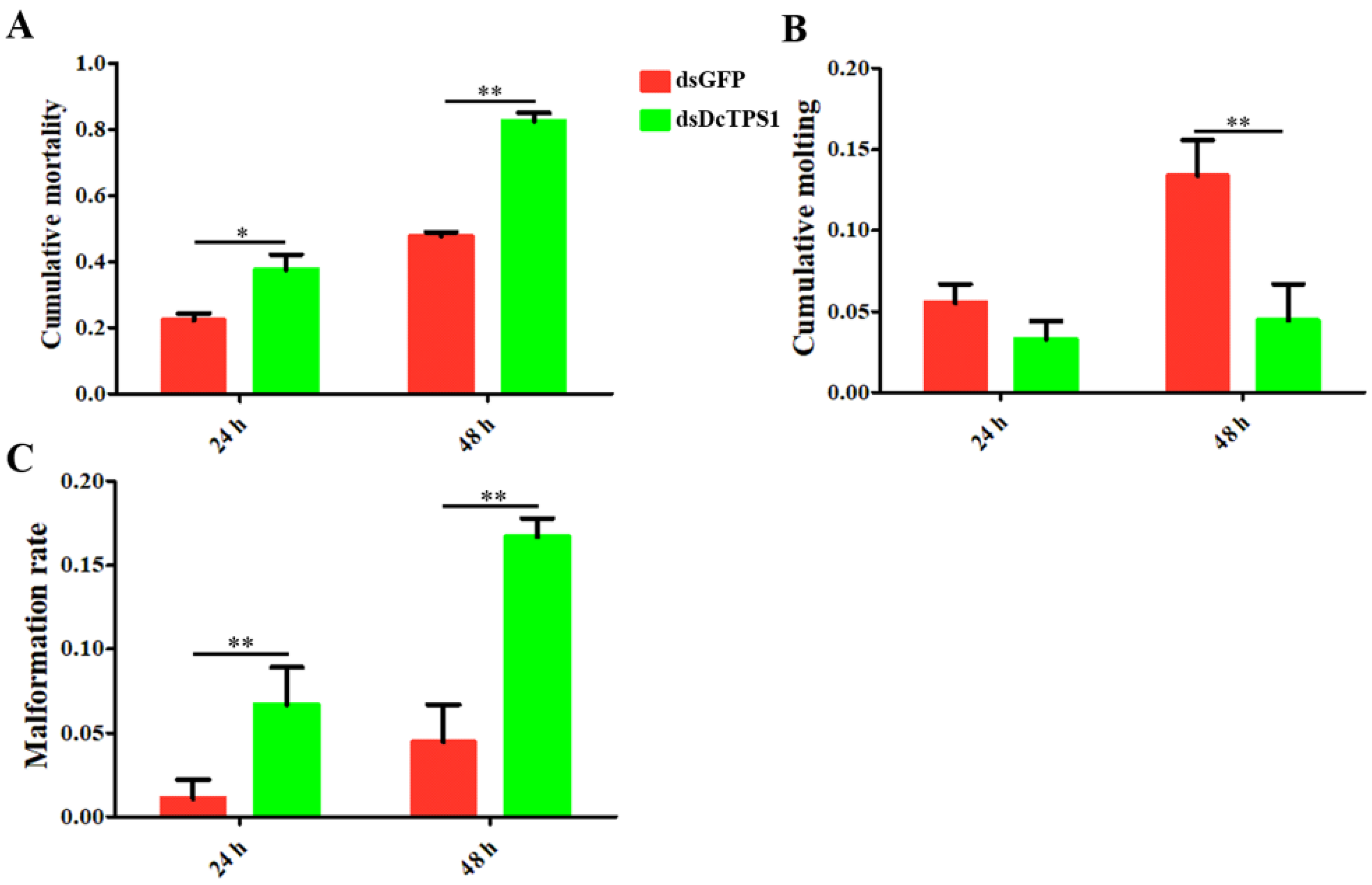
3.3. Analysis of Mortality, Molting and Malformation Rate after Inhibition of DcTPS1
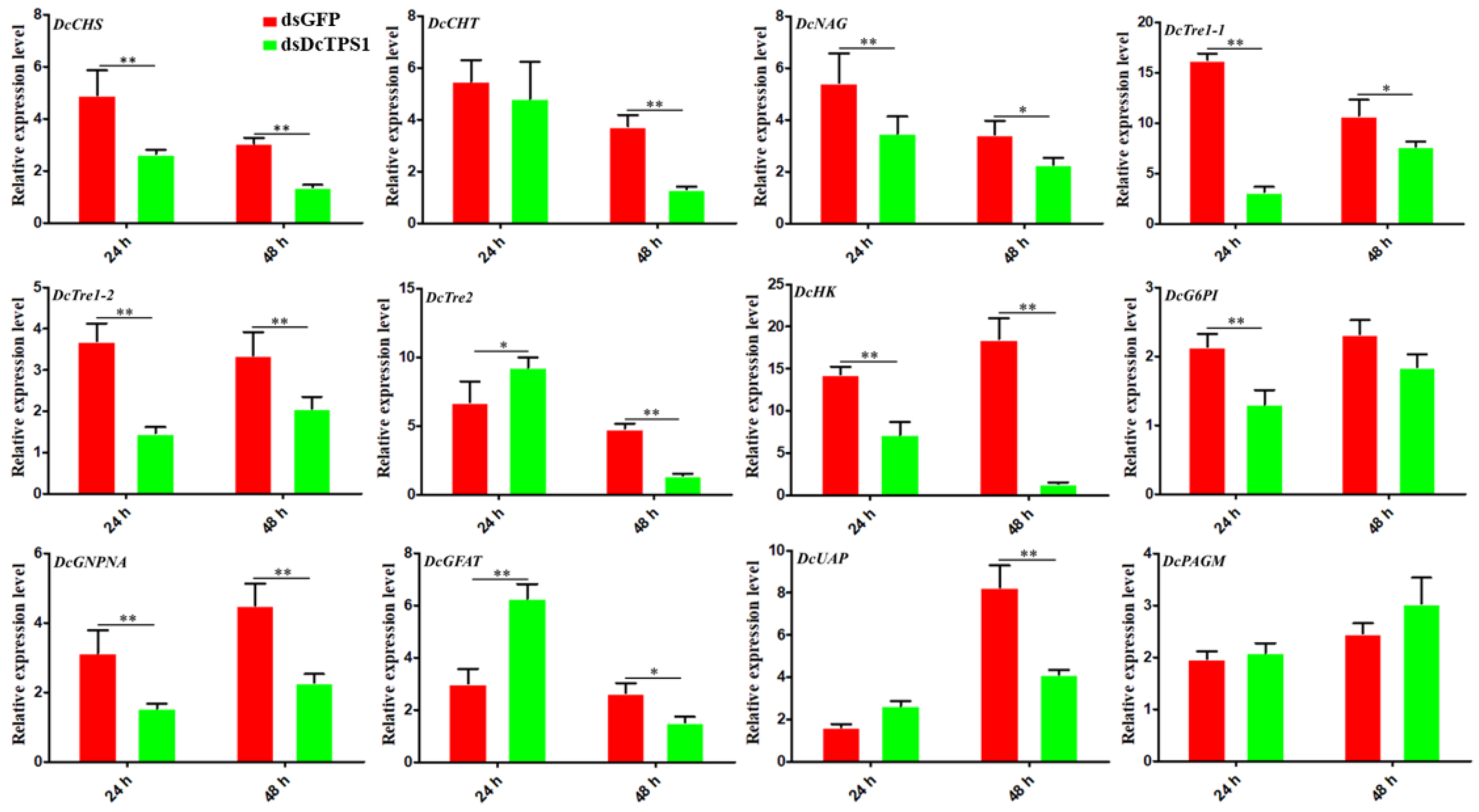
3.4. Analysis of the Effect on Chitin Metabolism after Silencing DcTPS1
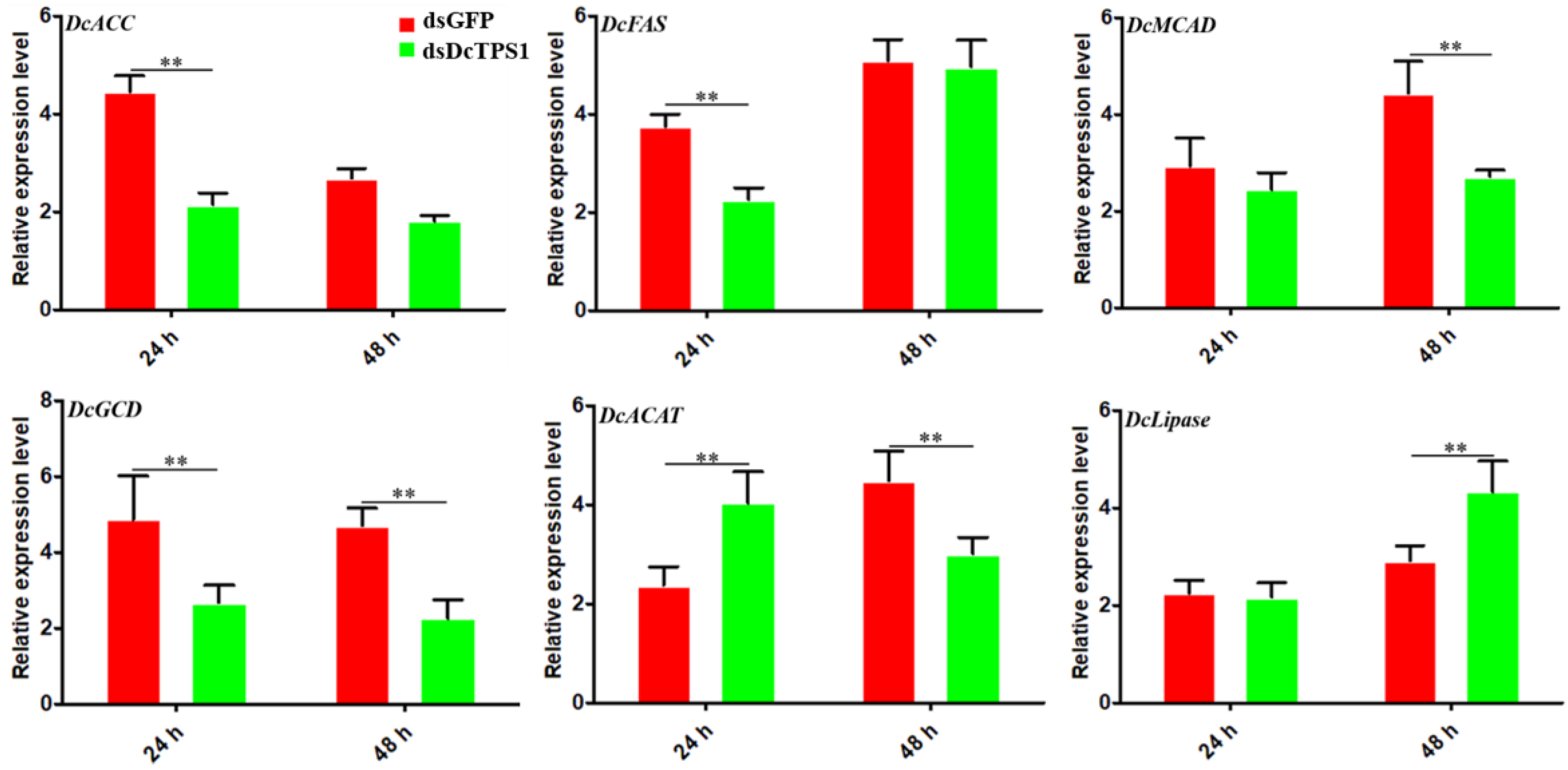
3.5. Analysis of the Effect on Fatty Acid Metabolism after Silencing of DcTPS1
3.6. Transcriptome Sequencing and Reads Assembly
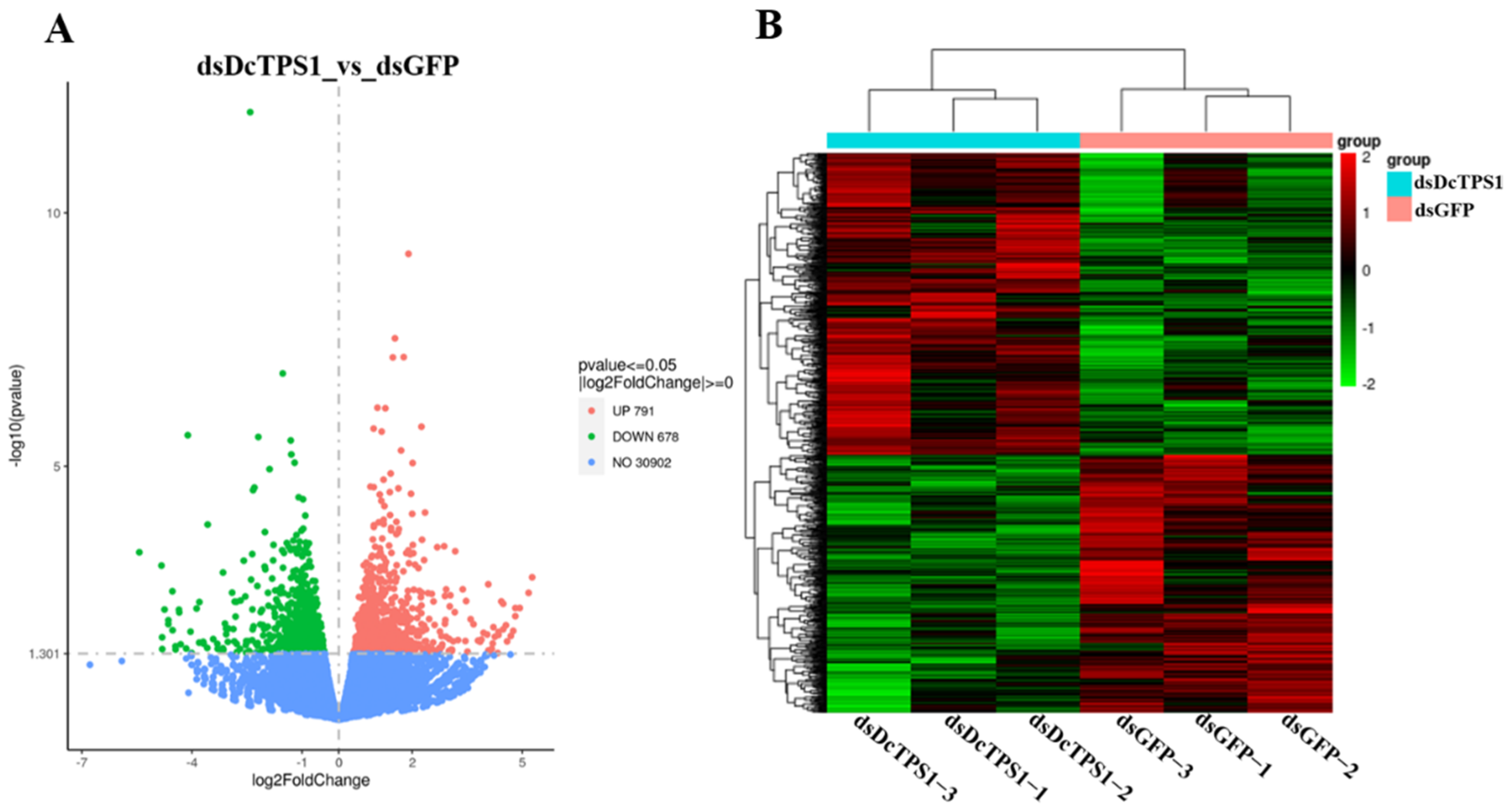
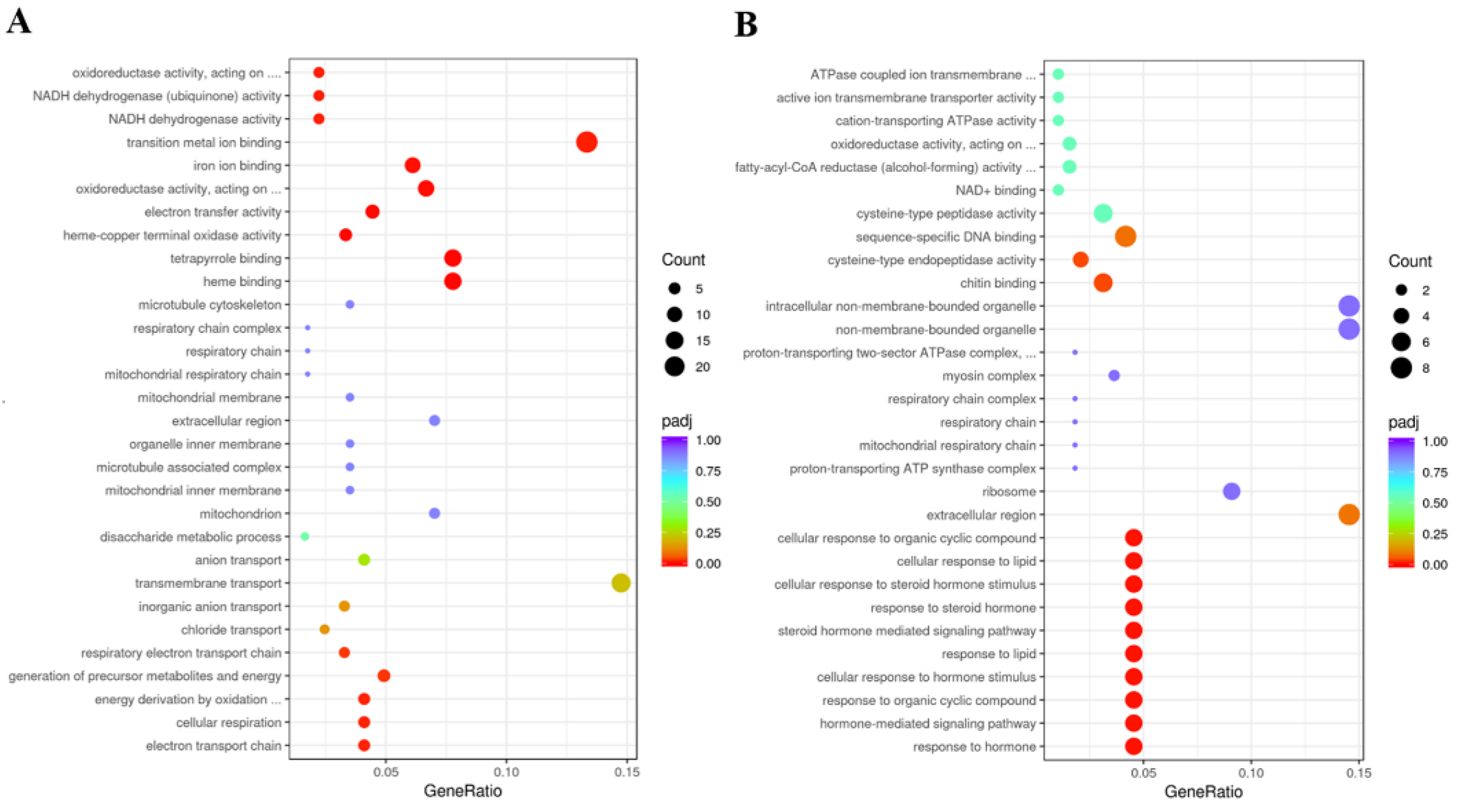
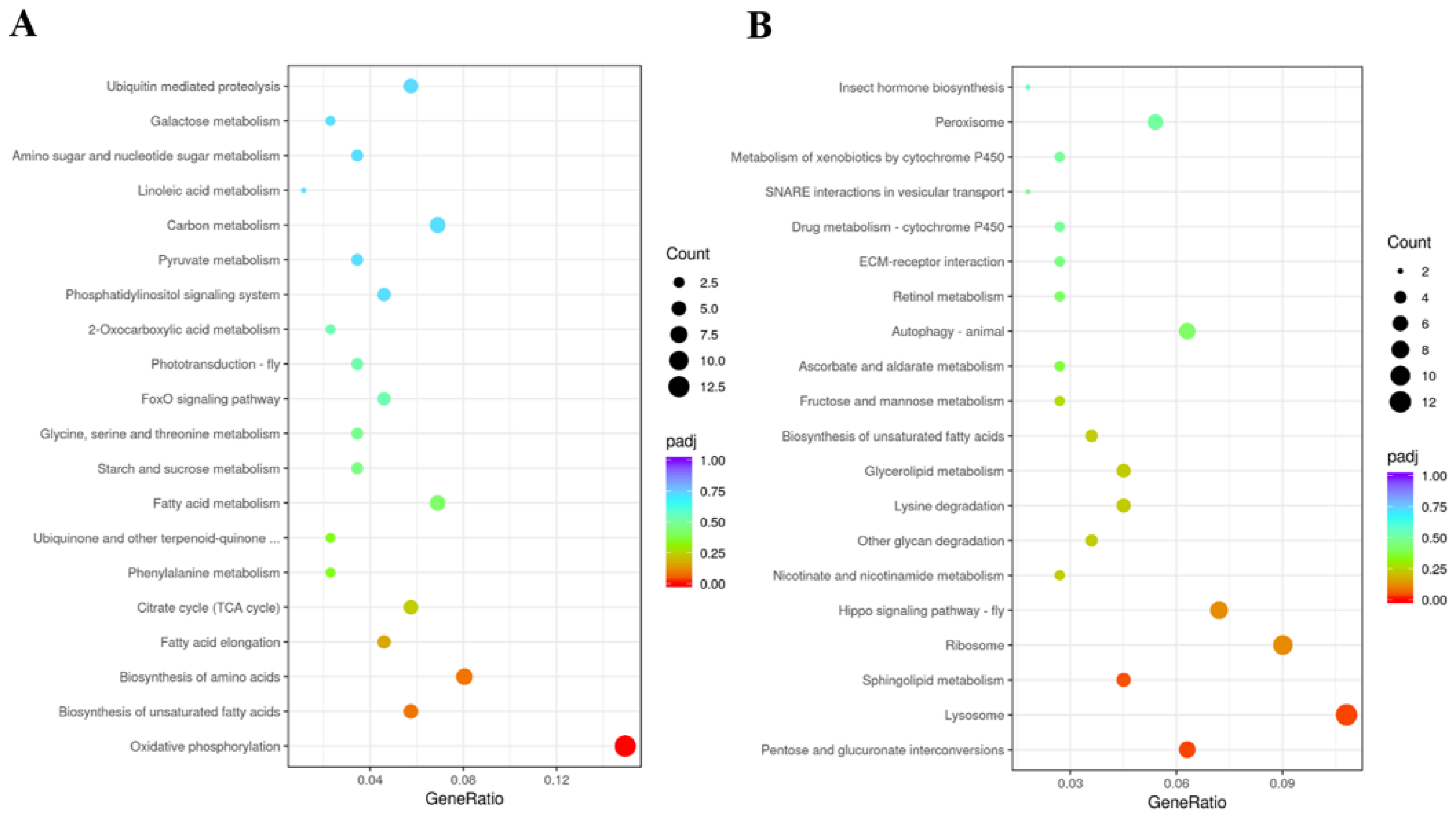
3.7. Identification of DEGs and Functional Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.F.; Haddad, G.G. Role of trehalose phosphate synthase and trehalose during hypoxia: From flies to mammals. J. Biol. Chem. 2004, 207, 3125–3129. [Google Scholar] [CrossRef] [Green Version]
- Xiong, K.C.; Wang, J.; Li, J.H.; Deng, Y.Q.; Pu, P.; Fan, H.; Liu, Y.H. RNA interference of a trehalose-6-phosphate synthase gene reveals its roles during larval-pupal metamorphosis in Bactrocera minax (Diptera: Tephritidae). J. Insect Physiol. 2016, 91–92, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Akiyama, Y.; Suzuki, M.; Hoshino, T.; Nakamura, N.; Ohno, H.; Morishima, K. Insect biofuel cells using trehalose included in insect hemolymph leading to an insect-mountable biofuel cell. Biomed. Microdevices 2012, 14, 1063–1068. [Google Scholar] [CrossRef]
- Karpova, E.K.; Eremina, M.A.; Pirozhkova, D.S.; Gruntenko, N.E. Stress-related hormones affect carbohydrate metabolism in Drosophila females. Arch. Insect Biochem. Physiol. 2019, 101, e21540. [Google Scholar] [CrossRef]
- Yu, H.Z.; Huang, Y.L.; Lu, Z.J.; Zhang, Q.; Su, H.N.; Du, Y.M.; Yi, L.; Zhong, B.L. Inhibition of trehalase affects the trehalose and chitin metabolism pathways in Diaphorina citri (Hemiptera: Psyllidae). Insect Sci. 2021, 28, 718–734. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.S.; Li, G.Y.; Liu, Y.K.; Luo, Y.J.; Xu, C.D.; Li, C.; Tang, B. Regulation of carbohydrate metabolism by trehalose-6-phosphate synthase 3 in the brown planthopper, Nilaparvata lugens. Front. Physiol. 2020, 11, 575485. [Google Scholar] [CrossRef]
- Wang, G.; Gou, Y.P.; Guo, S.F.; Zhou, J.J.; Liu, C.Z. RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 948. [Google Scholar] [CrossRef]
- Tang, B.; Chen, J.; Yao, Q.; Pan, Z.Q.; Xu, W.H.; Wang, S.G.; Zhang, W.Q. Characterization of a trehalose-6-phosphate synthase gene from Spodoptera exigua and its function identification through RNA interference. J. Insect Physiol. 2010, 56, 813–821. [Google Scholar] [CrossRef]
- Shi, J.F.; Xu, Q.Y.; Sun, Q.K.; Meng, Q.W.; Mu, L.L.; Guo, W.C.; Li, G.Q. Physiological roles of trehalose in Leptinotarsa larvae revealed by RNA interference of trehalose-6-phosphate synthase and trehalase genes. Insect Biochem. Mol. Biol. 2016, 77, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.W.; Jin, S.; Zhang, L.; Shen, Q.D.; Wei, P.; Wei, Z.M.; Wang, S.G.; Tang, B. Regulatory functions of trehalose-6-phosphate synthase in the chitin biosynthesis pathway in Tribolium castaneum (Coleoptera: Tenebrionidae) revealed by RNA interference. Bull. Entomol. Res. 2018, 108, 388–399. [Google Scholar] [CrossRef]
- Kern, C.; Wolf, C.; Bender, F.; Berger, B.; Noack, S.; Schmalz, S.; Ilg, T. Trehalose-6-phosphate synthase from the cat flea Ctenocephalides felis and Drosophila melanogaster: Gene identification, cloning, heterologous functional expression and identification of inhibitors by highthroughput screening. Insect Mol. Biol. 2012, 21, 456–471. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamada, T.; Yoshida, M.; Nishimura, T. Flies without trehalose. J. Biol. Chem. 2015, 290, 1244–1255. [Google Scholar] [CrossRef] [Green Version]
- Bonnett, T.R.; Robert, J.A.; Pitt, C.; Fraser, J.D.; Keeling, C.I.; Bohlmann, J.; Huber, D.P.W. Global and comparative proteomic profiling of overwintering and developing mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Curculionidae), larvae. Insect Biochem. Mol. Biol. 2012, 42, 890–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Y.; Zou, Z.W.; Zhang, C.; Liu, X.; Wang, J.; Xin, T.R.; Xia, B. Knockdown of the trehalose-6-phosphate synthase gene using RNA interference inhibits synthesis of trehalose and increases lethality rate in Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Insects 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Higgins, S.A.; Ramsey, J.; Howe, K.; Griggs, M.; Castrillo, L.; Heck, M. Proteomic polyphenism in color morphotypes of Diaphorina citri, insect vector of citrus greening disease. J. Proteome Res. 2021, 20, 2851–2866. [Google Scholar] [CrossRef] [PubMed]
- EI-Shesheny, I.; EI-Hawary, I.; Mesbah, I.; Killiny, N. Comparative proteomic analysis between fifth-instar nymphs and adults of Asian citrus psyllid Diaphorina citri. Physiol. Entomol. 2016, 41, 162–184. [Google Scholar] [CrossRef]
- Hijaz, F.; Lu, Z.J.; Killiny, N. Effect of host-plant and infection with ‘Candidatus Liberibacter asiaticus’ on honeydew chemical composition of the Asian citrus psyllid, Diaphorina citri. Entomol. Exp. Appl. 2016, 158, 34–43. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. PLoS ONE 2014, 9, e112331. [Google Scholar]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Kishk, A.; Anber, H.A.I.; AbdEI-Raof, T.K.; EI-Sherbeni, A.-H.D.; Hamed, S.; Gowda, S.; Killiny, N. RNA interference of carboxyesterases causes nymph mortality in the Asian citrus psyllid, Diaphorina citri. Arch. Insect Biochem. Physiol. 2017, 94, e21377. [Google Scholar] [CrossRef]
- Tiwari, S.; Clayson, P.J.; Kuhns, E.H.; Stelinski, L.L. Effects of buprofezin and diflubenzuron on various developmental stages of Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2012, 68, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Zha, W.J.; Peng, X.X.; Chen, R.Z.; Du, B.; Zhu, L.L.; He, G.C. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect. PLoS ONE 2011, 6, e20504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, D.R.G.; Gatehouse, J.A. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A.M.W.; Silver, K.; Zhang, J.Z.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.B.; Li, H.C.; Miao, X.X. Prediction of effective RNA interference targets and pathway-related genes in lepidopteran insects by RNA sequencing analysis. Insect Sci. 2018, 25, 356–367. [Google Scholar] [CrossRef]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-stranded RNA oral delivery methods to induce RNA interference in phloem and plant-sap-feeding hemipteran insects. JoVE-J. Vis. Exp. 2018, 135, 57390. [Google Scholar] [CrossRef] [Green Version]
- Yoshiyama, N.; Tojo, K.; Hatakeyama, M. A survey of the effectiveness of non-cell autonomous RNAi throughoutdevelopment in the sawfly, Athalia rosae (Hymenoptera). J. Insect Physiol. 2013, 59, 400–407. [Google Scholar] [CrossRef]
- Rangasamy, M.; Siegfried, B.D. Validation of RNA interference in western corn rootworm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) adults. Pest Manag. Sci. 2012, 68, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Y.; Jing, T.X.; Li, W.; Liu, X.Q.; Liu, T.Y.; Liu, Y.; Chen, M.L.; Jiang, R.X.; Yuan, G.R.; Dou, W.; et al. NADPH-cytochrome P450 reductase mediates the susceptibility of Asian citrus psyllid Diaphorina citri to imidacloprid and thiamethoxam. Pest Manag. Sci. 2021, 77, 677–685. [Google Scholar] [CrossRef]
- Kishk, A.; Hijaz, F.; Anber, H.A.; AbdEI-Raof, T.K.; EI-Sherbeni, A.-H.D.; Hamed, S.; Killiny, N. RNA interference of acetylcholinesterase in the Asian citrus psyllid, Diaphorina citri, increases its susceptibility to carbamate and organophosphate insecticides. Pestic. Biochem. Phys. 2017, 143, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.D.; Killiny, N. RNA interference of two glutathione S-transferase genes, DcGSTe2 and DcGSTd1, increases the susceptibility of Asian citrus psyllid (Hemiptera: Liviidae) to the pesticides, fenpropathrin and thiamethoxam. Pest Manag. Sci. 2018, 74, 638–647. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose-the insect ‘blood’ sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar]
- Doucet, D.; Retnakaran, A. Insect chitin: Metabolism, genomics and pest management. Adv. Insect Physiol. 2012, 43, 437–511. [Google Scholar]
- Xu, J.; Bao, B.; Zhang, Z.F.; Yi, Y.Z.; Xu, W.H. Identification of a novel gene encoding the trehalose phosphate synthase in the cotton bollworm, Helicoverpa armigera. Glycobiology 2009, 19, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Shen, Q.D.; Zeng, B.P.; Xiao, Z.J.; Qiu, L.Y.; Pan, B.Y.; Li, K.; Zhang, D.W. Characteristics, developmental expression and RNAi effect analysis of a novel trehalose-6-phosphate synthase gene in Nilaparvata lugens. Sci. Agric. Sin. 2019, 52, 466–477. [Google Scholar]
- Chen, J.; Zhang, D.W. Molecular cloning, tissue distribution and temperature-induced expression of two trehalose-6-phosphate synthase genes in Blattella germanica (Blattodea: Blattellidae). Acta Entomol. Sin. 2015, 58, 1046–1053. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Gans, U.; Subramanian, V.; Tan, B.H. Selective phagocytosis: A new concept in protein catabolism. Science 1968, 159, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yao, Q.; Zhang, X.; Dong, H.; Tian, H.; Chen, J.; Zhang, W. Feeding-based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Boina, D.R.; Rogers, M.E.; Wang, N.; Stelinski, L.L. Effect of pyriproxyfen, a juvenile hormone mimic, on egg hatch, nymph development, adult emergence and reproduction of the Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2010, 66, 349–357. [Google Scholar] [CrossRef]
- Souza-Ferreira, P.S.; Mansur, J.F.; Berni, M.; Moreira, M.F.; Dos Santos, R.E.; Araijo, H.M.M.; de Souza, W.; Ramos, I.B.; Masuda, H. Chitin deposition on the embryonic cuticle of Rhodnius prolixus: The reduction of CHS transcripts by CHS–dsRNA injection in females affects chitin deposition and eclosion of the first instar nymph. Insect Biochem. Mol. Biol. 2014, 51, 101–109. [Google Scholar] [CrossRef]
- Yang, M.M.; Zhao, L.N.; Shen, Q.D.; Xie, G.Q.; Wang, S.G.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef]
- Bianchi, A.; Evans, J.L.; Iverson, A.J.; Nordlund, A.C.; Watts, T.D.; Witters, L.A. Identification of an isozymic form of acetyl-CoA carboxylase. J. Biol. Chem. 1990, 265, 1502–1509. [Google Scholar] [CrossRef]
- Moriconi, D.E.; Dulbecco, A.B.; Juarez, M.P.; Calderon-Fernandez, G.M. A fatty acid synthase gene (FASN3) from the integument tissue of Rhodnius prolixus contributes to cuticle water loss regulation. Insect Mol. Biol. 2019, 28, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.A., Jr. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994, 4, 139–143. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.-C.; Lu, Z.-J.; Yi, L.; Yu, H.-Z. Functional Characterization of a Trehalose-6-Phosphate Synthase in Diaphorina citri Revealed by RNA Interference and Transcriptome Sequencing. Insects 2021, 12, 1074. https://doi.org/10.3390/insects12121074
Song J-C, Lu Z-J, Yi L, Yu H-Z. Functional Characterization of a Trehalose-6-Phosphate Synthase in Diaphorina citri Revealed by RNA Interference and Transcriptome Sequencing. Insects. 2021; 12(12):1074. https://doi.org/10.3390/insects12121074
Chicago/Turabian StyleSong, Jian-Chun, Zhan-Jun Lu, Long Yi, and Hai-Zhong Yu. 2021. "Functional Characterization of a Trehalose-6-Phosphate Synthase in Diaphorina citri Revealed by RNA Interference and Transcriptome Sequencing" Insects 12, no. 12: 1074. https://doi.org/10.3390/insects12121074
APA StyleSong, J.-C., Lu, Z.-J., Yi, L., & Yu, H.-Z. (2021). Functional Characterization of a Trehalose-6-Phosphate Synthase in Diaphorina citri Revealed by RNA Interference and Transcriptome Sequencing. Insects, 12(12), 1074. https://doi.org/10.3390/insects12121074





