The Impact of Ultraviolet-B Radiation on the Sugar Contents and Protective Enzymes in Acyrthosiphon pisum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture
2.2. Mode of UV-B Treatment
2.3. Determination of Trehalose and Glycogen Content
2.4. Preparation of Enzyme Solution
2.5. Protein Content Determination
2.6. Protective Enzyme Activities of Pea Aphids
2.7. Statistical Analysis
3. Results
3.1. Interaction between UV-B Treatment and Generation
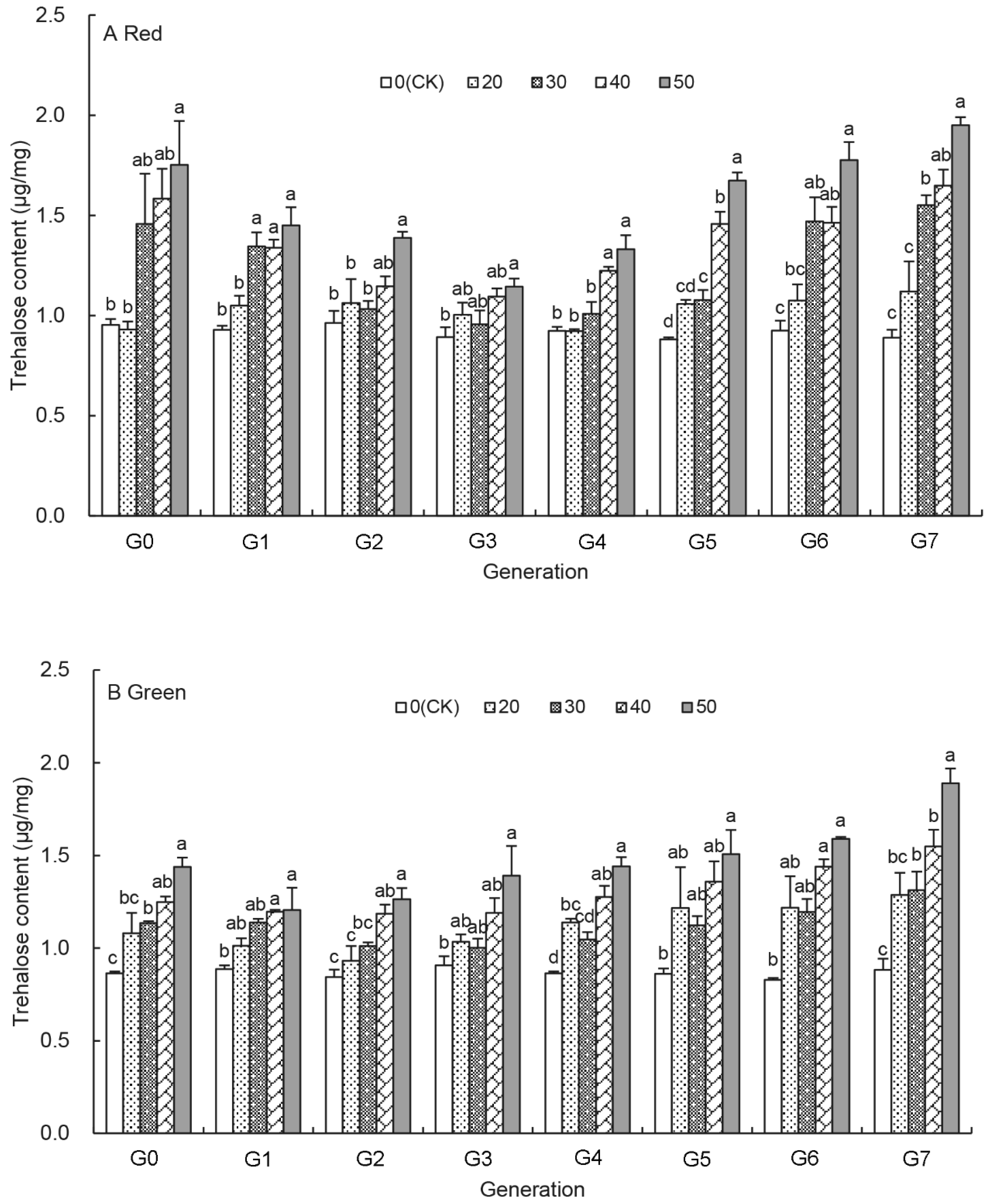
3.2. Effects of UV-B Treatments on the Trehalose Content of Aphids
3.3. Effects of UV-B Treatments on the Glycogen Content of Aphids
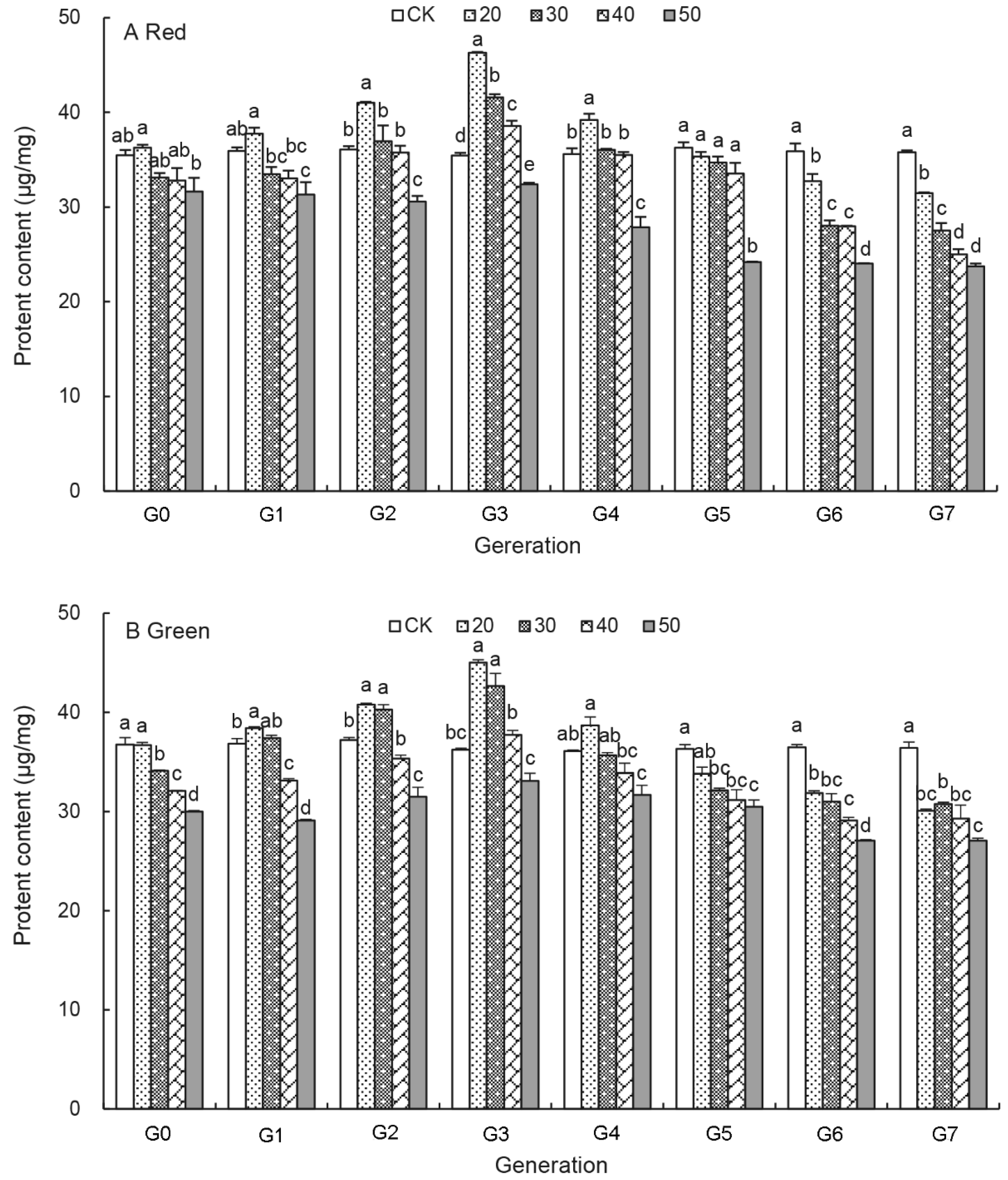
3.4. Effects of UV-B Treatments on the Protein Content of Aphids
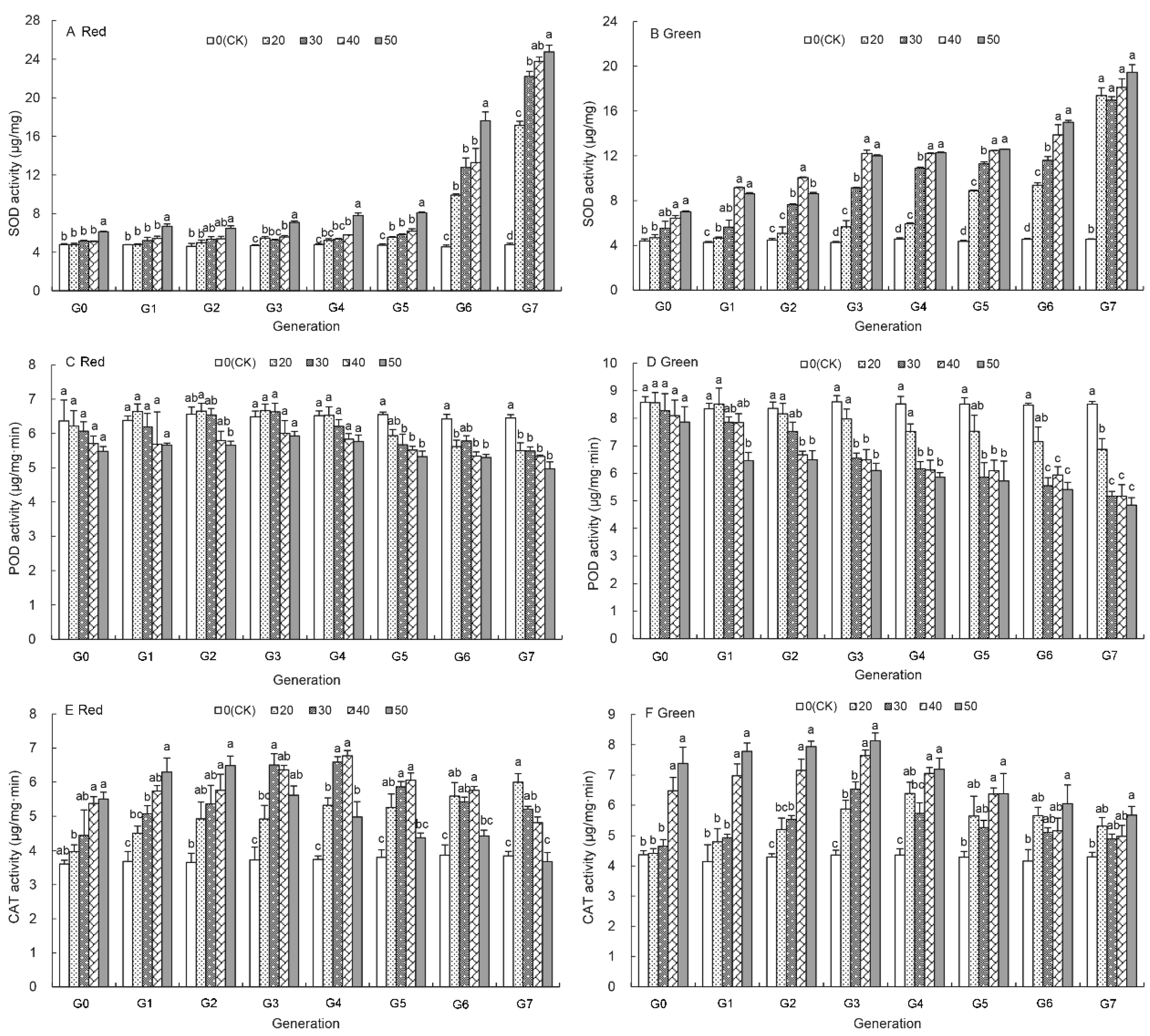
3.5. Effects of UV-B Treatment on SOD Activity of Aphids
3.6. Effects of UV-B Treatment on the POD Activity of Aphids
3.7. Effects of UV-B Treatment on the CAT Activity of Aphids
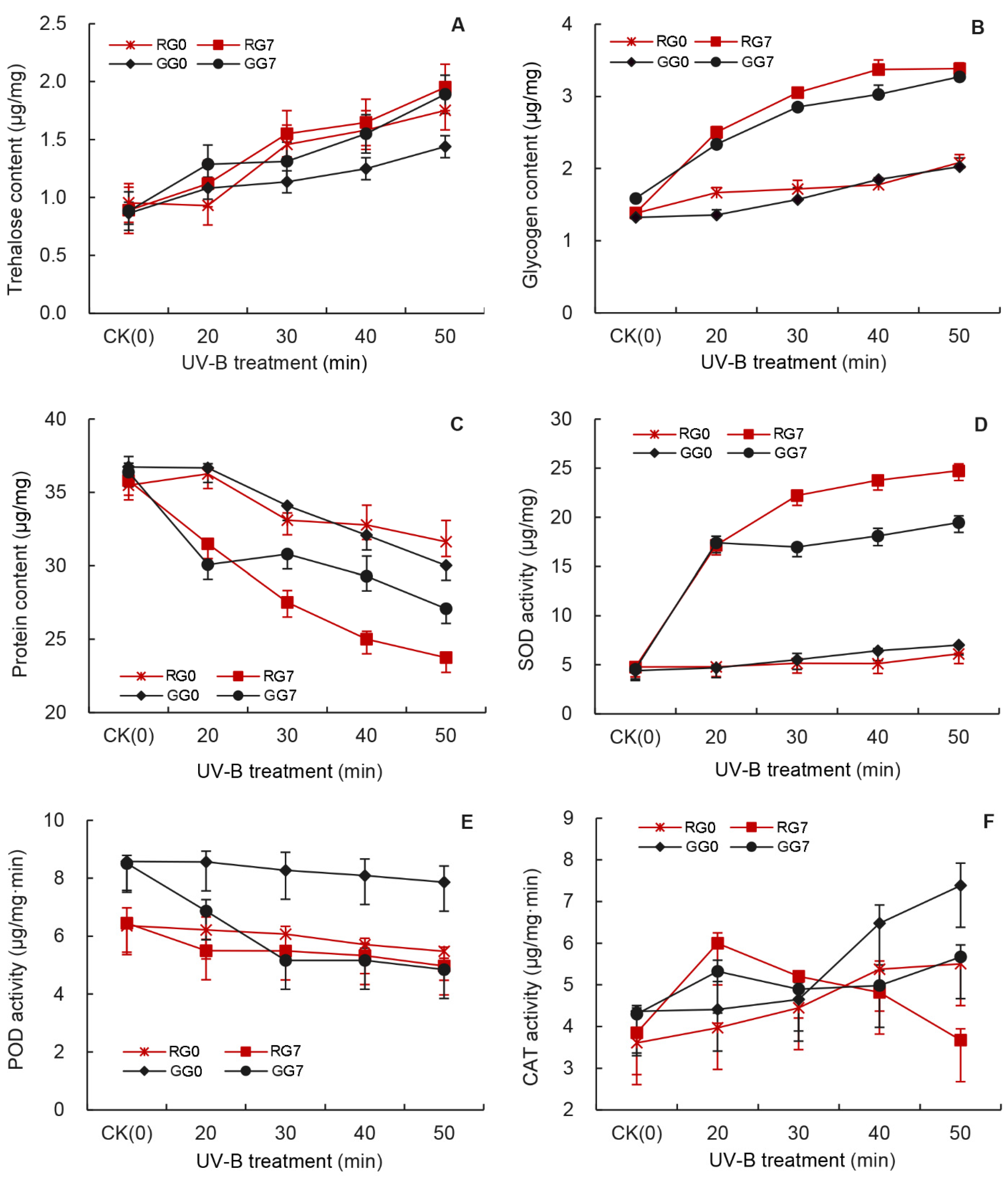
3.8. Comparison of UV-B Treatments on Sugar and the Enzyme Activity at G0 and G7 Generations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madronich, S.; McKenzie, R.L.; Caldwell, M.M.; Bjorn, L.O.; Li, W. The changes of surface ultraviolet radiation. AMBIO J. Hum. Environ. 1995, 24, 143–152. [Google Scholar]
- Long, C.S.; Miller, A.J.; Lee, H.T.; Wild, J.D.; Przywarty, R.C.; Hufford, D. Ultraviolet index forecasts issued by the National Weather Service. Bull. Am. Meteorol. Soc. 1996, 77, 729–748. [Google Scholar] [CrossRef]
- Potter, K.A.; Woods, H.A. Immobile and tough versus mobile and weak: Effects of ultraviolet B radiation on eggs and larvae of Manduca sexta. Physiol. Entomol. 2013, 38, 246–252. [Google Scholar] [CrossRef]
- Osakabe, M. Biological impact of ultraviolet-B radiation on spider mites and its application in integrated pest management. Appl. Entomol. Zool. 2021, 56, 139–155. [Google Scholar] [CrossRef]
- Villena, O.C.; Momen, B.; Sullivan, J.; Leisnham, P.T. Effects of ultraviolet radiation on metabolic rate and fitness of Aedes albopictus and Culex pipiens mosquitoes. PeerJ 2018, 6, e6133. [Google Scholar] [CrossRef] [Green Version]
- Crutzen, P.J. Ultraviolet on the increase. Nature 1992, 356, 104–105. [Google Scholar] [CrossRef]
- Madronich, S.; McKenzie, R.L.; Bjorn, L.O.; Caldwell, M.M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J. Photochem. Photobiol. B Biol. 1998, 46, 5–19. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, H.S.; Hu, X.; Torikai, A. Effects of increased solar ultraviolet radiation on materials. J. Photochem. Photobiol. 1998, 46, 96–103. [Google Scholar] [CrossRef]
- Rebollar, E.; Valadez-Graham, V.; Vázquez, M.; Reynaud, E.; Zurita, M. Role of the p53 homologue from Drosophila melanogaster in the maintenance of histone H3 acetylation and response to UV-light irradiation. FEBS Lett. 2006, 580, 642–648. [Google Scholar] [CrossRef] [Green Version]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Tuncbilek, A.S.; Ercan, F.S.; Canpolat, U. Effect of ionizing (gamma) and non-ionizing (UV) radiation on the development of Trichogramma euproctidis (Hymenoptera: Trichogrammatidae). Arch. Biol. Sci. 2012, 64, 287–295. [Google Scholar] [CrossRef]
- Lidon, F.C.; Teixeira, M.; Ramalho, J.C. Decay of the chloroplast pool of ascorbate switches on the oxidative burst in UV-B-irradiated rice. J. Agron. Crop Sci. 2012, 198, 130–144. [Google Scholar] [CrossRef]
- Zeni-Graiff, M.; Rios, A.C.; Maurya, P.K.; Rizzo, L.B.; Sethi, S.; Yamagata, A.S.; Mansur, R.B.; Pan, P.M.; Asevedo, E.; Cunha, G.R.; et al. Peripheral levels of superoxide dismutase and glutathione peroxidase in youths in ultra-high risk for psychosis: A pilot study. CNS Spectr. 2019, 24, 333–337. [Google Scholar] [CrossRef]
- Lozinskaya, Y.L.; Slepneva, I.A.; Khramtsov, V.V.; Glupov, V.V. Changes of the antioxidant status and system of generation of free radicals in hemolymph of Galleria mellonella larvae at microsporidiosis. J. Evol. Biochem. Physiol. 2004, 40, 119–125. [Google Scholar] [CrossRef]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Ghosh, P.; Kumar, A. Ultraviolet-B radiation stress-induced toxicity and alterations in proteome of Deinococcus radiodurans. Adv. Microb. Physiol. 2020, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.P.; Chen, Q.; Chen, Z.S.; Lu, H.; Xu, X.L.; Jing, F.L. Effects of heat stress on development, reproduction and activities of protective enzymes in Mononychellus mcgregori. Exp. Appl. Acarol. 2014, 63, 267–284. [Google Scholar] [CrossRef]
- Qin, D.Q.; Liu, B.J.; Zhang, P.W.; Zheng, Q.; Luo, P.R.; Ye, C.Y.; Zhao, W.H.; Zhang, Z.X. Treating green pea aphids, Myzus persicae, with azadirachtin affects the predatory ability and protective enzyme activity of harlequin ladybirds, Harmonia axyridis. Ecotoxicol. Environ. Saf. 2021, 212, 111984. [Google Scholar] [CrossRef]
- Huang, A.L.; Meng, L.Y.; Zhang, W.; Liu, J.Y.; Li, G.Y.; Tan, H.H.; Lu, W.; Zheng, X.L. Effects of five pesticides on toxicity, detoxifying and protective enzymes in Phauda flammans Walker (Lepidoptera: Zygaenidae). Pak. J. Zool. 2019, 51, 1457–1463. [Google Scholar] [CrossRef]
- Ding, J.N.; Zhang, H.H.; Chi, D.F. Effects of a pathogenic Beauveria bassiana (Hypocreales: Cordycipitaceae) strain on detoxifying and protective enzyme activities in Xylotrechus rusticus (Coleoptera: Cerambycidae) larvae. Fla. Entomol. 2015, 98, 1148–1156. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Martemyanov, V.V.; Vorontsova, Y.L.; Rantala, M.J.; Gryzanova, E.V.; Glupov, V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2008, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kayser, H.; Palivan, C.G. Stable free radicals in insect cuticles: Electron spin resonance spectroscopy reveals differences between melanization and sclerotization. Arch. Biochem. Biophys. 2006, 453, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Du, Y.M.; Yang, J.; Zhang, L.; Zhao, H.Y.; Hu, Z.Q.; Hu, X.S. Effects of UV-B radiation in successive generations on the activities of protective enzymes in the grain aphid, Sitobion avenae (Hemiptera: Aphididae). Acta Entomol. Sin. 2014, 57, 762–768. [Google Scholar]
- Bournoville, R. Insectes ravageurs des legumineuses. In Ennemiset Maladies des Prairies; Raynal, G., Gondran, J., Bournoville, R., Courtillot, M., Eds.; Editions Quae: Versailles, France, 1989; pp. 179–195. [Google Scholar]
- Harrington, C.D. Biological races of the pea aphid. J. Econ. Entomol. 1945, 38, 12–22. [Google Scholar] [CrossRef]
- Losey, J.E.; Ives, A.R.; Harmon, J.; Browm, C.; Ballantyne, F. A polymorphism maintained by opposite patterns of parasitism and predation. Nature 1997, 388, 269–272. [Google Scholar] [CrossRef]
- Caillaud, M.C.; Losey, J.E. Genetics of color polymorphism in the pea aphid, Acyrthosiphon pisum. J. Insect Sci. 2010, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.C.; Fukatsu, T. Symbiotic bacterium modifies aphid body color. Science 2010, 330, 1102–1104. [Google Scholar] [CrossRef]
- Sang, W.; Yu, L.; He, L.; Ma, W.H.; Zhu, Z.H.; Zhu, F.; Wang, X.P.; Lei, C.L. UVB radiation delays Tribolium castaneum metamorphosis by influencing ecdysteroid metabolism. PLoS ONE 2016, 11, e0151831. [Google Scholar] [CrossRef] [Green Version]
- Ryalls, J.M.W.; Riegler, M.; Moore, B.D.; Johnson, S.N. Biology and trophic interactions of lucerne aphids. Agric. For. Entomol. 2013, 15, 335–350. [Google Scholar] [CrossRef]
- Makkouk, K. Viruses and virus diseases of pea, lentil, faba bean and chickpea. World Crops: Cool Seas. Food Legum. 1988, 5, 591–615. [Google Scholar]
- Du, J.L.; Wu, D.G.; Zhang, T.W.; Qian, X.J.; Liu, C.Z. Effect of UV-B for different radiation durations on biological characteristics of two color morphs of pea aphid Acyrthosiphon pisum (Harris) offspring. Acta Agresti. Sin. 2012, 20, 961–966. [Google Scholar]
- Lv, N.; Wang, L.; Sang, W.; Liu, C.Z.; Qiu, B.L. Effects of endosymbiont disruption on the nutritional dynamics of the pea aphid Acyrthosiphon pisum. Insects 2018, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Barberà, M.; Rubén, C.C.; David, M.T. Insulin-like peptides in volved in photoperiodism in the aphid Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2019, 112, 103185. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.P.; Guo, S.F.; Zhou, J.J.; Liu, C.Z. RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, J.J.; Li, Y.; Guo, Y.P.; Quandahor, P.; Liu, C.Z. Trehalose and glucose levels regulate feeding behavior of the phloem-feeding insect, the pea aphid Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 15864. [Google Scholar] [CrossRef]
- Yuan, W.N.; Lv, N.; Sun, X.L.; Liu, C.Z. Effects of continuous UV-B stress on biological characteristics of green pea aphid. Chin. J. Eco-Agric. 2015, 23, 1020–1025. [Google Scholar]
- Yuan, W.N.; Zhu, Y.Y.; Sun, X.L.; Yang, Q.Y.; Liu, C.Z. Effects of UV-B stress on biological characteristics of red pea aphid Acyrthosiphon pisum Harris. Plant Prot. 2016, 42, 77–82. [Google Scholar]
- Handel, V.E.; Day, J.F. Assay of lipids, glycogen and sugars in individual mosquitoes: Correlations with wing length in field-collected Aedes vexans. J. Am. Mosq. Control. Assoc. 1988, 4, 549–550. [Google Scholar]
- Lowry, B.O.H.; Rosebroug, N.J.; Farr, A.L.; Randdall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 256–275. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, P.; Maehly, A.C. Assay of catalases and peroxidases. Meth. Enzymol. 1955, 11, 764–775. [Google Scholar]
- Shan, W.; Li, Y.L.; Zhu, J.J.; Liang, Y.H.; Wang, Z.M. Effect of different sulfur fumigation dosages on activity of browning enzymes and chemical constituents of Chrysanthemum morifolium cv. Boju. Chin. Med. J. 2019, 44, 4852–4856. [Google Scholar]
- Lease, H.M.; Wolf, B.O. Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol. Entomol. 2011, 36, 29–38. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T.; Van der Horst, D.J.; Van Marrewijk, W.J.A. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar] [CrossRef]
- O’Brien, D.M. Fuel use in flight and its dependence on nectar feeding in the hawkmoth Amphion floridensis. J. Exp. Biol. 1999, 202, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tsonev, L.I.; Tihova, M.G.; Brain, A.P.R.; Yu, Z.W.; Quinn, P.J. The effect of the cryoprotective sugar trehalose on the phase behaviour of mixed dispersions of dioleoyl derivatives of phosphatidylethanolamine and phosphatidylcholine. Liq. Cryst. 1994, 17, 717–728. [Google Scholar] [CrossRef]
- Womersley, C.Z.; Higa, L.M. Trehalose: Its role in the anhydrobiotic survival of Ditylenchus myceliophagus. Nematologica 1998, 44, 269–292. [Google Scholar]
- Li, C.C.; Sun, Q.; Gou, Y.P.; Zhang, K.X.; Zhang, Q.Y.; Zhou, J.J.; Liu, C.Z. Long-term effect of elevated CO2 on the development and nutrition contents of the Pea Aphid (Acyrthosiphon pisum). Front. Physiol. 2021, 12, 827. [Google Scholar] [CrossRef]
- Hawakawa, Y.; Chino, H. Temperature-dependent activation or inactivation of glycogen phosphorylase and synthase of fat body of the silkworm Philosamia cynthia: The possible mechanism of the temperature-dependent interconversion between glycogen and trehalose. Insect Biochem. 1982, 12, 361–366. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Freeze tolerance in animals. Physiol. Rev. 1988, 68, 27–84. [Google Scholar] [CrossRef]
- Uritani, M.; Takai, M.; Yoshinaga, K. Protective effect of disaccharides on restriction endonucleases during drying under vacuum. J. Biochem. 1995, 117, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef]
- Ahsaei, S.M.; Tabadkani, S.M.; Hosseininaveh, V.; Allahyari, H.; Bigham, M. Differential accumulation of energy by the colour morphs of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphididae) mirrors their ecological adaptations. Eur. J. Entomol. 2013, 110, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xuan, J.P.; Guo, H.L.; Liu, J.X. Seasonal changes of freezing tolerance and its relationship to the contents of carbohydrates, proline, and soluble protein of Zoysia. Acta Prataculturae Sin. 2011, 20, 98–107. [Google Scholar]
- Moeini, P.; Tahmasebi, A. Maize Iranian mosaic virus infection promotes the energy sources of its insect vector, Laodelphax striatellus. J. Appl. Entomol. 2018, 143, 271–276. [Google Scholar] [CrossRef]
- Wang, F.L.; Chambi, C.; Li, Z.Y.; Huang, C.; Ma, Y.K.; Li, C.R.; Tian, X.H.; Sangija, F.; Ntambo, M.S.; Kankonda, O.M.; et al. Influence of supplemental protein on the life expectancy and reproduction of the Chinese Citrus Fruit Fly, Bactrocera minax (Enderlein) (Tetradacus minax) (Diptera: Tephritidae). J. Insect Sci. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.M. A study of the germ cells of Aphis rosae and Aphis oenotherae. J. Exp. Zool. 1905, 2, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Markkula, M. Studies on the pea aphid, Acyrthosiphon pisum, with special reference to the differences in the biology of the green and red forms. Ann. Agric. Fenn. 1963, 2, 1–30. [Google Scholar]
- Valmalette, J.C.; Dombrovsky, A.; Brat, P.; Mertz, C.; Capovilla, M.; Robichon, A. Light-induced electron transfer and ATP synthesis in a carotene synthesizing insect. Sci. Rep. 2012, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisser, W.W.; Braendle, C. Body color and genetic variation in winged morph production in the pea aphid. Entomol. Exp. Appl. 2001, 99, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Wang, S.S.; He, C.G. Genetic diversities of Acyrthosiphon pisum (harris) (pink form) populations from different geographic regions in the northwest of China. Acta Agrestia Sin. 2013, 21, 406–412. [Google Scholar]
- Raikhel, A.S.; Dhadialla, T.S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992, 37, 217–251. [Google Scholar] [CrossRef]
- Wei, H.; Zhikuan, J.; Qingfang, H. Effects of herbivore stress by Aphis medicaginis Koch on the malondialdehyde contents and the activities of protective enzymes in different alfalfa varieties. Acta Ecol. Sin. 2007, 27, 2177–2183. [Google Scholar] [CrossRef]
- Orr, W.C.; Sohal, R.S. Extension of life span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef]
- Orr, W.C.; Sohal, R.S. Does overexpression of Cu, Zn-SOD extend life span in Drosophila melanogaster? Exp. Gerontol. 2003, 38, 227–230. [Google Scholar] [CrossRef]
- Sohal, R.S.; Agarwal, A.; Agarwal, S.; Orr, W.C. Simultaneous overexpression of copper- and Zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem. 1995, 270, 15671–15674. [Google Scholar] [CrossRef] [Green Version]
- Bruce, R.J.; West, C.A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragnents in suspension cultures caster bean. Plant Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef]

| Measurement | Morph | Type III Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Trehalose | Red | 1.61 | 28 | 0.06 | 2.85 | <0.001 |
| Green | 0.80 | 28 | 0.03 | 1.48 | 0.089 | |
| Glycogen | Red | 4.62 | 28 | 0.17 | 3.89 | <0.001 |
| Green | 2.66 | 28 | 0.10 | 4.88 | <0.001 | |
| Protein | Red | 489.78 | 28 | 17.49 | 12.09 | <0.001 |
| Green | 352.13 | 28 | 12.58 | 12.91 | <0.001 | |
| SOD | Red | 701.47 | 28 | 25.05 | 54.56 | <0.001 |
| Green | 300.34 | 28 | 10.73 | 31.71 | <0.001 | |
| POD | Red | 3.28 | 28 | 0.12 | 0.54 | 0.966 |
| Green | 20.08 | 28 | 0.72 | 1.78 | 0.024 | |
| CAT | Red | 36.92 | 28 | 1.32 | 4.90 | <0.001 |
| Green | 27.46 | 28 | 0.98 | 2.88 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yuan, W.; Gou, Y.; Zhang, K.; Zhang, Q.; Zhou, J.-J.; Liu, C. The Impact of Ultraviolet-B Radiation on the Sugar Contents and Protective Enzymes in Acyrthosiphon pisum. Insects 2021, 12, 1053. https://doi.org/10.3390/insects12121053
Li C, Yuan W, Gou Y, Zhang K, Zhang Q, Zhou J-J, Liu C. The Impact of Ultraviolet-B Radiation on the Sugar Contents and Protective Enzymes in Acyrthosiphon pisum. Insects. 2021; 12(12):1053. https://doi.org/10.3390/insects12121053
Chicago/Turabian StyleLi, Chunchun, Weining Yuan, Yuping Gou, Kexin Zhang, Qiangyan Zhang, Jing-Jiang Zhou, and Changzhong Liu. 2021. "The Impact of Ultraviolet-B Radiation on the Sugar Contents and Protective Enzymes in Acyrthosiphon pisum" Insects 12, no. 12: 1053. https://doi.org/10.3390/insects12121053
APA StyleLi, C., Yuan, W., Gou, Y., Zhang, K., Zhang, Q., Zhou, J.-J., & Liu, C. (2021). The Impact of Ultraviolet-B Radiation on the Sugar Contents and Protective Enzymes in Acyrthosiphon pisum. Insects, 12(12), 1053. https://doi.org/10.3390/insects12121053





