The Ant-like Tachydromia Complex in the Iberian Peninsula—Insights from Habitat Suitability Modelling for the Conservation of an Endemism (Diptera: Hybotidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
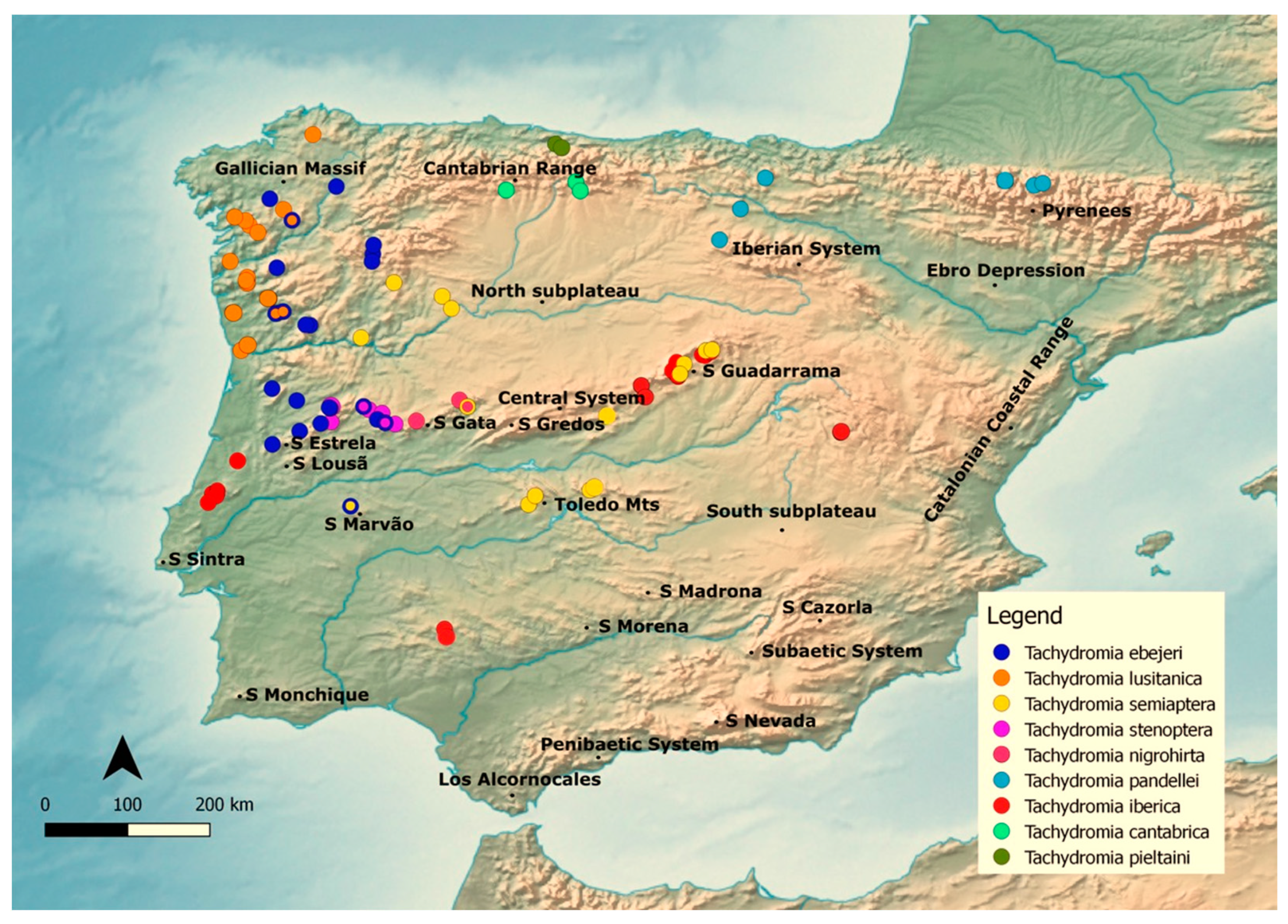
2.2. Target Taxa and Data Sources
2.3. Habitat Suitability/Distribution Model Development
2.4. Environmental Variables Selection
2.5. Model Fitting and Evaluation
2.6. Model Ensembles
2.7. Habitat Similarity and Clustering Analysis
3. Results
3.1. Predictive Model Performance
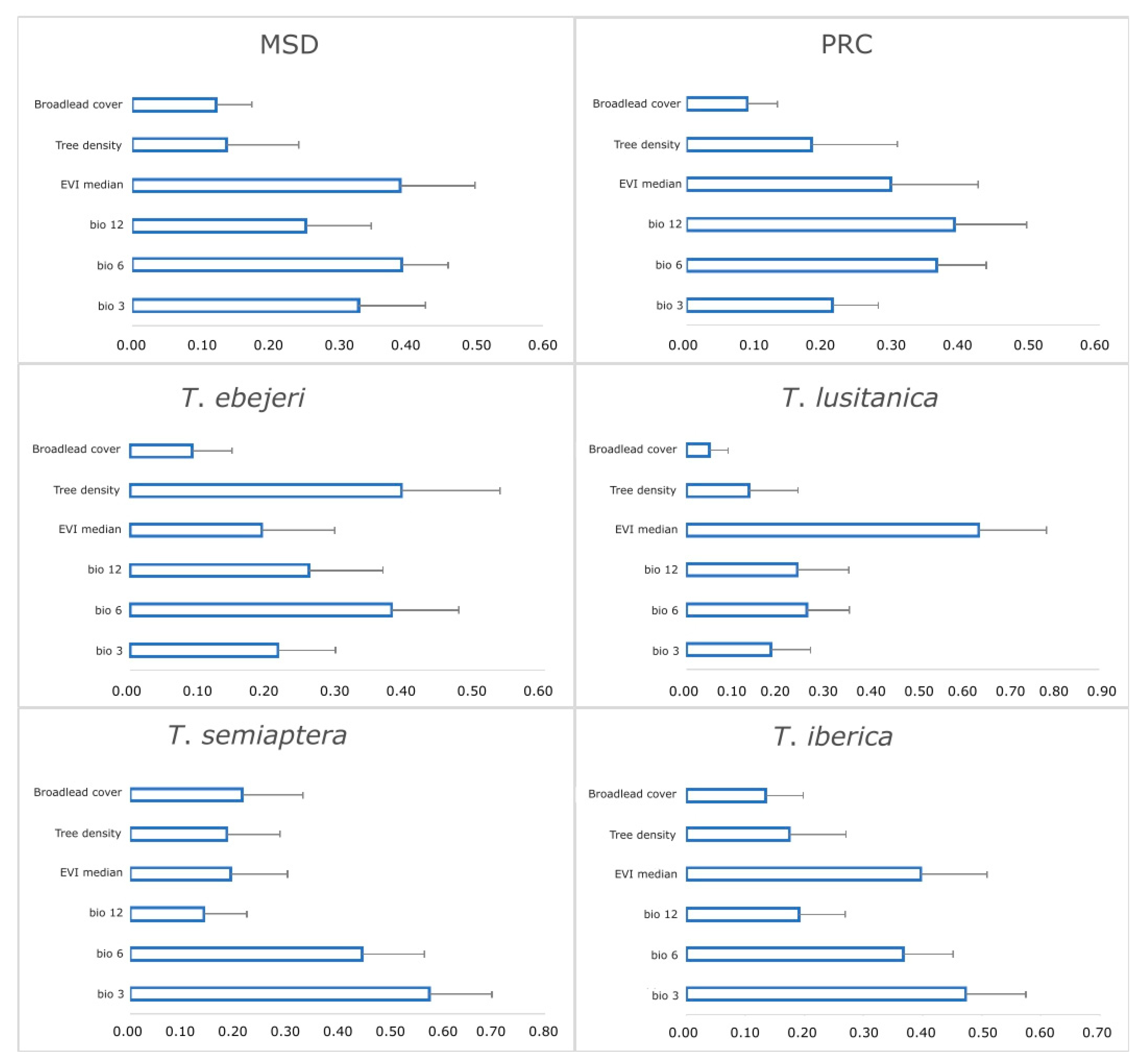
3.2. Relative Importance of Environmental Variables
3.3. Species Hierarchical Clustering
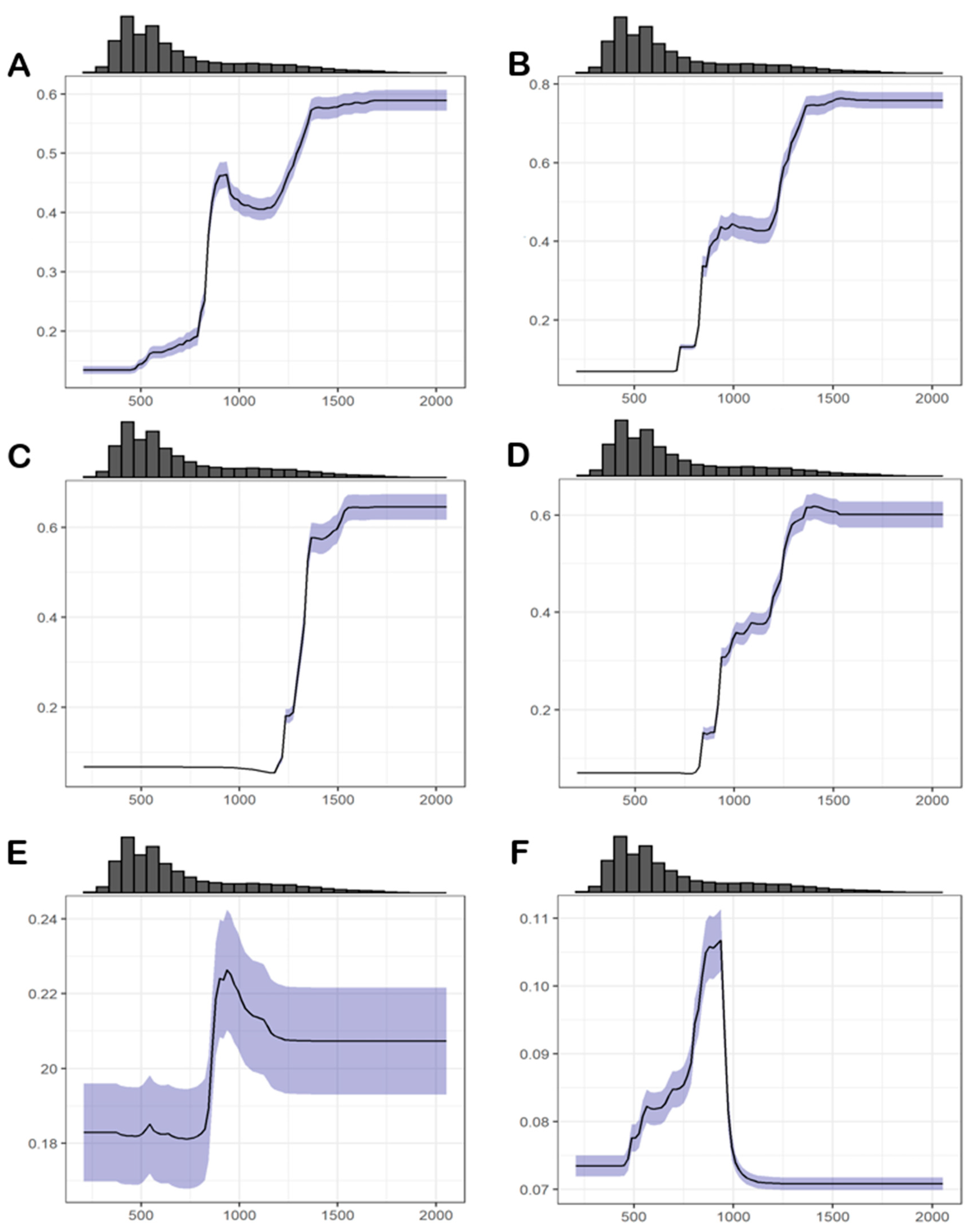
3.4. Environmental Suitability
3.4.1. Abiotic Factors
3.4.2. Biotic Factors
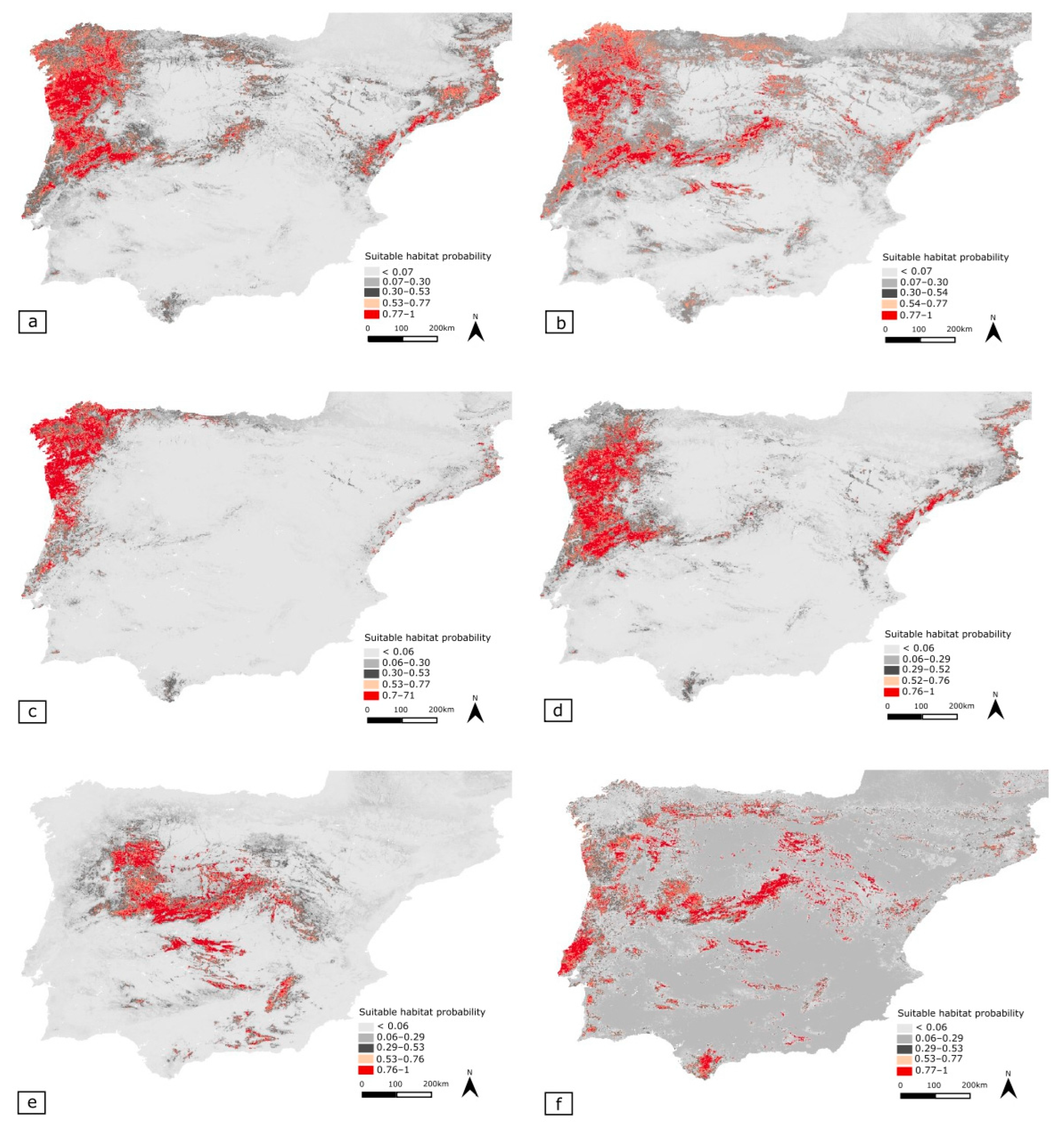
3.5. Potential Distribution Geographic Patterns
3.5.1. Merged Species Dataset (MSD)
3.5.2. Phylogenetically Most Related Cluster (PRC)
- Tachydromia lusitanica
- Tachydromia ebejeri
- Tachydromia semiaptera
- Tachydromia iberica
3.5.3. Distribution Overlap of Tachydromia with Quercus Species and Life Zones
4. Discussion
4.1. Multi-Scale Influence of Environmental Variables on Habitat Suitability
4.2. The Influence of Abiotic (Coarse-Scale) Variables
4.3. The Influence of Biotic (Fine-Scale) Factors
4.4. Spatial Overlap between Ant-like Flies, Oak Forests, and Life Zones in the Iberian Peninsula
4.5. Conservation and Future Study Guidelines
4.6. Study Limitations and Future Improvements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Scale | Main Environmental Factors Determining Habitat Suitability for the Target Species in Iberia | |
|---|---|---|
| Global | Climate | Atlantic |
| Continental | ||
| Submediterranean | ||
| Regional | Elevation | Mountainous regions |
| Coastal areas | ||
| Temperature | Mild in Spring and/or Summer | |
| Precipitation | Medium/high | |
| Local | Soil | Leaflitter present |
| High organic content | ||
| High humidity | ||
| Forest type | Deciduous | |
| Marcescent | ||
| Tree cover | Partial to allow sunlight penetration | |
| Type | Variable Name | Data Sources |
|---|---|---|
| Climate | BIO1 = Annual Mean Temperature | WolrdClim bioclimatic indices v-2.1/https://www.worldclim.org/data/worldclim21.html. Accessed: 26 May 2020 |
| Climate Geomorphology | BIO2 = Mean Diurnal Range (Mean of monthly (max temp–min temp)) | WolrdClim bioclimatic indices v-2.1/https://www.worldclim.org/data/worldclim21.html. Accessed: 26 May 2020. SRTM 90 m Digital Elevation Database v4.1: https://cgiarcsi.community/data/srtm-90m-digital-elevation-database-v4-1/. Accessed: 26 May 2020 |
| BIO3 = Isothermality (BIO2/BIO7) (×100) | ||
| BIO4 = Temperature Seasonality (standard deviation ×100) | ||
| BIO5 = Max Temperature of Warmest Month | ||
| BIO6 = Min Temperature of Coldest Month | ||
| BIO7 = Temperature Annual Range (BIO5-BIO6) | ||
| BIO8 = Mean Temperature of Wettest Quarter | ||
| BIO9 = Mean Temperature of Driest Quarter | ||
| BIO10 = Mean Temperature of Warmest Quarter | ||
| BIO11 = Mean Temperature of Coldest Quarter | ||
| BIO12 = Annual Precipitation | ||
| BIO13 = Precipitation of Wettest Month | ||
| BIO14 = Precipitation of Driest Month | ||
| BIO15 = Precipitation Seasonality (Coefficient of Variation) | ||
| BIO16 = Precipitation of Wettest Quarter | ||
| BIO17 = Precipitation of Driest Quarter | ||
| BIO18 = Precipitation of Warmest Quarter | ||
| BIO19 = Precipitation of Coldest Quarter | ||
| Topographic Wetness Index (TWI) | ||
| Soil attributes | % of sand | Topsoil physical properties for Europe (based on LUCAS topsoil data)/EU JRC: https://esdac.jrc.ec.europa.eu/content/topsoil-physical-properties-europe-based-lucas-topsoil-data. Accessed on 4 October 2018 |
| Soil attributes | % of clay | Topsoil physical properties for Europe (based on LUCAS topsoil data)/EU JRC: https://esdac.jrc.ec.europa.eu/content/topsoil-physical-properties-europe-based-lucas-topsoil-data. Accessed on 4 October 2018. Soil pH in Europe/EU JRC: https://esdac.jrc.ec.europa.eu/content/soil-ph-europe. Accessed on 4 October 2018 |
| % of silt | ||
| % coarse fragments | ||
| Bulk Density | ||
| Available Water Capacity (AWC) | ||
| Soil pH | ||
| Topsoil organic matter content | European Soil Database & soil properties/EU JRC: https://esdac.jrc.ec.europa.eu/resource-type/european-soil-database-soil-properties. Accessed on 4 October 2018 | |
| Root depth | European Soil Database & soil properties/EU JRC: https://esdac.jrc.ec.europa.eu/resource-type/european-soil-database-soil-properties. Accessed on 4 October 2018 Terra/MODIS product MOD13Q1 v-006/250m: https://lpdaac.usgs.gov/products/mod13q1v006/. Accessed on 4 October 2018 | |
| Enhanced Vegetation Index (EVI)—annual median | ||
| Remotely sensed vegetation attributes | Enhanced Vegetation Index (EVI)—annual interquartile range | Terra/MODIS product MOD13Q1 v-006/250m: https://lpdaac.usgs.gov/products/mod13q1v006/. Accessed on 4 October 2018 Global tree density map/Crowther, T. W., Glick, H. B., Covey, K. R., et al. (2015). Mapping tree density at a global scale. Nature, 525(7568), 201–205. doi:10.1038/nature14967. Available online: https://elischolar.library.yale.edu/yale_fes_data/1/. Accessed on 4 October 2018 |
| Global Tree Density/~1 Km | ||
| % cover of coniferous forest | Copernicus High Resolution Layers https://land.copernicus.eu/pan-european/high-resolution-layers. Accessed on 4 October 2018 | |
| % cover of broadleaf forest | Copernicus High Resolution Layers https://land.copernicus.eu/pan-european/high-resolution-layers. Accessed on 4 October 2018 Terra/MODIS product MOD16A2 v-006/1000m: https://lpdaac.usgs.gov/products/mod16a2v006/. Accessed on 4 October 2018 | |
| Evapotranspiration—annual median | ||
| Remotely sensed water cycle attributes | Evapotranspiration—annual interquartile range | Terra/MODIS product MOD16A2 v-006/1000m: https://lpdaac.usgs.gov/products/mod16a2v006/. Accessed on 4 October 2018 Terra/MODIS product MOD09A1 v-006/500m: https://lpdaac.usgs.gov/products/mod09a1v006/. Accessed on 4 October 2018 |
| Normalized Difference Water Index (NDWI)—annual median | ||
| Normalized Difference Water Index (NDWI)—annual interquartile range | Terra/MODIS product MOD09A1 v-006/500m: https://lpdaac.usgs.gov/products/mod09a1v006/. Accessed on 4 October 2018 |
Appendix B





References
- Gonçalves, A.R.; Grootaert, P.; Andrade, R.; Paulo, O.S.; Mengual, X. Revision of the Morphology, Phylogenetic Relationships, Behaviour and Diversity of the Iberian and Italian Ant-like Tachydromia Meigen, 1803 (Diptera: Hybotidae). Eur. J. Taxon. 2021, 732, 1–56. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter Decomposition in Forest Ecosystems: A Review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Ngai, J.T.; Srivastava, D.S. Predators Accelerate Nutrient Cycling in a Bromeliad Ecosystem. Science 2006, 314, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivula, M.; Punttila., P.; Haila, Y.; Niemelä, J. Leaf Litter and the Small-Scale Distribution of Carabid Beetles (Coleoptera, Carabidae) in the Boreal Forest. Ecography 1999, 22, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Frouz, J. Use of Soil Dwelling Diptera (Insecta, Diptera) as Bioindicators: A Review of Ecological Requirements and Response to Disturbance. Agric. Ecosyst. Environ. 1999, 74, 167–186. [Google Scholar] [CrossRef]
- Nielsen, B.O.; Nielsen, L.B. Soil Diptera of a Beech Stand and an Arable Field: A Comparison of Dipteran Emergence in Neighbouring Sites. Pedobiologia 2007, 51, 33–43. [Google Scholar] [CrossRef]
- Cumming, J.M.; Cooper, B.E. Techniques for Obtaining Adult-Associated Immature Stages of Predacious Tachydromiine Flies (Diptera: Empidoidea), with Implications for Rearing and Biocontrol. Entomol. News 1993, 104, 93–101. [Google Scholar]
- Vila-Viçosa, C.; Gonçalves, J.; Honrado, J.; Silva, R.; Vazquez, F.; Stephan, J.; García, C. Past, Present, and Future of Marcescent Mediterranean Forests. Biodivers. Inf. Sci. Stand. 2019, 3, 3. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Gonçalves, J.; Honrado, J.; Lomba, Â.; Almeida, R.S.; Vázquez, F.M.; Garcia, C. Late Quaternary Range Shifts of Marcescent Oaks Unveil the Dynamics of a Major Biogeographic Transition in Southern Europe. Sci. Rep. 2020, 10, 21598. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Vila-Viçosa, C.; Marcos, B.; Garcia, C.; Gonçalves, J. Biogeography from Space—A New Tool to Disentangle Iberian Forests Ecology and Distribution. In Proceedings of the SIBECOL. 1st Meeting of the Iberian Ecological Society & XIV AEET Meeting, Barcelona, Spain, 4–17 February 2019. [Google Scholar]
- Richardson, D.M.; Whittaker, R.J. Conservation Biogeography—Foundations, Concepts and Challenges. Divers. Distrib. 2010, 16, 313–320. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V.; New, T.R. The Seven Impediments in Invertebrate Conservation and How to Overcome Them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Moreno, J.M.; Pineda, F.D.; Rivas-Martinez, S. Climate and Vegetation at the Eurosiberian-Mediterranean Boundary in the Iberian Peninsula. J. Veg. Sci. 1990, 1, 233–244. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2020. Available online: http://www.Rstudio.Com/ (accessed on 4 October 2018).
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized Linear and Generalized Additive Models in Studies of Species Distributions: Setting the Scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Biau, G. Analysis of a Random Forests Model. J. Mach. Learn. Res. 2012, 13, 1063–1095. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Ferrier, S.; Huettmann, F.; Leathwick, J. The Evaluation Strip: A New and Robust Method for Plotting Predicted Responses from Species Distribution Models. Ecol. Model. 2005, 186, 280–289. [Google Scholar] [CrossRef]
- Axelrod, D.I. Biogeography of Oaks in the Arcto-Tertiary Province. Ann. Mo. Bot. Gard. 1983, 70, 629. [Google Scholar] [CrossRef]
- Carrero, C.; Jerome, D.; Beckman, E.; Byrne, A.; Coombes, A.J.; Deng, M.; González-Rodríguez, A.; Hoang, V.S.; Khoo, E.; Nguyen, N.; et al. The Red List of Oaks 2020; The Morton Arboretum: Lisle, IL, USA, 2020. [Google Scholar]
- Manos, P.S.; Cavender-Bares, J.; Eaton, D.A.R.; Hipp, A.A.L.; Beulke, A.; González-Rodríguez, A. Phylogeny and Biogeography of the American Live Oaks (Quercus virentes): A Genomic and Population Genetics Approach. Mol. Ecol. 2015, 24, 3668–3687. [Google Scholar] [CrossRef]
- Rivas Martínez, S.; Penas, Á.; Díaz, T.E.; del Río, S.; Cantó, P.; Herrero, L.; Pinto-Gomes, C.; Costa, J.C. Biogeography of Spain and Portugal. Typologycal Synopsis. Int. J. Geobot. Res. 2014, 4, 1–64. [Google Scholar]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An Updated Infrageneric Classification of the Oaks: Review of Previous Taxonomic Schemes and Synthesis of Evolutionary Patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- García-Mijangos, I.; Campos, J.A.; Biurrun, I.; Herrera, M.; Loidi, J. Marcescent Forests of the Iberian Peninsula: Floristic and Climatic Characterization. In Warm-Temperate Deciduous Forests around the Northern Hemisphere; Springer: Cham, Stitzerland, 2015. [Google Scholar]
- Vila-Viçosa, C.; Arenas-Castro, S.; Marcos, B.; Honrado, J.; García, C.; Vázquez, F.M.; Almeida, R.; Gonçalves, J. Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus L.). ISPRS Int. J. Geo-Inf. 2020, 9, 735. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; Díaz González, T.E.; Cantó, P.; del Río, S.; Costa, J.C.; Herrero, L.; Molero, J. Biogeographic Units of the Iberian Peninsula and Baelaric Islands to District Level. A Concise Synopsis. In The Vegetation of the Iberian Peninsula; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of Climate Warming and Altered Precipitation Patterns for Plant-Insect and Multitrophic Interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsignore, C.P.; Vizzari, G.; Vono, G.; Bernardo, U. Short-Term Cold Stress Affects Parasitism on the Asian Chestnut Gall Wasp Dryocosmus kuriphilus. Insects 2020, 11, 841. [Google Scholar] [CrossRef]
- Weltzin, J.; Loik, M.; Schwinning, S.; Williams, D.; Fay, P.; Haddad, B.; Harte, J.; Huxman, T.; Knapp, A.; Lin, G.; et al. Assessing the Response of Terrestrial Ecosystems to Potential Changes in Precipitation. BioScience 2003, 10, 941–952. [Google Scholar] [CrossRef]
- Sehler, R.; Li, J.; Reager, J.; Ye, H. Investigating Relationship Between Soil Moisture and Precipitation Globally Using Remote Sensing Observations. J. Contemp. Water Res. Educ. 2019, 168, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Yoon, T.K.; Noh, N.J.; Han, S.; Lee, J.; Son, Y. Soil Moisture Effects on Leaf Litter Decomposition and Soil Carbon Dioxide Efflux in Wetland and Upland Forests. Soil Sci. Soc. Am. J. 2014, 78, 1804–1816. [Google Scholar] [CrossRef]
- Xing, K.; Ma, C.-S.; Zhao, F.; Han, J.-C. Effects of Large Temperature Fluctuations on Hatching and Subsequent Development of the Diamondback Moth (Lepidoptera: Plutellidae). Fla. Entomol. 2015, 98, 651–659. [Google Scholar] [CrossRef]
- Lepage, M.P.; Bourgeois, G.; Brodeur, J.; Boivin, G. Effect of Soil Temperature and Moisture on Survival of Eggs and First-Instar Larvae of Delia Radicum. Environ. Entomol. 2012, 41, 159–165. [Google Scholar] [CrossRef]
- de la Serna, B.V.; Sánchez-Mata, D.; Gavilán, R.G. Marcescent Quercus pyrenaica Forest on the Iberian Peninsula. In Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European Beech Forests—A Review with Recommendations for Sustainable Forest Management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Vila-Viçosa, C.; Mendes, P.; del Rio, S.; Meireles, C.; Quinto-Canas, R.; Arsénio, P.; Pinto-Gomes, C. Temporihygrophilous Quercus broteroi Forests in Southern Portugal: Analysis and Conservation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012, 146, 298–308. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Vázquez, F.M.; Mendes, P.; del Rio, S.; Musarella, C.; Cano-Ortiz, A.; Meireles, C. Syntaxonomic Update on the Relict Groves of Mirbeck’s Oak (Quercus canariensis Willd. and Q. marianica C. Vicioso) in Southern Iberia. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2015, 149, 512–526. [Google Scholar] [CrossRef]
- Ward, A. Fog at North Front, Gibraltar. Meteorol. Mag. 1952, 81, 272–279. [Google Scholar]
- Periago, M.C.; Lana, X.; Serra, C.; Mills, G.F. Precipitation Pegionalization: An Application Using a Meteorological Network in Catalonia (Ne Spain). Int. J. Climatol. 2007, 11, 529–543. [Google Scholar] [CrossRef]
- Wheeler, D. Factors Governing Sunshine in South-West Iberia: A Review of Western Europe’s Sunniest Region. Weather 2001, 56, 189–197. [Google Scholar] [CrossRef]
- Ninyerola, M.; Pons, X.; Roure, J.M. Atlas Climático Digital de La Península Ibérica: Metodología y Aplicaciones En Bioclimatología y Geobotánica; Universitat Autònoma de Barcelona: Barcelona, Spain, 2005; ISBN 932860-8-7. [Google Scholar]
- Amigo, J.; Rodríguez-Guitián, M.A.; Pradinho Honrado, J.J.; Alves, P. The Lowlands and Midlands of Northwestern Atlantic Iberia. In The Vegetation of the Iberian Peninsula; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Cardoso, P.; Borges, P.A.V.; Triantis, K.A.; Ferrández, M.A.; Martín, J.L. Adapting the IUCN Red List Criteria for Invertebrates. Biol. Conserv. 2011, 144, 2432–2440. [Google Scholar] [CrossRef] [Green Version]
- Strandsbjerg Tristan Pedersen, J.; Duarte Santos, F.; van Vuuren, D.; Gupta, J.; Encarnação Coelho, R.; Aparício, B.A.; Swart, R. An Assessment of the Performance of Scenarios against Historical Global Emissions for IPCC Reports. Glob. Environ. Chang. 2021, 66, 102199. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Arraiano-Castilho, R.; Hipp, A.; Vázquez, F.M.; Almeida, R.; García, C.; Beja-Pereira, A.; Azevedo, H. RAD-Seq Unveils the Phylogenetic Backbone of the Iberian White Oak (Quercus L. Section Quercus) Syngameon. In Proceedings of the 1st Spanish Botanical Congress, Toledo, Spain, 8–10 September 2021. [Google Scholar]
- Aoki, K.; Kato, M.; Murakami, N. Phylogeographical Patterns of a Generalist Acorn Weevil: Insight into the Biogeographical History of Broadleaved Deciduous and Evergreen Forests. BMC Evol. Biol. 2009, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Bonal, R.; Muñóz, A.; Aparicio, J.M.; Santoro, M.; Espelta, J.M. Phylogeography, Historical Factors and Host-Parasite Specificity: Comparative Study of the Communities of Acorn Feeding Insects (Curculio spp.) in the Iberian Peninsula and California. Ecosistemas 2019, 28, 15–25. [Google Scholar] [CrossRef]
- Stone, G.N.; White, S.C.; Csóka, G.; Melika, G.; Mutun, S.; Pénzes, Z.; Sadeghi, S.E.; Schönrogge, K.; Tavakoli, M.; Nicholls, J.A. Tournament ABC Analysis of the Western Palaearctic Population History of an Oak Gall Wasp, Synergus umbraculus. Mol. Ecol. 2017, 26, 6685–6703. [Google Scholar] [CrossRef] [Green Version]
- Reut, M.; Chrabąszcz, M.; Moniuszko, H. Timing Is Everything. Temporal and Spatial Niche Segregation in Curculio spp. (Coleoptera: Curculionidae) Associated with Oak Trees. Insects 2021, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.M.; Egan, S.P.; Hood, G.R.; Rull, J.; Aluja, M.; Feder, J.L. Phylogeography of Walnut-Infesting Rhagoletis suavis (Diptera: Tephritidae) Flies. Insect Syst. Divers. 2018, 2, 2. [Google Scholar] [CrossRef]
- Vieites-Blanco, C.; González-Prieto, S.J. Invasiveness, Ecological Impacts and Control of Acacias in Southwestern Europe—A Review. Web Ecol. 2020, 20, 33–51. [Google Scholar] [CrossRef]
- Vicente, J.R.; Gonçalves, J.; Honrado, J.P.; Randin, C.F.; Pottier, J.; Broennimann, O.; Lomba, A.; Guisan, A. A Framework for Assessing the Scale of Influence of Environmental Factors on Ecological Patterns. Ecol. Complex. 2014, 20, 151–156. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Alonso, Á.; Saldaña-López, A.; Granda, E. Effects of Widespread Non-Native Trees on Regulating Ecosystem Services. Sci. Total Environ. 2021, 778, 146141. [Google Scholar] [CrossRef]

| Dataset Number | Dataset Designation | Species Name and Total Number | Number of Presence Records |
|---|---|---|---|
| 1 | Merged species dataset (MSD) | T. cantabrica T. ebejeri T. iberica T. lusitanica T. nigrohirta T. pandellei T. pieltaini T. semiaptera T. stenoptera (9) | 100 |
| 2 | Cluster of most phylogenetically related species (PRC) | T. cantabrica T. ebejeri T. lusitanica T. nigrohirta (4) | 53 |
| 3 | Tachydromia lusitanica | (1) | 20 |
| 4 | Tachydromia ebejeri | (1) | 20 |
| 5 | Tachydromia semiaptera | (1) | 14 |
| 6 | Tachydromia iberica | (1) | 20 |
| Type | Description | Source |
|---|---|---|
| Climate (coarse scale) | BIO 3—Isothermality (Mean Diurnal Range /Temperature Annual Range) (×100) | WorldClim v2.1 https://worldclim.org (accessed on 20 November 2021) |
| BIO 6—Minimum Temperature of Coldest Month | ||
| BIO 12—Annual Precipitation | ||
| Vegetation/land cover (fine scale) | % cover of broadleaf forests | Copernicus High Resolution Layers https://land.copernicus.eu/pan-european/high-resolution-layers (accessed on 20 November 2021) |
| Tree density (nr/km2) | Crowther et al., 2016 | |
| Enhanced Vegetation Index (EVI)—annual median | Terra/MODIS MOD13Q1 product (v6) https://lpdaac.usgs.gov/products/mod13q1v006/USGS/NASA (accessed on 20 November 2021) |
| MSD | PRC | T. ebejeri | T. lusitanica | T. semiaptera | T. iberica | |
|---|---|---|---|---|---|---|
| KAPPA | 0.55 | 0.60 | 0.56 | 0.66 | 0.75 | 0.73 |
| TSS | 0.79 | 0.87 | 0.96 | 0.95 | 0.95 | 0.96 |
| ROC | 0.96 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 |
| Sensitivity | 89.9 | 96.4 | 100 | 100 | 100 | 100 |
| Specificity | 88.9 | 90.1 | 95.8 | 95.2 | 95.1 | 96.2 |
| Qbro | Qcan | Qcer | Qest | Qfag | Qlus | Qmar | Qoro | Qpet | Qpub | Qpyr | Qrob | Qsub | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSD | 0.03 | 0.02 | 0.00 | 0.03 | 0.10 | 0.03 | 0.04 | 0.09 | 0.37 | 0.21 | 0.62 | 0.54 | 0.16 |
| T. lusitanica | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 | 0.00 | 0.06 | 0.00 | 0.64 | 0.96 | 0.00 |
| T. ebejeri | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.01 | 0.09 | 0.01 | 0.85 | 0.93 | 0.00 |
| T. semiaptera | 0.00 | 0.00 | 0.00 | 0.01 | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.41 | 0.00 |
| T. iberica | 0.14 | 0.08 | 0.00 | 0.14 | 0.29 | 0.09 | 0.13 | 0.14 | 0.50 | 0.34 | 0.61 | 0.06 | 0.33 |
| PRC | 0.00 | 0.00 | 0.00 | 0.01 | 0.12 | 0.03 | 0.00 | 0.05 | 0.19 | 0.06 | 0.68 | 0.77 | 0.03 |
| Mediterranean | Temperate Eurosiberian | Submediterranean | Eurosiberian Submediterranean Transition | |
|---|---|---|---|---|
| MSD | 0.07 | 0.64 | 0.16 | 0.13 |
| T. lusitanica | 0.04 | 0.82 | 0.00 | 0.14 |
| T. ebejeri | 0.03 | 0.75 | 0.03 | 0.19 |
| T. semiaptera | 0.00 | 0.37 | 0.17 | 0.47 |
| T. iberica | 0.08 | 0.36 | 0.43 | 0.12 |
| PRC | 0.08 | 0.69 | 0.06 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.R.; Vila-Viçosa, C.; Gonçalves, J. The Ant-like Tachydromia Complex in the Iberian Peninsula—Insights from Habitat Suitability Modelling for the Conservation of an Endemism (Diptera: Hybotidae). Insects 2021, 12, 1068. https://doi.org/10.3390/insects12121068
Gonçalves AR, Vila-Viçosa C, Gonçalves J. The Ant-like Tachydromia Complex in the Iberian Peninsula—Insights from Habitat Suitability Modelling for the Conservation of an Endemism (Diptera: Hybotidae). Insects. 2021; 12(12):1068. https://doi.org/10.3390/insects12121068
Chicago/Turabian StyleGonçalves, Ana Rita, Carlos Vila-Viçosa, and João Gonçalves. 2021. "The Ant-like Tachydromia Complex in the Iberian Peninsula—Insights from Habitat Suitability Modelling for the Conservation of an Endemism (Diptera: Hybotidae)" Insects 12, no. 12: 1068. https://doi.org/10.3390/insects12121068
APA StyleGonçalves, A. R., Vila-Viçosa, C., & Gonçalves, J. (2021). The Ant-like Tachydromia Complex in the Iberian Peninsula—Insights from Habitat Suitability Modelling for the Conservation of an Endemism (Diptera: Hybotidae). Insects, 12(12), 1068. https://doi.org/10.3390/insects12121068







