Effectiveness of Different Soft Acaricides against Honey Bee Ectoparasitic Mite Varroa destructor (Acari: Varroidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Honey Bee Colonies
2.3. Soft Acaricide Treatments
2.3.1. Formic Acid (AnalaR 98/100% ‘Safe-Break’)
2.3.2. Oxalic Acid (AnalaR)
2.3.3. Thymol (GPR)
2.4. Mite Collection
2.5. Honey Yield
2.6. Statistical Analysis
3. Results
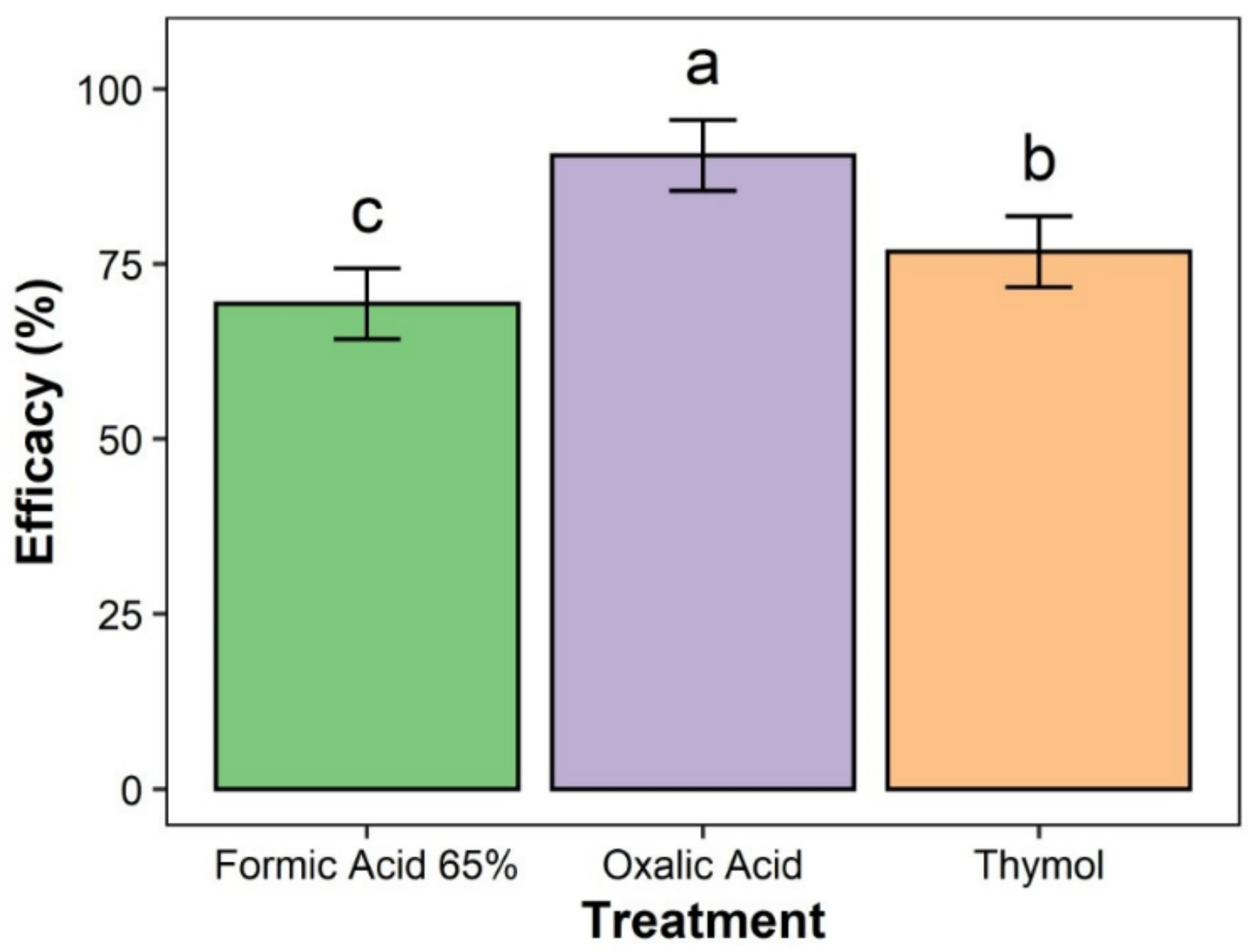
3.1. Treatment Effectiveness among Major Groups (Formic Acid 65%, Oxalic Acid, and Thymol)
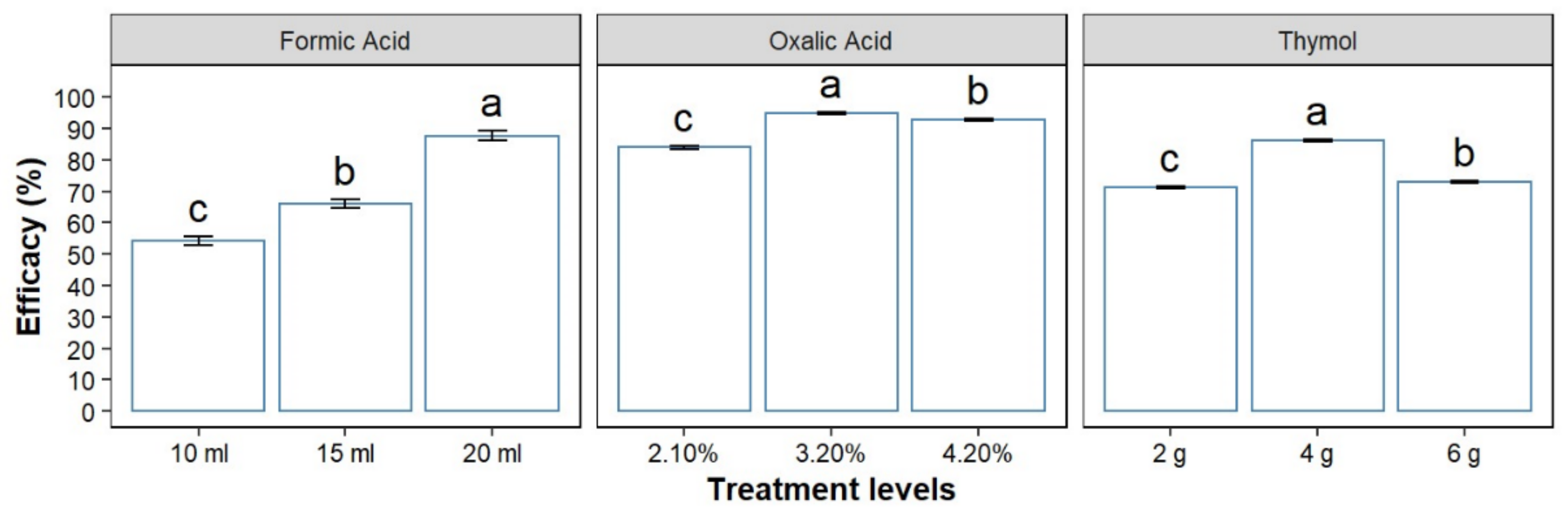
3.2. Treatment Effectiveness within All Concentrations/Quantities
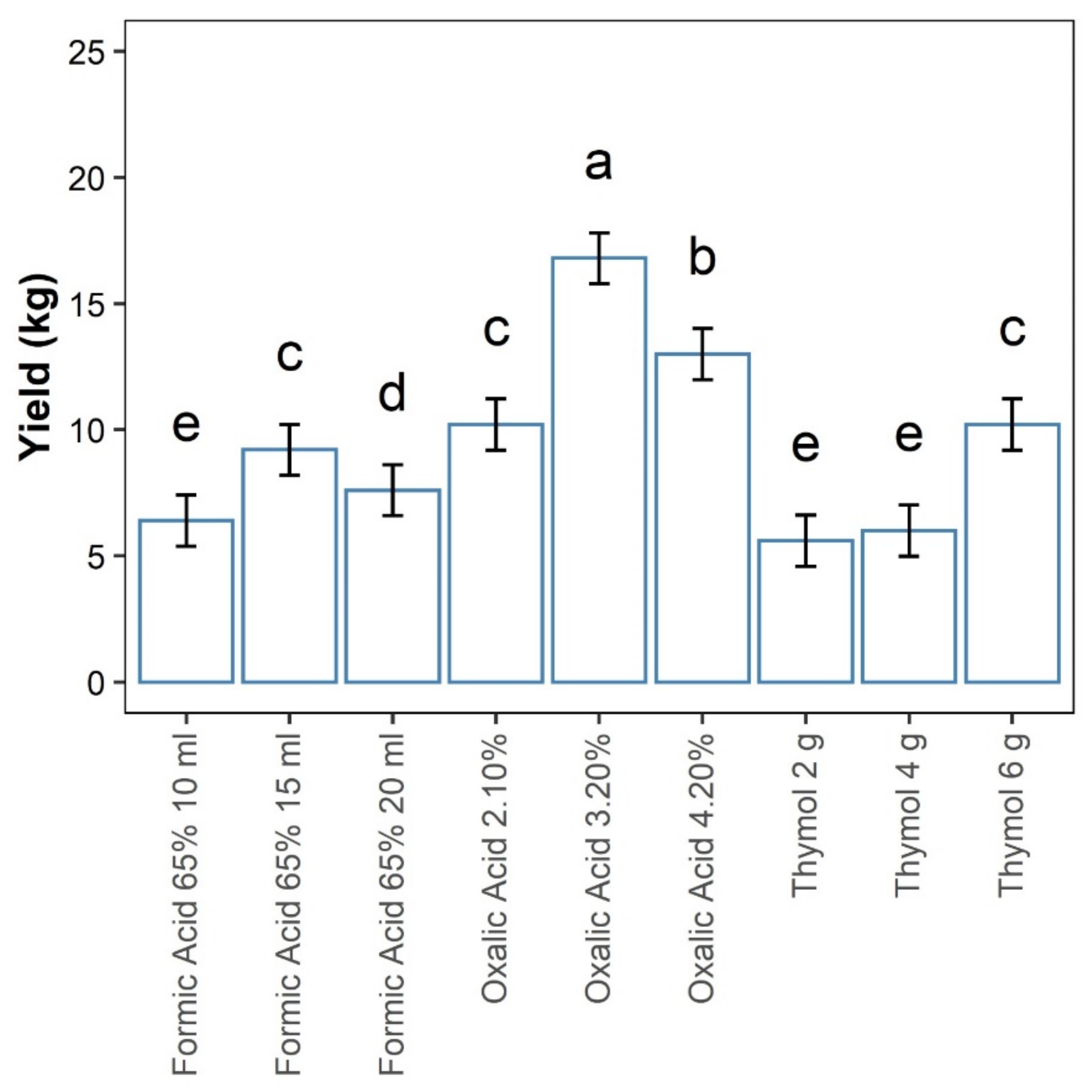
3.3. Honey Yield
All Concentrations/Quantities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, R.; Dhami, M.; Mathur, V.; Bisht, B. Efficacy of Animal Origin Products and Ajwain Powder against Honey Bee Diseases in Apis mellifera (Linnaeus) Colonies in Uttarakhand-A Novel Eco-Friendly Approach. JANS 2014, 6, 68–75. [Google Scholar] [CrossRef]
- Bakar, M.A.; Aqueel, M.A.; Raza, A.B.M.; Mahmood, R.; Qadir, Z.A.; Arshad, M.; Sohail, M. Evaluation of Essential Oils for the Management of Parasitic Bee Mites, Varroa destructor (Acari: Varroidae) In Vitro. PJAR 2019, 32, 566–571. [Google Scholar] [CrossRef]
- Muzaffar, N.; Wells, H. Beekeeping in Pakistan. Am. Bee J. 2002, 142, 339–342. [Google Scholar]
- PARC. Annual Report 2010–2011; Pakistan Agricultural Research Council: Islamabad, Pakistan, 2011; p. 98.
- Mahmood, R.; Wagchoure, E.S.; Raja, S.; Sarwar, G. Control of Varroa destructor Using Oxalic Acid, Formic Acid and Bayvarol Strip in Apis mellifera (Hymenoptera: Apidae) Colonies. Pak. J. Zool. 2012, 44, 1473–1476. [Google Scholar]
- Comtrade, U. Download Trade Data | UN Comtrade: International Trade Statistics. Available online: https://comtrade.un.org/data/ (accessed on 5 October 2021).
- Chantawannakul, P.; de Guzman, L.I.; Li, J.; Williams, G.R. Parasites, Pathogens, and Pests of Honeybees in Asia. Apidologie 2016, 47, 301–324. [Google Scholar] [CrossRef]
- Sajid, Z.N.; Aziz, M.A.; Bodlah, I.; Rana, R.M.; Ghramh, H.A.; Khan, K.A. Efficacy Assessment of Soft and Hard Acaricides against Varroa destructor Mite Infesting Honey Bee (Apis mellifera) Colonies, through Sugar Roll Method. Saudi J. Biol. Sci. 2020, 27, 53–59. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Alattal, Y.; Al-Ghamdi, A.; Single, A.; Ansari, M.J.; Alkathiri, H. Fertility and Reproductive Rate of Varroa Mite, Varroa destructor, in Native and Exotic Honeybee, Apis mellifera L., Colonies under Saudi Arabia Conditions. Saudi J. Biol. Sci. 2017, 24, 992–995. [Google Scholar] [CrossRef]
- De Jong, D. Mites: Varroa and Other Parasites of Brood. In Honey Bee Pests, Predators, and Diseases, 2nd ed.; Morse, R.A., Flottum, K., Eds.; A.I. Root Co.: Medina, OH, USA, 1997; pp. 279–327. [Google Scholar]
- Ahmad, R. Honeybee parasitic mites and their control in Pakistan. In Progressive Farming; Pakistan Agricultural Research Council-Publications: Islamabad, Pakistan, 1988; Volume 8, pp. 34–36. [Google Scholar]
- Wagchoure-Camphor, E.S.; Martin, S.J. Beekeeping in Pakistan: A Bright Future in a Troubled Land. Am. Bee J. 2008, 148, 726–728. [Google Scholar]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Boecking, O.; Genersch, E. Varroosis–the Ongoing Crisis in Bee Keeping. J. Verbr. Lebensm. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Rademacher, E.; Harz, M. Oxalic Acid for the Control of Varroosis in Honey Bee Colonies–A Review. Apidologie 2006, 37, 98–120. [Google Scholar] [CrossRef]
- Khan, M.S.; Shah, M.M.; Mahmood, Q.; Hassan, A.; Akbar, K. Assessment of Pesticide Residues on Selected Vegetables of Pakistan. J. Chem. Soc. Pak. 2011, 33, 816–821. [Google Scholar]
- Idrees, A.; Qasim, M.; Ali, H.; Qadir, Z.A.; Idrees, A.; Bashir, M.H. Acaricidal Potential of Some Botanicals against the Stored Grain Mites, Rhizoglyphus tritici. J. Entomol. Zool. Stud. 2016, 4, 611–617. [Google Scholar]
- Idrees, A.; Zhangh, H.; Luo, M.; Thu, M.; Cai, P.; Islam, W.; Hussain, M.; Chen, J.; JI, Q. Protein Baits, Volatile Compounds and Irradiation Influence the Expression Profiles of Odorant Binding Protein Genes in Bactrocera dorsalis (Diptera: Tephritidae). Appl. Ecol. Env. Res. 2017, 15, 1883–1899. [Google Scholar] [CrossRef]
- Cai, P.; Gu, X.; Yao, M.; Zhang, H.; Huang, J.; Idress, A.; Ji, Q.; Chen, J.; Yang, J. The Optimal Age and Radiation Dose for Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) Eggs as Hosts for Mass-Reared Fopius arisanus (Sonan) (Hymenoptera: Braconidae). Biol. Control 2017, 108, 89–97. [Google Scholar] [CrossRef]
- Gu, X.; Cai, P.; Yang, Y.; Yang, Q.; Yao, M.; Idrees, A.; Ji, Q.; Yang, J.; Chen, J. The Response of Four Braconid Parasitoid Species to Methyl Eugenol: Optimization of a Biocontrol Tactic to Suppress Bactrocera dorsalis. Biol. Control 2018, 122, 101–108. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, H.; Du, Y.; Idrees, A.; He, L.; Chen, J.; Ji, Q. Molecular Identification of Cultivable Bacteria in the Gut of Adult Bactrocera tau (Walker) and Their Trapping Effect: Trapping Effect of Cultivable Bacteria from Bactrocera tau. Pest. Manag. Sci. 2018, 74, 2842–2850. [Google Scholar] [CrossRef]
- Loglio, G.; Plebani, G. Valutazione Dell’efficacia Dell’Apistan. Apic.-Mod. 1992, 83, 95–98. [Google Scholar]
- Milani, N. Possible Presence of Fluvalinate-Resistant Strains of Varroa jacobsoni in Northern Italy. In New perspectives on Varroa, Proceedings of the International Meeting, Prague, Czech Republic, November 1993; Matheson, A., Ed.; International Bee Research Association: Cardiff, UK, 1994; p. 87. Available online: https://hal.archives-ouvertes.fr/hal-00891580/document (accessed on 24 September 2021).
- Elzen, P.J.; Eischen, F.A.; Baxter, J.R.; Elzen, G.W.; Wilson, W.T. Detection of Resistance in US Varroa jacobsoni Oud. (Mesostigmata: Varroidae) to the Acaricide Fluvalinate. Apidologie 1999, 30, 13–17. [Google Scholar] [CrossRef]
- Elzen, P.J.; Baxter, J.R.; Spivak, M.; Wilson, W.T. Amitraz Resistance in Varroa: New Discovery in North America. Am. Bee J. 1999, 139, 362. [Google Scholar]
- Lodesani, M.; Colombo, M.; Spreafico, M. Ineffectiveness of Apistan Treatment against the Mite Varroa jacobsoni Oud in Several Districts of Lombardy (Italy). Apidologie 1995, 26, 67–72. [Google Scholar] [CrossRef]
- Milani, N. The Resistance of Varroa jacobsoni Oud. to Acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Trouiller, J. Monitoring Varroa jacobsoni Resistance to Pyrethroids in Western Europe. Apidologie 1998, 29, 537–546. [Google Scholar] [CrossRef]
- Gashout, H.A.; Guzman-Novoa, E.; Goodwin, P.H. Synthetic and Natural Acaricides Impair Hygienic and Foraging Behaviors of Honey Bees. Apidologie 2020, 51, 1155–1165. [Google Scholar] [CrossRef]
- Flamini, G. Acaricides of Natural Origin, Personal Experiences and Review of Literature (1990-2001). Stud. Nat. Prod. Chem. 2003, 28, 381–451. [Google Scholar] [CrossRef]
- TECA-FAO. Varroosis: A Parasitic Disease of the Brood and Adult Honeybees. Available online: https://www.fao.org/teca/en/technologies/8416 (accessed on 6 November 2021).
- Islam, N.; Amjad, M.; Ehsan-ul-Haq, S.E.; Naz, F. Management of Varroa destructor by essential oils and formic acid in Apis mellifera Linn. Colonies. J. Entomol. Zool. Stud. 2016, 4, 97–104. [Google Scholar]
- Fries, I. Treatment of Sealed Honey-Bee Brood with Formic-Acid for Control of Varroa jacobsoni. Am. Bee J. 1991, 131, 313–314. [Google Scholar]
- Bogdanov, S.; Imdorf, A.; Kilchenmann, V. Residues in Wax and Honey after Apilife VAR Treatment. Apidologie 1998, 513–524. [Google Scholar] [CrossRef]
- Bogdanov, S.; Charrière, J.-D.; Imdorf, A.; Kilchenmann, V.; Fluri, P. Determination of Residues in Honey after Treatments with Formic and Oxalic Acid under Field Conditions. Apidologie 2002, 33, 399–409. [Google Scholar] [CrossRef]
- Floris, I.; Satta, A.; Cabras, P.; Garau, V.L.; Angioni, A. Comparison Between Two Thymol Formulations in the Control of Varroa destructor: Effectiveness, Persistence, and Residues. J. Econ. Entomol. 2004, 97, 5. [Google Scholar] [CrossRef]
- Baggio, A.; Arculeo, P.; Nanetti, A.; Marinelli, E.; Mutinelli, F. Field Trials with Different Thymol-Based Products for the Control of Varroosis. Am. Bee J. 2004, 144, 395–400. [Google Scholar]
- Imdorf, A.; Charrière, J.-D.; Bachofen, B. Efficency Checking of the Varroa jacobsoni Control Methods by Means of Oxalic Acid. Apiacta 1997, 3, 89–91. [Google Scholar]
- Brødsgaard, C.J.; Jansen, S.E.; Hansen, C.W.; Hansen, H. Spring Treatment with Oxalic Acid in Honey Bee Colonies as Varroa Control. DIAS Rep. Hortic. 1999, 6, 16. [Google Scholar]
- Gregorc, A.; Adamczyk, J.; Kapun, S.; Planinc, I. Integrated Varroa Control in Honey Bee (Apis mellifera carnica ) Colonies with or without Brood. J. Apic. Res. 2016, 55, 253–258. [Google Scholar] [CrossRef]
- Mahmood, R.; Wagchoure, E.S.; Raja, S.; Sarwar, G.; Aslam, M. Effect of Thymol and Formic Acid Against Ectoparasitic Brood Mite Tropilaelaps clareae in Apis mellifera Colonies. Pak. J. Zool. 2011, 43, 91–95. [Google Scholar]
- Prandin, L.; Dainese, N.; Girardi, B.; Damolin, O.; Piro, R.; Mutinelli, F. A Scientific Note on Long-Term Stability of a Home-Made Oxalic Acid Water Sugar Solution for Controlling Varroosis. Apidologie 2001, 32, 451–452. [Google Scholar] [CrossRef][Green Version]
- Mahmood, R.; Wagchoure, E.S.; ul Mohsin, A.; Raja, S.; Sarwar, G. Control of Ectoparasitic Mites in Honeybee (Apis mellifera L.) Colonies by Using Thymol and Oxalic Acid. Pak. J. Zool. 2012, 44, 985–989. [Google Scholar]
- Bakar, M.A.; Aqueel, M.A.; Raza, A.B.M.; Arshad, M.; Mahmood, R.; Qadir, Z.A. Comparative Efficacy of Five Commercial Synthetic Acaricides against Varroa destructor (Anderson and Trueman) in Apis mellifera L. Colonies. PJZ 2018, 50, 857–861. [Google Scholar] [CrossRef]
- Mahmood, R.; Asad, S.; Ahmad, W.; Sarwar, G.; Rafique, M.K.; Islam, N.; Qadir, Z.A.; Abiden, Z.U. Efficacy of Screen Bottom Board Tray with and without Soft Chemicals for Controlling Varroa destructor in Honeybee Colonies. PJZ 2017, 49, 9–13. [Google Scholar] [CrossRef]
- Mahmood, R.; Abu Bakar, M.; Raza, M.F.; Qadir, Z.A.; Yahya, M. Efficacy of Naturally Occurring Chemicals for the Integrated Control of Varroa destructor (Anderson and Trueman) in Honeybee Colonies. PJZ 2021, 53, 1173–1176. [Google Scholar] [CrossRef]
- Rashid, M.; Wagchoure, E.S.; Mohsin, A.U.; Raja, S.; Sarwar, G. Control of Ectoparasitic Mite Varroa destructor in Honeybee (Apis mellifera L.) Colonies by Using Different Concentrations of Oxalic Acid. J. Anim. Plant Sci. 2012, 22, 72–76. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.1. 0 2019. Available online: https://cran.r-project.org/web/packages/DHARMa/index.html (accessed on 20 October 2020).
- Russell, L. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.4. 2 2019. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 15 August 2020).
- Jelihovschi, E.; Faria, J.C.; Allaman, I.B. ScottKnott: A Package for Performing the Scott-Knott Clustering Algorithm in R. Tend. Mat. Apl. Comput. 2014, 15, 3. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2-Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Papežíková, I.; Palíková, M.; Kremserová, S.; Zachová, A.; Peterová, H.; Babák, V.; Navrátil, S. Effect of Oxalic Acid on the Mite Varroa destructor and Its Host the Honey Bee Apis mellifera. J. Apic. Res. 2017, 56, 400–408. [Google Scholar] [CrossRef]
- Toomemaa, K. The Synergistic Effect of Weak Oxalic Acid and Thymol Aqueous Solutions on Varroa mites and Honey Bees. J. Apic. Res. 2019, 58, 37–52. [Google Scholar] [CrossRef]
- Barbero, R.; Panella, F.; Bonizzoni, L. Ácido Oxálico y El Tratamiento de Limpieza Radical de Otoño-Invierno. Vida Apícola 1997, 85, 8–13. [Google Scholar]
- Imdorf, A.; Kilchenmann, V.; Bogdanov, S.; Bachofen, B.; Beretta, C. Toxizität von Thymol, Campher, Menthol und Eucalyptol auf Varroa jacobsoni Oud und Apis mellifera L im Labortest. Apidologie 1995, 26, 27–31. [Google Scholar] [CrossRef]
- Calderone, N.W.; Wilson, W.T.; Spivak, M. Plant Extracts Used for Control of the Parasitic Mites Varroa jacobsoni (Acari: Varroidae) and Acarapis woodi (Acari: Tarsonemidae) in Colonies of Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 1997, 90, 1080–1086. [Google Scholar] [CrossRef]
- Imdorf, A.; Charrière, J.-D.; Kilchenmann, V.; Bogdanov, S.; Fluri, P. Alternative Strategy in Central Europe for the Control of Varroa destructor in Honey Bee Colonies. APIACTA 2003, 38, 258–278. [Google Scholar]
- Imdorf, A.; Calderone, N.W. Use of Essential Oils for the Control of Varroa jacobsoni Oud. in Honey Bee Colonies. Apidologie 1999, 30, 209–228. [Google Scholar] [CrossRef]
- Melathopoulos, A.P.; Gates, J. Comparison of Two Thymol-Based Acaricides, Apilifevar and Apiguard, for the Control of Varroa Mites. Am. Bee J. 2003, 143, 489–493. [Google Scholar]
- Rashid, M.; Asad, S.; Sarwar, G.; Ahmad, W. Control of Varroa destructor Mite by Using Oxalic Acid, Formic Acid in Honey Bee Apis mellifera L. Colonies in Pakistan. World Appl. Sci. J. 2013, 26, 5. [Google Scholar]
- Fries, I. Short-Interval Treatments with Formic Acid for Control of Varroa jacobsoni in Honey Bee (Apis mellifera) Colonies in Cold Climates. Swed. J. Agric. Res. 1989, 19, 213–216. [Google Scholar]
- Underwood, R.M.; Currie, R.W. The Effects of Temperature and Dose of Formic Acid on Treatment Efficacy against Varroa destructor (Acari: Varroidae), a Parasite of Apis mellifera (Hymenoptera: Apidae). Exp. Appl. Acarol. 2003, 29, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.A.; Parkman, J.P.; Studer, M.D. Evaluation of Honey Bee Miticides, Including Temporal and Thermal Effects on Formic Acid Gel Vapours, in the Central South-Eastern USA. J. Apic. Res. 2001, 40, 81–89. [Google Scholar] [CrossRef]
- Maul, V.; Petersen, N.; Wisssen, W. Field Trials Using Formic Acid as a Varroatosis Therapy. Allg. Dtsch. Imkerztg. 1980, 14, 155–157. [Google Scholar]
- Eischen, F.A. Trials (and Tribulations) with Formic Acid for Varroa Control. Am. Bee J. 1998, 138, 734–735. [Google Scholar]
- Giusti, M.; Sabelli, C.; Di Donato, A.; Lamberti, D.; Paturzo, C.E.; Polignano, V.; Lazzari, R.; Felicioli, A. Efficacy and Safety of Varterminator, a New Formic Acid Medicine against the Varroa mite. J. Apic. Res. 2017, 56, 162–167. [Google Scholar] [CrossRef]
- Genc, F.; Aksoy, A. Some of the Correlations between the Colony Development and Honey Production on the Honeybee (Apis mellifera L.) Colonies. Apiacta 1993, 28, 33–41. [Google Scholar]
- Tlak Gajger, I.; Svečnjak, L.; Bubalo, D.; Žorat, T. Control of Varroa destructor Mite Infestations at Experimental Apiaries Situated in Croatia. Diversity 2020, 12, 12. [Google Scholar] [CrossRef]
- Pietropaoli, M.; Tlak Gajger, I.; Costa, C.; Gerula, D.; Wilde, J.; Adjlane, N.; Aldea-Sánchez, P.; Smodiš Škerl, M.I.; Bubnič, J.; Formato, G. Evaluation of Two Commonly Used Field Tests to Assess Varroa destructor Infestation on Honey Bee (Apis mellifera) Colonies. Appl. Sci. 2021, 11, 4458. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, Z.A.; Idrees, A.; Mahmood, R.; Sarwar, G.; Bakar, M.A.; Ahmad, S.; Raza, M.M.; Li, J. Effectiveness of Different Soft Acaricides against Honey Bee Ectoparasitic Mite Varroa destructor (Acari: Varroidae). Insects 2021, 12, 1032. https://doi.org/10.3390/insects12111032
Qadir ZA, Idrees A, Mahmood R, Sarwar G, Bakar MA, Ahmad S, Raza MM, Li J. Effectiveness of Different Soft Acaricides against Honey Bee Ectoparasitic Mite Varroa destructor (Acari: Varroidae). Insects. 2021; 12(11):1032. https://doi.org/10.3390/insects12111032
Chicago/Turabian StyleQadir, Ziyad Abdul, Atif Idrees, Rashid Mahmood, Ghulam Sarwar, Muhammad Abu Bakar, Saboor Ahmad, Muhammad Mohsin Raza, and Jun Li. 2021. "Effectiveness of Different Soft Acaricides against Honey Bee Ectoparasitic Mite Varroa destructor (Acari: Varroidae)" Insects 12, no. 11: 1032. https://doi.org/10.3390/insects12111032
APA StyleQadir, Z. A., Idrees, A., Mahmood, R., Sarwar, G., Bakar, M. A., Ahmad, S., Raza, M. M., & Li, J. (2021). Effectiveness of Different Soft Acaricides against Honey Bee Ectoparasitic Mite Varroa destructor (Acari: Varroidae). Insects, 12(11), 1032. https://doi.org/10.3390/insects12111032






