Updating Ecological and Behavioral Aspects of the Sandfly Fauna in the Vale do Ribeira Region, São Paulo State, Brazil
Abstract
Simple Summary
Abstract
1. Introduction
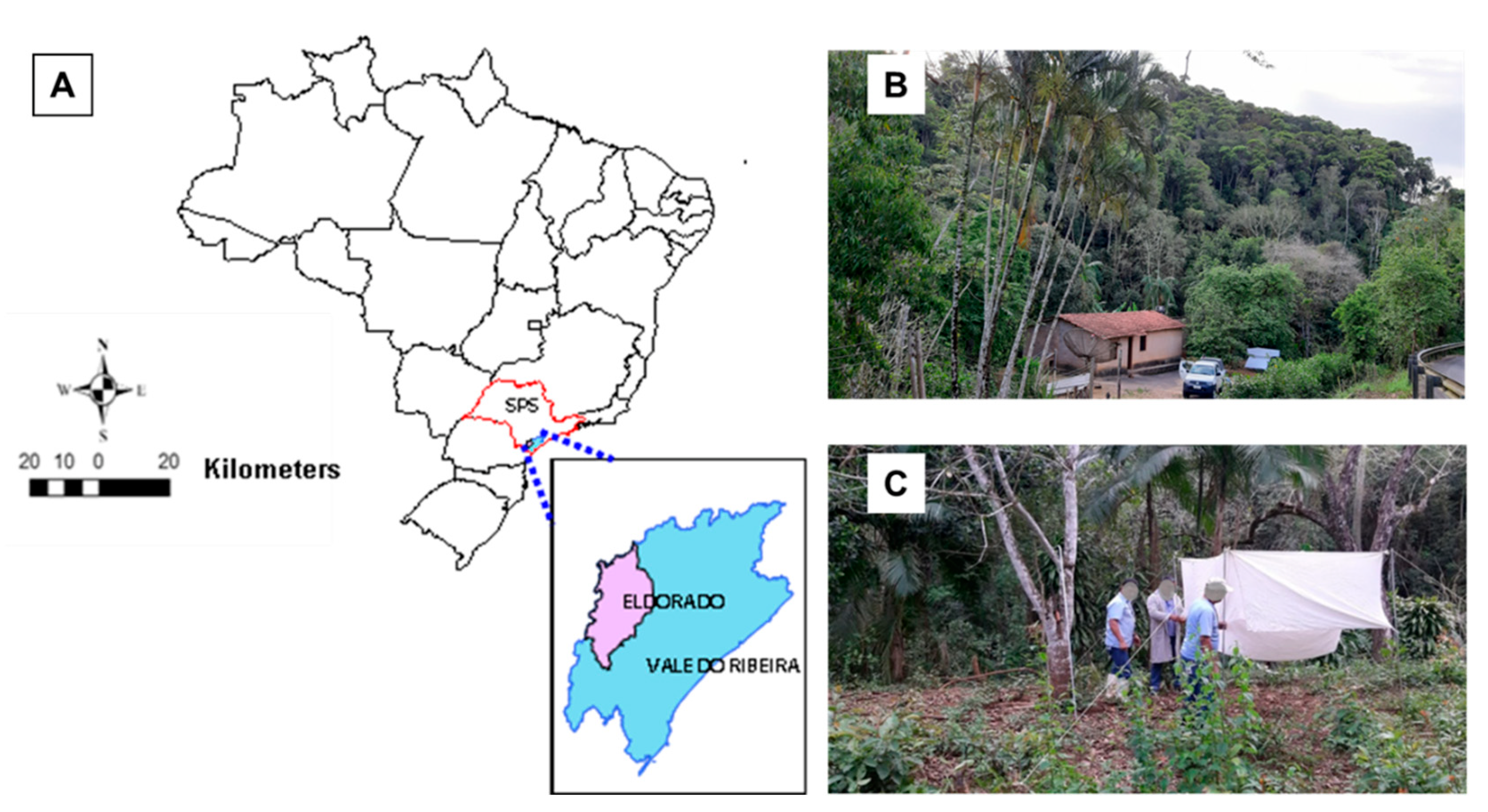
2. Materials and Methods
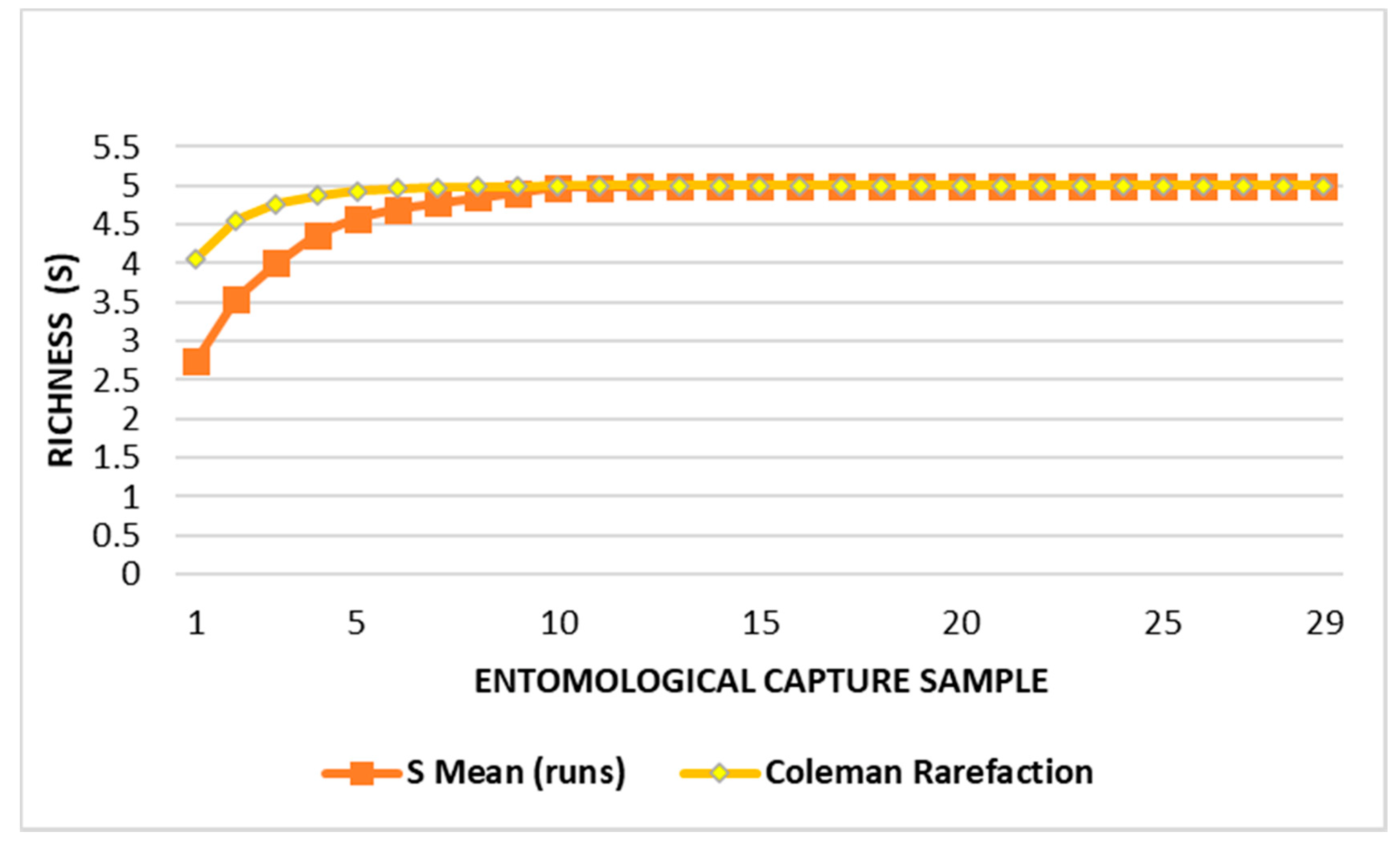
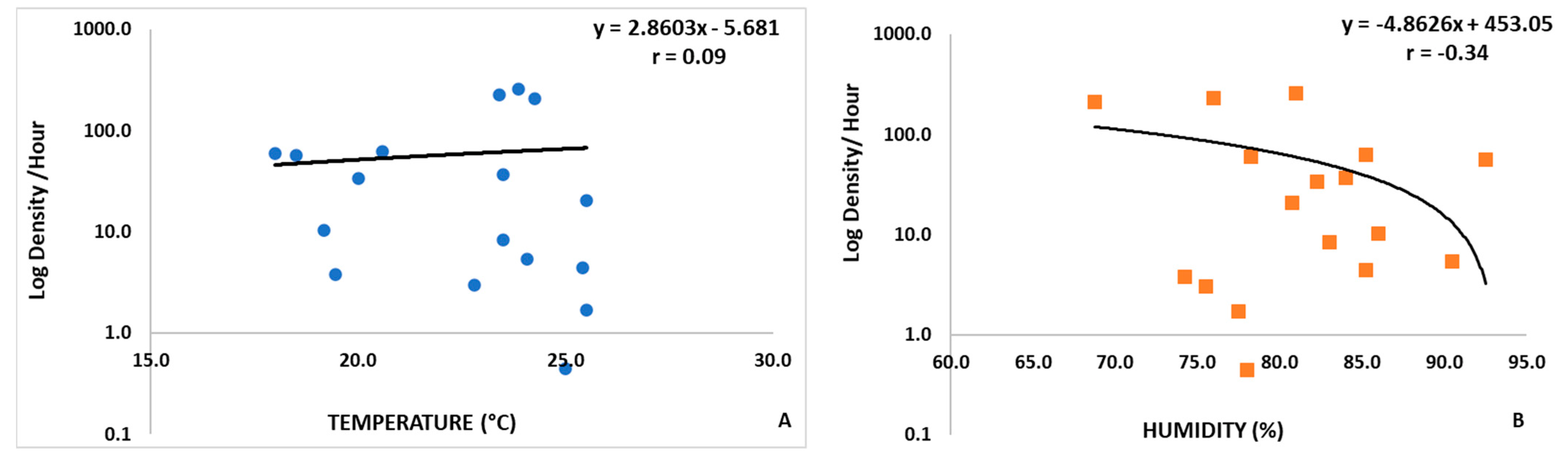
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Guimarães, L.H.; Machado, P.R.L.; Lessa, H.A.; Lessa, M.; D’Oliveira, A., Jr.; Carvalho, E.M. Aspectos Clínicos da Leishmaniose Tegumentar. Gaz. Médica Bahia 2005, 75, 66–74. [Google Scholar]
- Camargo-Neves, V.L.F.; Gomes, A.C.; Antunes, J.L.F. Correlação da presença de espécies de flebotomíneos (Diptera: Psychodidae) com registros de casos da leishmaniose tegumentar americana no Estado de São Paulo, Brasil. Rev. Soc. Bras. de Med. Trop. 2002, 35, 299–306. [Google Scholar] [CrossRef][Green Version]
- BRASIL. Ministério da Saúde (MS). Casos de Leishmaniose Tegumentar. Brasil, Grandes Regiões e Unidades Federadas. 1990 a 2019. [Internet]. 2019. Available online: https://antigo.saude.gov.br/images/pdf/2020/August/25/LT-Casos.pdf (accessed on 1 March 2021).
- Forattini, O.P.; Oliveira, O. Um foco de leishmaniose tegumentar na zona sul do Estado de São Paulo, Brasil. Arq. Fac. Hig. Saúde Pública 1957, 11, 23–34. [Google Scholar] [CrossRef]
- Gomes, A.C.; Camargo-Neves, V.L.F. Estratégias e perspectivas de controle da leishmaniose tegumentar americana no Estado de São Paulo, Brasil. Rev. Soc. Bras. Med. Trop. 1998, 31, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Da-Silva, L.M.R.; Cunha, P.R. A urbanização da leishmaniose tegumentar americana no município de Campinas—São Paulo (SP) e região: Magnitude do problema e desafios. An. Bras. Dermatol. 2007, 82, 515–519. [Google Scholar] [CrossRef]
- Condino, M.L.F.; Sampaio, S.M.P.; Henriques, L.F.; Galati, E.A.B.; Wanderley, D.M.V.; Corrêa, F.M.A. Leishmaniose tegumentar americana: Flebotomíneos de área de transmissão no município de Teodoro Sampaio, região sudoeste do estado de São Paulo, Brasil. Rev. Soc. Bras. Med. Trop. 1998, 31, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.F.; Carreri-Bruno, G.C.; Ciaravolo, R.M.C.; Galati, E.A.B.; Wanderley, D.M.V.; Corrêa, F.M.A. Leishmaniose tegumentar americana: Flebotomíneos de área de transmissão, no município de Pedro de Toledo, região sul do Estado de São Paulo, Brasil. Rev. Soc. Bras. Med. Trop. 1998, 31, 425–432. [Google Scholar] [CrossRef][Green Version]
- BRASIL. Climate-Data.org. Clima: Eldorado [Internet]. 2021. Available online: https://pt.climate-data.org/ (accessed on 1 March 2021).
- IBGE—Instituto Brasileiro de Geografia e Estatísticas. [Internet]. 2021. Available online: www.ibge.gov.br/cidadesat (accessed on 1 March 2021).
- Lepsch, I.F.; Saraiva, I.R.; Donzeli, P.L.; Marinho, M.A.; Sakai, E.; Guillaumon, J.R.; Pfeifer, R.M.; Mattos, I.F.A.; Andrade, W.J.; Silva, C.E.F. Macrozoneamento das Terras da Região do Rio Ribeira de Iguape, SP; Governo do Estado de São Paulo, Secretaria do Agricultura e Abastecimento, Coordenadoria da Pesquisa Agropecuária, Instituto Agronômico: São Paulo, Brasil, 1990; Volume 19, pp. 1–181. ISSN 0102-2032. [Google Scholar]
- Ferraz, M.V.; Barletta, G.A.; Tosetti, L.L.; Cofani-Nunes, J.V. Diagnóstico da arborizaçãourbana de Eldorado, São Paulo. Rev. Tree Dimens. ProFloresta 2017, 2, 22. [Google Scholar] [CrossRef]
- Shannon, R. Métodos para coleta e alimentação de mosquitos em estudos de febre amarela na selva. Am. J. Trop. Med. 1939, 19, 131–140. [Google Scholar] [CrossRef]
- Galati, E.A.B. Morfologia e taxonomia: Classificação de Phlebotominae. In Flebotomíneos do Brasil; Rangel, E.F., Lainson, R., Eds.; Editora Fiocruz: Rio de Janeiro, Brazil, 2003; pp. 23–51. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. User’s Guide and Application. 2013. Available online: http://purl.oclc.org/estimates (accessed on 9 March 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 9 March 2021).
- Sokal, R.R.; Rohlf, F.J. Biometria, Principios y Métodos Estadísticos em la Investigación Biológica; H Blume: Madrid, Spain, 1979; Volume XII, 832p, ISBN 84-721466-7. [Google Scholar]
- Forattini, O.P.; Rabello, E.X.; Serra, O.P.; Galati, E.A.B.; Barata, J.M.S. Observações sobre a transmissão da leishmaniose tegumentar no Estado de São Paulo, Brasil. Rev. Saúde Pública 1976, 10, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Forattini, O.P.; Santos, M.R. Nota sobre infecção natural de Phlebotomus intermedius Lutz & Neiva, 1912, por formas leptomonas, em um foco ativo de leishmaniose tegumentar americana. Arq. Hig. São Paulo 1952, 17, 171–174. [Google Scholar]
- Gomes, A.C.; Rabello, E.X.; Santos, J.L.F.; Galati, E.A.B. Aspectos ecológicos da leishmaniose tegumentar americana. 1- Estudo experimental da frequência de flebotomíneos em ecótopos artificiais com referência especial a Ps. intermedius. Rev. Saúde Pública 1980, 14, 540–546. [Google Scholar] [CrossRef]
- Gomes, A.C.; Galati, E.A.B. Aspectos ecológicos da leishmaniose tegumentar americana. 7. Capacidade vetorial flebotomínea em ambiente florestal primário do sistema da Serra do Mar, região do Vale do Ribeira, Estado de São Paulo, Brasil. Rev. Saúde Pública 1989, 23, 136–142. [Google Scholar] [CrossRef]
- Marcondes, C.B. A redescription of Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912), and resurrection of L. neivai (Pinto, 1926) (Diptera, Psychodidae, Phlebotominae). Memórias Inst. Oswaldo Cruz 1996, 91, 457–462. [Google Scholar] [CrossRef]
- Gomes, A.C.; Santos, J.L.F.; Galati, E.A.B. Ecological aspects of American cutâneos leishmaniasis. 4. Observations on the endophilic behavior of the sandfly and vectorial role of Psychodopygus intermedius in the RibeiraValey region of the S. Paulo State, Brazil. Rev. Saúde Pública 1986, 20, 280–287. [Google Scholar] [CrossRef]
- Gomes, A.C.; Rabello, E.X.; Santos, J.L.F.; Galati, E.A.B. Aspectos ecológicos da leishmaniose tegumentar americana. 2. Ecótopo artificial como abrigo de Psychodopygus intermedius e observações sobre alimentação e reprodução sob influência de fatores físicos naturais. Rev. Saúde Pública 1982, 16, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Camargo, N.J.; Santos, S.R.; Massafera, R.; Ferreira, A.C.; Postai, C.; Cristovao, E.C.; Konolsaisen, J.F.; Bisetto, A., Jr.; Perinazo, R.; et al. Diversidade, distribuição e abundância de flebotomíneos (Diptera: Psychodidae) no Paraná. Neotrop. Entomol. 2008, 37, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.S.; França-Silva, J.C.; Silva, J.C.; Monteiro, E.M.; Paula, K.M.; Gonçalves, C.M.; Barata, R.A. Flebotomíneos (Diptera: Psychodidae) de um foco de leishmaniose tegumentar no Estado de Minas Gerais. Rev. Soc. Bras. Med. Trop. 2007, 40, 49–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massafera, R.; Silva, A.L.; Carvalho, A.P.; Santos, D.R.; Galati, E.A.B.; Teodoro, U. Fauna de flebotomíneos do município de Bandeirantes, no Estado do Paraná. Rev. Saúde Pública 2005, 39, 571–577. [Google Scholar] [CrossRef][Green Version]
- Lainson, R.; Rangel, E.F. Ecologia das leishmanioses: Transmissores de leishmaniose tegumentar americana. In Flebotomíneos do Brasil; Rangel, E.F., Lainson, R., Eds.; Fiocruz: Rio de Janeiro, Brazil, 2003; pp. 291–309. [Google Scholar]
- Teodoro, U.; La Salvia Filho, E.M.L.; Misuta, N.M.; Verginassi, T.G.; Ferreira, M.E.M.C. Leismaniose tegumentar americana: Flebotomíneos de área de transmissão no Norte do Paraná, Brasil. Rev. Saúde Pública 1991, 25, 129–133. [Google Scholar] [CrossRef][Green Version]
- Andrade Filho, J.D.; Galati, E.A.B.; Falcão, A.L. Polymorphism, inter-population and inter-specific variation in Nyssomyia intermedia (Lutz & Neiva) and Nyssomyianeivai (Pinto) (Diptera, Psychodidae, Phlebotominae). Rev. Bras. Entomol. 2006, 50, 385–393. [Google Scholar] [CrossRef][Green Version]
- Andrade-Filho, J.D.; Galati, E.A.B.; Falcão, A.L. Nyssomyia intermedia (Lutz & Neiva, 1912) and Nyssomyia neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae) geographical distribution and epidemiological importance. Memórias Inst. Oswaldo Cruz 2007, 102, 481–487. [Google Scholar] [CrossRef]
- Forattini, O.P.; Pattoli, D.B.G.; Rebello, E.X.; Ferreira, A.O. Infecção natural de flebotomíneos em foco enzoótico de leishmaniose no estado de São Paulo, Brasil. Rev. Saúde Públ. 1972, 6, 431–433. Available online: https://www.scielosp.org/article/rsp/1972.v6n4/431-433/ (accessed on 9 March 2021).
- Rangel, E.F.; Sousa, N.A.; Wermelinger, E.D.; Barbosa, A.F. Infecção natural de Lutzomyia intermedia Lutz & Neiva, 1912, em área endêmica de leishmaniose tegumentar no Rio de Janeiro. Mem. Inst. Oswaldo Cruz 1984, 79, 395–396. [Google Scholar] [CrossRef]
- Casanova, C.; Mayo, R.C.; Rangel, O.; Mascarini, L.M.; Pignatti, M.G.; Galati, E.A.B.; Gomes, A.C. Natural Lutzomyia intermedia (Lutz & Neiva) infection in the Valley of the Mogi Guaçú River, State of São Paulo, Brazil. Bol. Dir. Malariol. San. Amb. 1995, 35, 77–84. Available online: https://scholar.google.com/citations?user=nNEEE4QAAAAJ&hl=pt-PT (accessed on 9 March 2021).
- Silva, A.C.; Gomes, A.C. Estudo da Competência vetorial de Lutzomyia intermedia (Lutz & Neiva, 1912) para Leishmania (Viannia) braziliensis, Vianna, 1911. Rev. Soc. Bras. Med. Trop. 2001, 34, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Pita-Pereira, D.; Alves, C.R.; Souza, M.B.; Brazil, R.P.; Bertho, A.L.; Barbosa, A.F.; Britto, C.C. Identifications of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridization assay. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 905–913. [Google Scholar] [CrossRef]
- Silva, O.S.; Grunewald, J. Contribution to the sandfly fauna (Diptera: Phlebotominae) of Rio Grande do Sul, Brazil and Leishmania (Viannia) infections. Mem. Inst. Oswaldo Cruz 1999, 94, 579–582. [Google Scholar] [CrossRef][Green Version]
- Cordoba-Lanus, E.; De Grosso, M.L.; Pinero, J.E.; Valladares, B.; Salomón, O.D. Natural infection of Lutzomyia neivai with Leishmania spp. in northwestwern Argentina. Acta Trop. 2006, 98, 1–5. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Reinhold-Castro, K.R.; Bernal, M.V.Z.; Legriffon, C.M.O.; Lonardoni, M.V.C.; Teodoro, U.; Silveira, T.G.V. Natural infection of Nyssomyia neivai by Leishmania (Viannia) spp. in the State of Paraná, Southern Brazil, detected by multiplex polymerase chain reaction. Vector-Borne Zoonotic Dis. 2011, 11, 137–143. [Google Scholar] [CrossRef]
- Marcondes, C.B.; Bittencourt, I.A.; Stoco, P.H.; Eger, I.; Grisard, E.C.; Steindel, M. Natural infection of Nyssomyia neivai (Pinto, 1926) (Diptera: Psychodidae, Phlebotominae) by Leishmania (Viannia) spp. in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1093–1097. [Google Scholar] [CrossRef]
- Casanova, C.; Costa, A.I.P.; Natal, D. Dispersal pattern of the sandfly Lutzomyia neivai (Diptera: Psychodidae) in a cutaneous leishmaniasis endemic rural area in Southeastern Brazil. Mem. Inst. Oswaldo Cruz 2005, 100. [Google Scholar] [CrossRef][Green Version]
- Gomes, A.C.; Barata, J.M.S.; Rocha e Silva, E.O.; Galati, E.A.B. Aspectos ecológicos da leishmaniose tegumentar americana. 6. Fauna flebotomínea antropofílica de matas residuais situadas na região centro-nordeste do estado de São Paulo. Rev. Inst. Med. Trop. São Paulo 1989, 31, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Galati, E.A.B.; Casanova, C.; Domingos, M.F.; Marques, G.R.A.M.; Camargo-Neves, V.L.F. Analysis of the geographical distribution of leishmaniasis vectors in the state of São Paulo Brazil. Bol. Dir. Malariol. San. Amb. 1995, 35 (Suppl. 1), 143–146. Available online: https://scholar.google.com.br/scholar?hl=pt-BR&as_sdt=0,5&cluster=5861626702299990072 (accessed on 9 March 2021).
- Gomes, A.C.; Galati, E.A.B. Aspectos ecológicos da leishmaniose tegumentar americana. 5. Estratificação de atividade espacial e estacional de Phlebotominae (Diptera, Psychodidae) em áreas de cultura agrícola da região do Vale do Ribeira, Estado de São Paulo, Brasil. Mem. Inst. Oswaldo Cruz 1987, 82, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.C.R.; Rangel, E.F.; Queiroz, R.G. Lutzomyia migonei (França, 1920) naturalmente infectado por flagelados peripilares em Baturité, foco de leishmaniose tegumentar no Estado do Ceará, Brasil. Mem. Inst. Oswaldo Cruz 1990, 85, 479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Queiroz, R.G.; Vasconcelos, I.A.B.; Vasconcelos, A.W.W.; Souza, R.N.; Fé Filho, N.M.; David, J.R. Infecções naturais em flebotomíneos de uma zona endêmica de leishmaniose tegumentar americana na serra de Baturité, Estado do Ceará, Nordeste do Brasil. Mem. Inst. Oswaldo Cruz 1991, 86, 256. [Google Scholar]
- Pessoa, S.B.; Coutinho, J.O. Infecção natural e experimental dos flebótomos pela Leishmania braziliensis, no Estado de São Paulo. O Hospital 1941, 20, 25–35. [Google Scholar]
- Young, D.G.; Arias, J.R. Flebotomíneos Vectores de Leishmaniasis en las Americas. OPAS Caderno Técnico 1992, 3, 28. [Google Scholar]
- Galati, E.A.B.; Fonseca, M.B.; Marassá, A.M.; Bueno, E.F.M. Dispersal and survival of Nyssomyia intermedia and Nyssomyia neivai (Diptera: Psychodidae, Phlebotominae) in a cutaneous leishmaniasis endemic area of the speleological province of Ribeira Valley, São Paulo State, Brasil. Mem. Inst. Oswaldo Cruz 2009, 104, 1148–1158. [Google Scholar] [CrossRef]
- Pita-Pereira, D.; Souza, G.D.; Pereira, T.A.; Zwetsch, A.; Brito, C.; Rangel, E.F. Lutzomyia (Pintomyia) fischeri (Diptera: Psychodidae: Phlebotominae), a probable vector of American Cutaneous Leishmaniasis: Detection of natural infection by Leishmania (Viannia) DNA in specimens from the municipality of Porto Alegre (RS), Brazil, using multiplex PCR assay. Acta Trop. 2011, 120, 273–275. [Google Scholar] [CrossRef]
- Diniz, M.M.C.D.S.L.; GalvisOvallos, F.; de Castro Gomes, C.M.; de Oliveira Lavitschka, C.; Galati, E.A.B. Host-biting rate and susceptibility of some suspected vectors to Leishmania braziliensis. Parasites Vectors 2014, 7, 139. [Google Scholar] [CrossRef]
- Leal MMCS. Estudo da Capacidade Vetorial de Pintomyua Fischeri (Pinto) (Diptera: Psychodidae) para Leishmania(Vianna) Braziliensis Viana. Brasil [Dissertação de Mestrado]; Faculdade de Saúde Pública Universidade de São Paulo: São Paulo, Brazil, 2013; Available online: https://teses.usp.br/teses/disponiveis/6/6132/tde-15082014-104825/pt-br.php (accessed on 9 March 2021).
- Moschin, J.C.; Ovallos, F.G.; Sei, I.A.; Galati, E.A.B. Ecological aspects of phlebotomine fauna (Diptera, Psychodidae) of Serra da Cantareira, Greater São Paulo Metropolitan region, state of São Paulo, Brazil. Rev. Bras. Epidemiol. 2013, 16, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, V.C.F.V.; Pruzinova, K.; Sadlova, J.; Volfova, V.; Myskova, J.; Brandão Filho, S.P.; Volf, P. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasites Vectors 2016, 9, 159. [Google Scholar] [CrossRef]
- Galvis-Ovallos, F.; Ueta, A.E.; Marques, G.O.; Sarmento, A.M.C.; Araujo, G.; Sandoval, C.; Tomokane, T.Y.; da Matta, V.L.R.; Laurenti, M.D.; Galati, E. Aparecida Bianchi Detection of Pintomyia fischeri (Diptera: Psychodidae) With Leishmania infantum (Trypanosomatida: Trypanosomatidae) Promastigotes in a Focus of Visceral Leishmaniasis in Brazil. J. Med. Entomol. 2021, 58, 830–836. [Google Scholar] [CrossRef]
- Galvis-Ovallos, F.; Silva, M.; Bispo, G.; Oliveira, A.; Gonçalves Neto, J.R.; Malafronte, R. “Canine visceral leishmaniasis in the metropolitan area of São Paulo: Pintomyia fischeri as potential vector of Leishmania infantum.” “Leishmaniose viscérale canine dans la région métropolitaine de São Paulo: Pintomyia fischeri comme vecteur potentiel de Leishmania infantum. Parasite 2017, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, P.H.F.; Galati, E.A.B. Lista de espécies de Phlebotominae (Diptera, Psychodidae) do Estado de São Paulo, Brasil, com comentários sobre sua distribuição geográfica. Biota Neotro 2011, 11 (Suppl. 1), 685–704. [Google Scholar] [CrossRef][Green Version]
- Camargo-Neves, V.L.F. Características da transmissão da Leishmaniose Tegumentar Americana no Estado de São Paulo, Brasil [Dissertação de Mestrado]; Faculdade de Saúde Pública Universidade de São Paulo: São Paulo, Brazil, 1999; Available online: https://scholar.google.com.br/citations?user=B4_Dz7cAAAAJ&hl=pt-BR (accessed on 9 March 2021).
- Eckert, J.; Souza, G.D. Flebotomíneos (Diptera: Psychodidae: Phlebotominae) no município de Estrela e primeiro registro de Lutzomyia pascalei (Coutinho & Barretto) no Rio Grande do Sul. Rev. Bras. Bioci. 2010, 8, 399–402. Available online: http://www.ufrgs.br/seerbio/ojs/index.php/rbb/article/view/1555 (accessed on 9 March 2021).
- Salomón, O.D.; Rossi, G.C.; Spinelli, G.R. Ecological aspects of phlebotomine ((Diptera, Psychodidae) in an endemic area of tegumentary leishmaniasis in the Northeastern Argentina, 1993–1998. Mem. Inst. Oswaldo Cruz 2002, 97, 163–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scorza, J.V.; Ortiz, I.; Gomez, I. Observaciones biologicas sobre algunos flebotomos de Rancho Grande (Venezuela). 6- Sobre los factores microclimáticos que determinan la endemicidad de la flebotomo fauna de “Rancho Grande”. Acta Biol. Venez. 1968, 6, 76–83. [Google Scholar]
- Miscevic, Z. Dependence of the flight of sandflies (Diptera, Phlebotomidae) in artificial light on the temperature and relative humidity. Acta Vet. 1981, 31, 32–39. [Google Scholar]
- Aguiar, A.M.; Soucasaux, T. Aspectos da ecologia dos flebótomos do Parque Nacional da Serra dos Órgãos, Rio de Janeiro: I- Frequência mensal em isca humana (Diptera, Psychodidae, Phlebotominae). Mem. Inst. Oswaldo Cruz 1984, 79, 179–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeledon, R.; Murrillo, J.; Gutierrez, H. Observaciones sobre la ecologia de Lutzomyia longipalpis (Lutz & Neiva, 1912) y posibilidades de existencia de leishmaniasis visceral en Costa Rica. Mem. Inst. Oswaldo Cruz 1984, 79, 455–459. [Google Scholar] [CrossRef][Green Version]
- Barata, R.A.; França-Silva, J.C.; Fortes-Dias, C.L.; Costa, R.T.; Silva, J.C.; Vieira, E.P.; Prata, A.; Michalsky, E.M.; Dias, E.S. Phlebotomine sandflies in Porteirinha, an endemic area of American visceral leishmaniasis in the State of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2004, 99, 481–487. Available online: https://www.scielo.br/j/mioc/a/ZWGRfh78ZM7N3CHj6Cr7Nzp/?lang=en&format=pdf (accessed on 9 March 2021).
- Rutledge, L.C.; Ellenwood, D.A. Production of phlebotomine sandflies on the open forest floor in Panama: The species complement. Environ. Entomol. 1975, 4, 71–77. [Google Scholar] [CrossRef]
| SPECIES | ♀ | ♂ | ∑ | % |
|---|---|---|---|---|
| Nyssomyia intermedia | 3851 | 4633 | 8484 | 73.3 |
| Nyssomyia neivai | 1280 | 1676 | 2956 | 25.5 |
| Mygonemyia migonei | 61 | 27 | 88 | 0.8 |
| Pintomyia fischeri | 23 | 13 | 36 | 0.3 |
| Psathyromyia pascalei | 14 | 0 | 14 | 0.1 |
| TOTAL | 5229 | 6349 | 11578 | 100.0 |
| Shannon index (H) | 0.63 | |||
| Simpson index (λ) | 0.60 | |||
| Gini–Simpson index (1 − λ) | 0.40 | |||
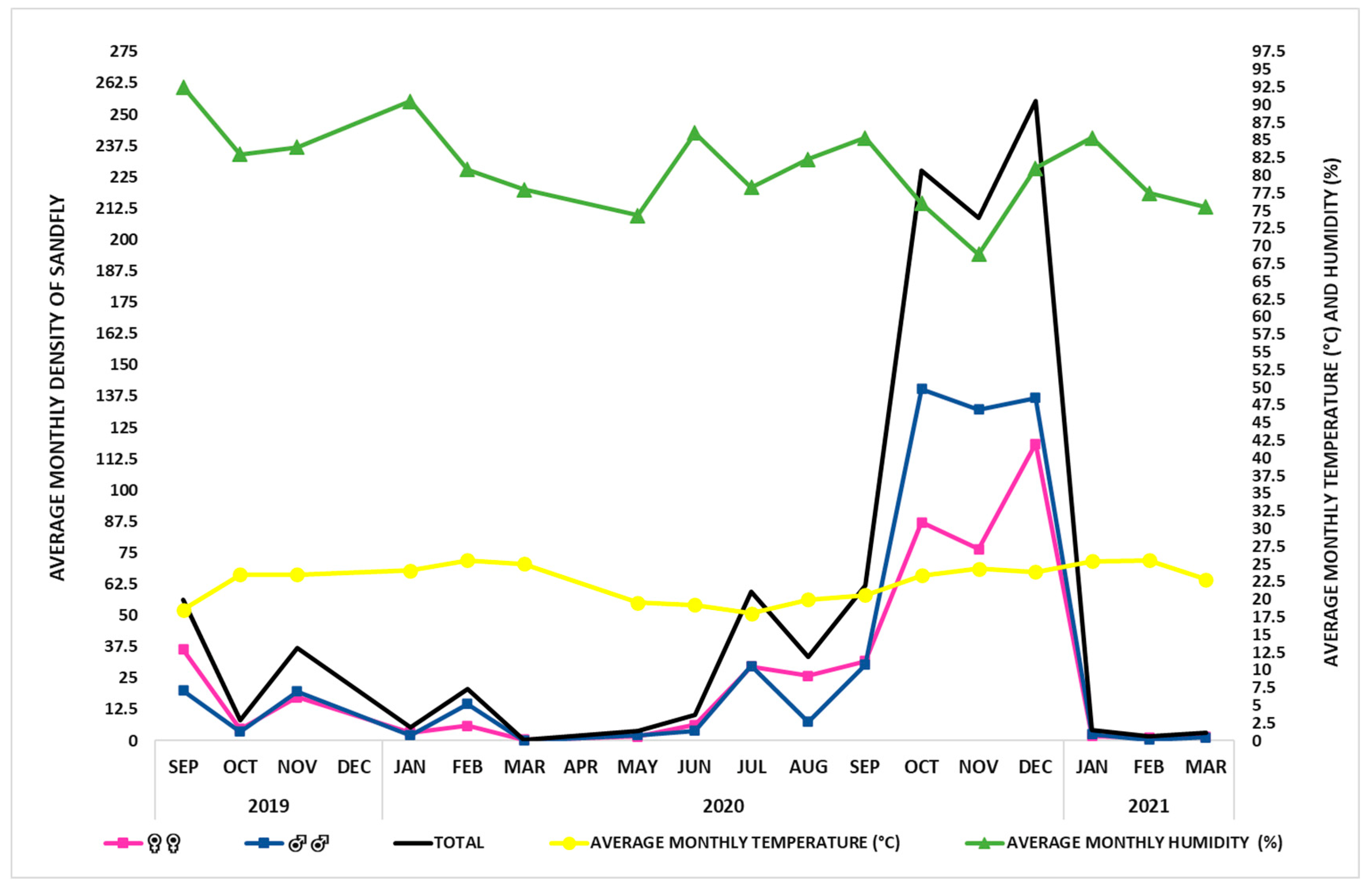
| Year | Month | Monthly Average | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Humidity (%) | Nyssomyia intermedia | Nyssomyia neivai | Sandfly Total | ||
| 2019 | SEP | 18.5 | 92.5 | 36.9 | 19.2 | 56.4 |
| OCT | 23.5 | 83.0 | 4.6 | 3.4 | 8.3 | |
| NOV | 23.5 | 84.0 | 24.6 | 9.2 | 37.0 | |
| DEC | - | - | - | - | - | |
| 2020 | JAN | 24.1 | 90.5 | 2.9 | 0.2 | 5.3 |
| FEB | 25.5 | 80.8 | 16.2 | 4.1 | 20.6 | |
| MAR | 25.0 | 78.0 | 0.4 | 0.0 | 0.4 | |
| APR | - | - | - | - | - | |
| MAY | 19.5 | 74.3 | 3.4 | 0.4 | 3.8 | |
| JUN | 19.2 | 86.0 | 7.6 | 2.6 | 10.3 | |
| JUL | 18.0 | 78.3 | 51.7 | 6.6 | 59.4 | |
| AUG | 20.0 | 82.3 | 28.9 | 4.4 | 33.4 | |
| SEP | 20.6 | 85.3 | 44.4 | 17.3 | 62.1 | |
| OCT | 23.4 | 76.0 | 160.3 | 62.3 | 227.5 | |
| NOV | 24.3 | 68.8 | 157.7 | 49.3 | 208.5 | |
| DEC | 23.9 | 81.0 | 178.9 | 72.2 | 255.3 | |
| 2021 | JAN | 25.4 | 85.3 | 2.4 | 2.1 | 4.4 |
| FEB | 25.5 | 77.5 | 1.1 | 0.6 | 1.7 | |
| MAR | 22.8 | 75.5 | 3.0 | 1.2 | 3.0 | |
| Average total | 22.4 | 81.1 | 42.6 | 15.0 | 58.7 | |
| Minimum and Maximum values | (18.0–25.5) | (68.8–92.5) | (0.4–178.9) | (0.0–72.2) | (0.4–255.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, B.F.G.; de Fátima Domingos, M.; Ovallos, F.G.; de Camargo-Neves, V.L.F. Updating Ecological and Behavioral Aspects of the Sandfly Fauna in the Vale do Ribeira Region, São Paulo State, Brazil. Insects 2021, 12, 988. https://doi.org/10.3390/insects12110988
Oliveira BFG, de Fátima Domingos M, Ovallos FG, de Camargo-Neves VLF. Updating Ecological and Behavioral Aspects of the Sandfly Fauna in the Vale do Ribeira Region, São Paulo State, Brazil. Insects. 2021; 12(11):988. https://doi.org/10.3390/insects12110988
Chicago/Turabian StyleOliveira, Byara Freitas Guedes, Maria de Fátima Domingos, Fredy Galvis Ovallos, and Vera Lucia Fonseca de Camargo-Neves. 2021. "Updating Ecological and Behavioral Aspects of the Sandfly Fauna in the Vale do Ribeira Region, São Paulo State, Brazil" Insects 12, no. 11: 988. https://doi.org/10.3390/insects12110988
APA StyleOliveira, B. F. G., de Fátima Domingos, M., Ovallos, F. G., & de Camargo-Neves, V. L. F. (2021). Updating Ecological and Behavioral Aspects of the Sandfly Fauna in the Vale do Ribeira Region, São Paulo State, Brazil. Insects, 12(11), 988. https://doi.org/10.3390/insects12110988







