The Influence of Temperature and Host Gender on Bacterial Communities in the Asian Citrus Psyllid
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Psyllid Samples
2.2. Temperature Treatments
2.3. Psyllid Collection
2.4. DNA Extraction
2.5. PCR Amplification and Sequencing of 16S rRNA Amplicons
2.6. Bioinformatic Processing of Amplicon Datasets
2.7. Statistical Analyses
2.8. Phylogenetic Analysis
2.9. Determination of Bacterial Abundance by Quantitative Real-Time PCR (RT-qPCR)
3. Results
3.1. Assessment of the Amplicon Dataset Obtained from D. citri
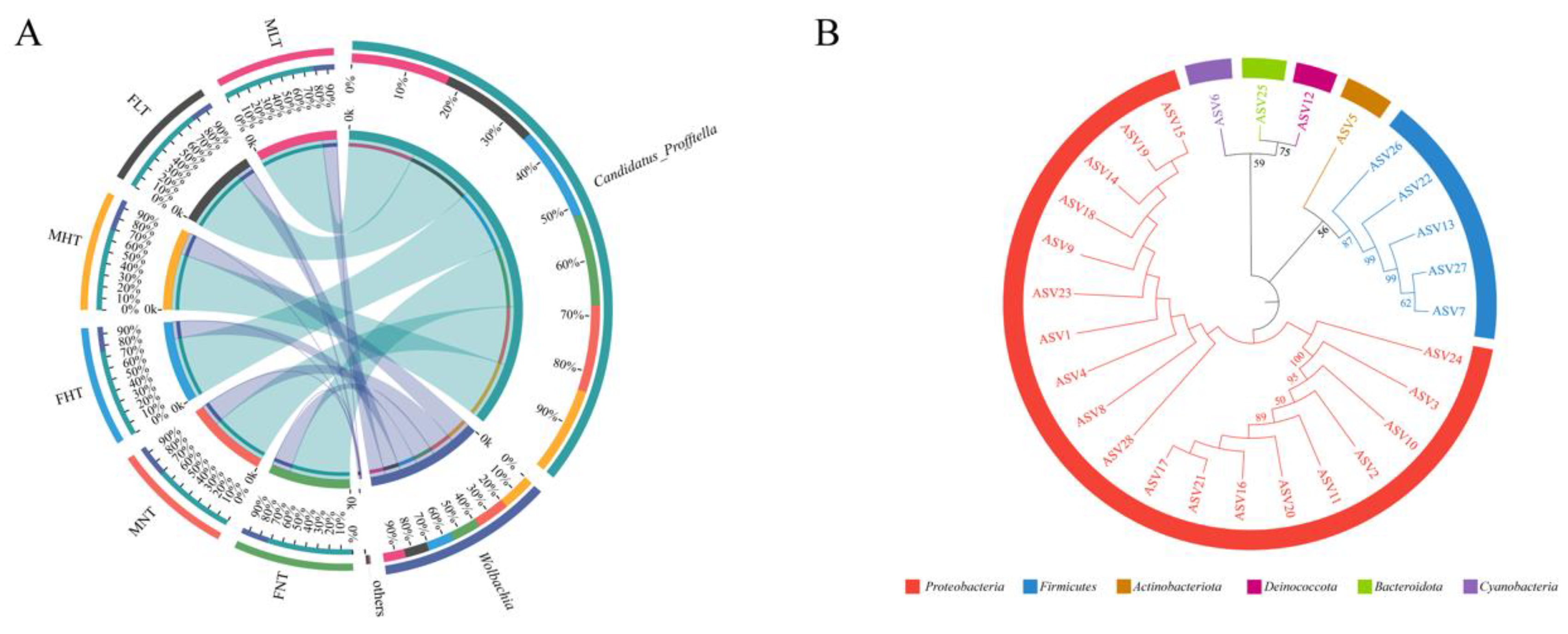
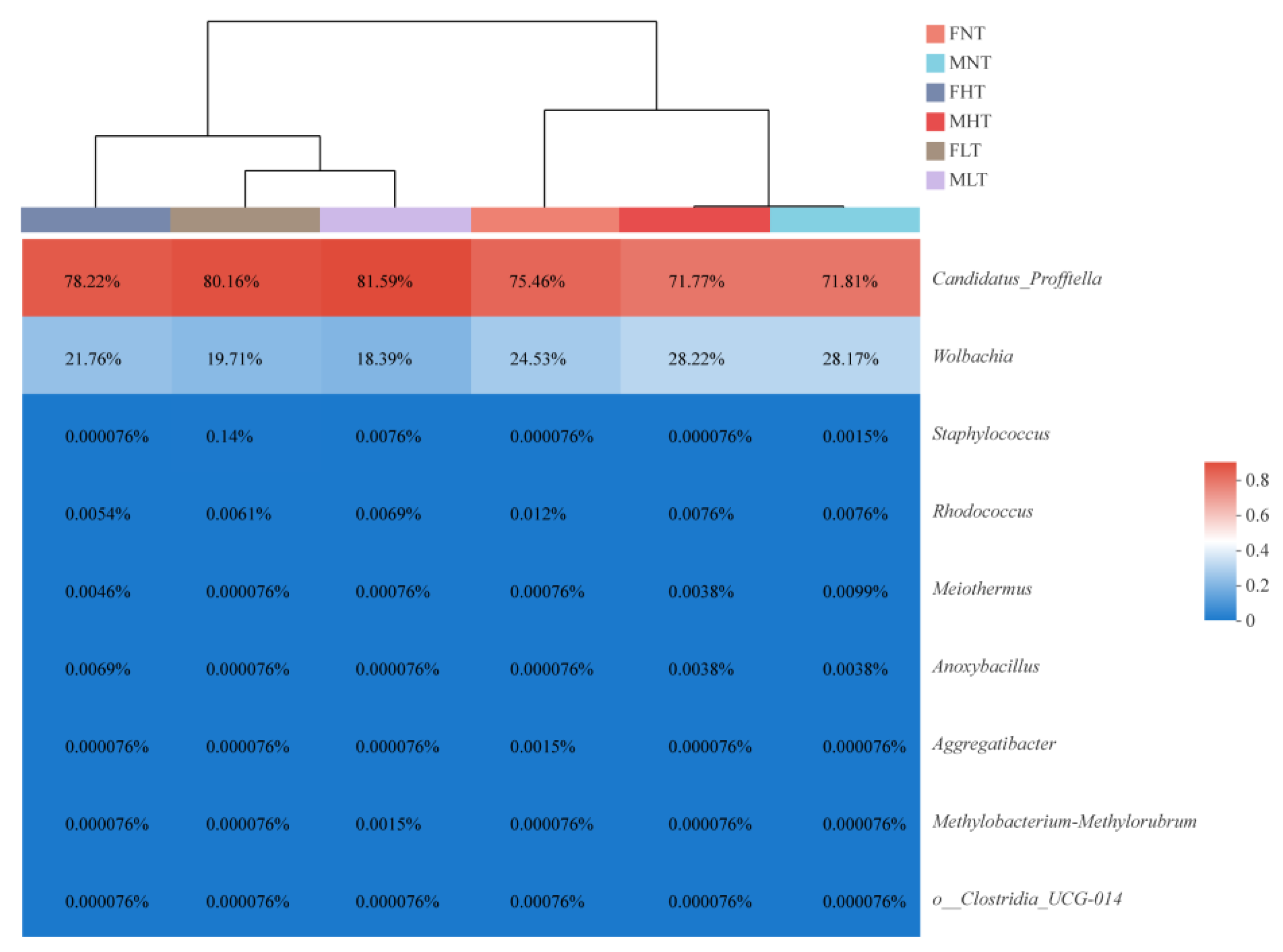
3.2. Taxonomic Composition of the Bacterial Community
3.3. Bacterial Diversity of D. citri under Different Temperature Treatments
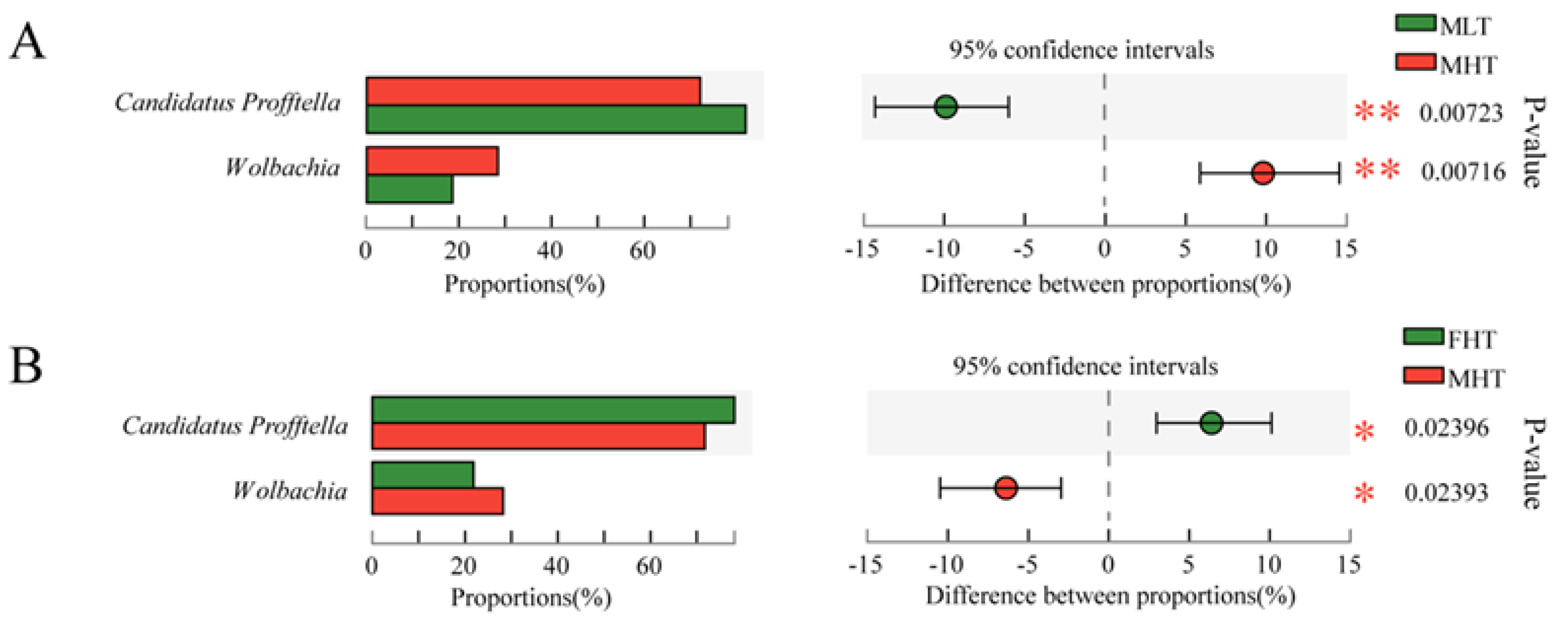
3.4. Symbiotic Community Dynamics of D. citri Are Affected by Temperature and Gender
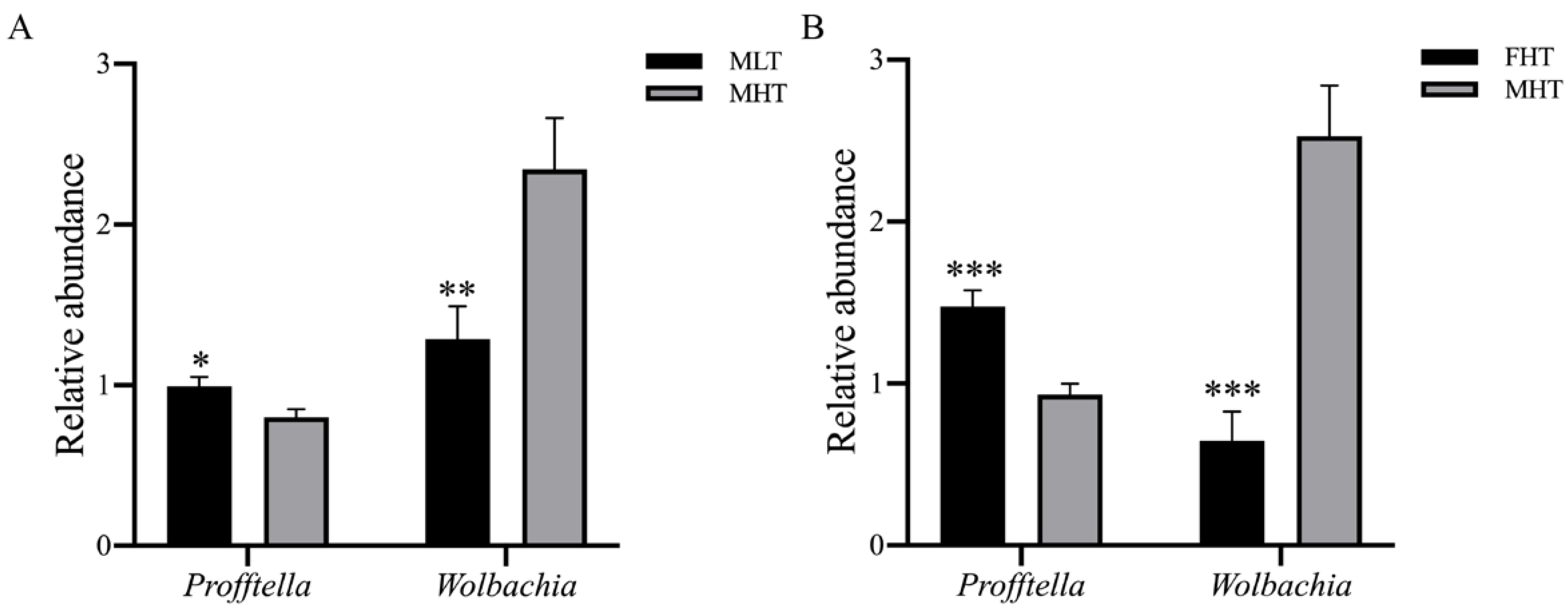
3.5. RT-qPCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsuura, Y.; Kikuchi, Y.; Hosokawa, T.; Koga, R.; Meng, X.Y.; Kamagata, Y.; Nikoh, N.; Fukatsu, T. Evolution of symbiotic organs and endosymbionts in lygaeid stinkbugs. ISME J. 2012, 6, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Manfredini, F.; Dimopoulos, G.; Schneider, S.D. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [Green Version]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Philipp, E.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Shigenobu, S.; Stern, D.L. Aphids evolved novel secreted proteins for symbiosis with bacterial endosymbiont. Proc. R. Soc. B. 2013, 280, 20121952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Han, X.; Hong, X.Y. Various infection status and molecular evidence for horizontal transmission and recombination of Wolbachia and Cardinium among rice planthoppers and related species. Insect Sci. 2013, 20, 329–344. [Google Scholar] [CrossRef]
- Cai, T.W.; Zhang, Y.H.; Liu, Y.; Deng, X.Q.; He, S.; Li, J.H.; Wan, H. Wolbachia enhances expression of NlCYP4CE1 in Nilaparvata lugens in response to imidacloprid stress. Insect Sci. 2021, 28, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Bayles, B.R.; Thomas, S.M.; Simmons, G.S.; Grafton-Cardwell, E.E.; Daugherty, M.P. Spatiotemporal dynamics of the Southern California Asian citrus psyllid (Diaphorina citri) invasion. PLoS ONE 2017, 12, e0173226. [Google Scholar] [CrossRef] [Green Version]
- Boykin, L.M.; Bagnall, R.A.; Frohlich, D.R.; Hall, D.G.; Hunter, W.B.; Katsar, C.S.; Mckenzie, C.L.; Rosell, R.; Shatters, R.G. Twelve polymorphic microsatellite loci from the Asian citrus psyllid, Diaphorina citri Kuwayama, the vector for citrus greening disease, huanglongbing. Mol. Ecol. Notes 2007, 7, 1202–1204. [Google Scholar] [CrossRef]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Thomas, S.M.; Simmons, G.S.; Daugherty, M.P. Spatiotemporal distribution of an invasive insect in an urban landscape: Introduction, establishment and impact. Landsc. Ecol. 2017, 32, 2041–2057. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Huang, C.Y.; Araujo, K.; Sáncheza, J.N.; Kund, G.; Thumble, J.; Roper, C.; Godfrey, K.E.; Jin., H.L. A stable antimicrobial peptide with dual functions of treating and preventing citrus Huanglongbing. Proc. Natl. Acad. Sci. USA 2021, 118, e2019628118. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Hubhachen, Z.; Madden, R.D.; Dillwith, J.W. Influence of rearing temperature on triacylglycerol storage in the pea aphid, Acyrthosiphon pisum. Arch. Insect Biochem. 2018, 99, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yuan, M.Y.; Liu, Y.; Guo, C.L.; Liu, T.X.; Zhang, Y.J.; Chu, D. First evidence for thermal tolerance benefits of the bacterial symbiont Cardinium in an invasive whitefly, Bemisia tabaci. Pest Manag. Sci. 2021, 77, 5021–5031. [Google Scholar] [CrossRef]
- Martini, X.; Pelz-Stelinski, K.S.; Stelinski, L.L. Factors affecting the overwintering abundance of the Asian citrus psyllid (Hemiptera: Liviidae) in Florida citrus (Sapindales: Rutaceae) orchards. Fla. Entomol. 2016, 99, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Degnan, P.H.; Ochman, H. Illumina-based analysis of microbial community diversity. ISME J. 2012, 6, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenny, J.G.F.; Diana, N.D.G.; Nelson, T.P. Bacterial communities of Aphis gossypii and Myzus persicae (Hemiptera: Aphididae) from pepper crops (Capsicum sp.). Sci. Rep. 2019, 9, 5776. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, S.; Luo, J.Y.; Wang, C.Y.; Lv, L.M.; Cui, J.J. Bacterial communities of the cotton aphid Aphis gossypii associated with Bt cotton in northern China. Sci. Rep. 2016, 6, 22958. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.G.; Hentz, M.G. Asian citrus psyllid (Hemiptera: Liviidae) tolerance to heat. Ann. Entomol. Soc. Am. 2014, 107, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.M.; He, S.Y.; Wu, W.; Huang, Y.J.; Zhu, C.Y.; Xiao, F.; Liu, Z.Y.; Zeng, J.W. Survival and lifespan of Diaphorina citri on non-host plants at various temperatures. Crop Pro. 2019, 124, 104841. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.W.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellemans, J.; Mortier, G.; De-Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Corbin, C.; Heyworth, E.R.; Ferrari, J.; Hurst, G. Heritable symbionts in a world of varying temperature. Heredity 2016, 118, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macmillan, H.A. Dissecting cause from consequence: A systematic approach to thermal limits. J. Exp. Biol. 2019, 222, jeb191593. [Google Scholar] [CrossRef] [Green Version]
- Dossi, F.; Consoli, F.L. Gross morphology and ultrastructure of the female reproductive system of Diaphorina citri (Hemiptera: Liviidae). Zoologia 2014, 31, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Shams-Bakhsh, M.; Mann, M.; Fattah-Hosseini, S.; Bagheri, A.; Mehrabadi, M.; Heck, M. Distribution and variation of bacterial endosymbiont and “Candidatus Liberibacter asiaticus” titer in the Huanglongbing insect vector, Diaphorina citri Kuwayama. Microb. Ecol. 2019, 78, 206–222. [Google Scholar] [CrossRef]
- Nakabachi, A.; Piel, J.; Malenovsky, I.; Hirose, Y. Comparative genomics underlines multiple roles of Profftella, an obligate symbiont of psyllids: Providing toxins, vitamins, and carotenoids. Genome Biol. Evol. 2020, 12, 1975–1987. [Google Scholar] [CrossRef] [PubMed]
- Nakabachi, A.; Fujikami, M. Concentration and distribution of diaphorin, and expression of diaphorin synthesis genes during Asian citrus psyllid development. J. Insect Physiol. 2019, 118, 103931. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; O’Hara, F.P.; Werren, J.H. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 2001, 409, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira1, L.A.; Ormaetxe, I.I.; Frentiu1, F.D.; McMeniman1, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Zhang, X. Wolbachia Supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef]
- Ghanim, M.; Fattah-Hosseini, S.; Levy, A.; Cilia, M. Morphological abnormalities and cell death in the Asian citrus psyllid (Diaphorina citri) midgut associated with Candidatus Liberibacter asiaticus. Sci. Rep. 2016, 6, 33418. [Google Scholar] [CrossRef]
- Hoffmann, M.; Coy, M.R.; Kingdom, G.; Pelz-Stelinski, K.S. Wolbachia infection density in populations of the Asian citrus psyllid (Hemiptera: Liviidae). Environ. Entomol. 2014, 43, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Strunov, A.; Kiseleva, E.; Gottlieb, Y. Spatial and temporal distribution of pathogenic Wolbachia strain wMelPop in Drosophila melanogaster central nervous system under different temperature conditions. J. Inverteb. Pathol. 2013, 114, 22–30. [Google Scholar] [CrossRef]
- Hussain, M.; Akutse, K.S.; Ravindran, K.; Lin, Y.; Bamisile, B.S.; Qasim, M.; Chandra, K.D.; Wang, L. Effects of different temperature regimes on survival of Diaphorina citri and its endosymbiotic bacterial communities. Environ. Microbiol. 2017, 19, 3439–3449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Song, Z.R.; Zhang, Y.Y.; Hoffmann, A.A.; Hong, X.Y. Spider mites singly infected with either Wolbachia or Spiroplasma have reduced thermal tolerance. Front. Microbiol. 2021, 12, 706321. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.-X.; Shang, F.; Jiang, H.-B.; Dou, W.; Cernava, T.; Wang, J.-J. The Influence of Temperature and Host Gender on Bacterial Communities in the Asian Citrus Psyllid. Insects 2021, 12, 1054. https://doi.org/10.3390/insects12121054
Jiang R-X, Shang F, Jiang H-B, Dou W, Cernava T, Wang J-J. The Influence of Temperature and Host Gender on Bacterial Communities in the Asian Citrus Psyllid. Insects. 2021; 12(12):1054. https://doi.org/10.3390/insects12121054
Chicago/Turabian StyleJiang, Rui-Xu, Feng Shang, Hong-Bo Jiang, Wei Dou, Tomislav Cernava, and Jin-Jun Wang. 2021. "The Influence of Temperature and Host Gender on Bacterial Communities in the Asian Citrus Psyllid" Insects 12, no. 12: 1054. https://doi.org/10.3390/insects12121054
APA StyleJiang, R.-X., Shang, F., Jiang, H.-B., Dou, W., Cernava, T., & Wang, J.-J. (2021). The Influence of Temperature and Host Gender on Bacterial Communities in the Asian Citrus Psyllid. Insects, 12(12), 1054. https://doi.org/10.3390/insects12121054







