Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Candidate Reference Genes
2.3. Phytosanitary Treatments of B. dorsalis
2.3.1. Heat Treatment
2.3.2. Cold Treatment
2.3.3. Irradiation Treatment
2.3.4. MB Fumigation
2.4. Total RNA Extraction and cDNA Synthesis
2.5. RT-qPCR
2.6. Data Analysis
3. Results
3.1. Response of B. dorsalis to Various Phytosanitary Treatments
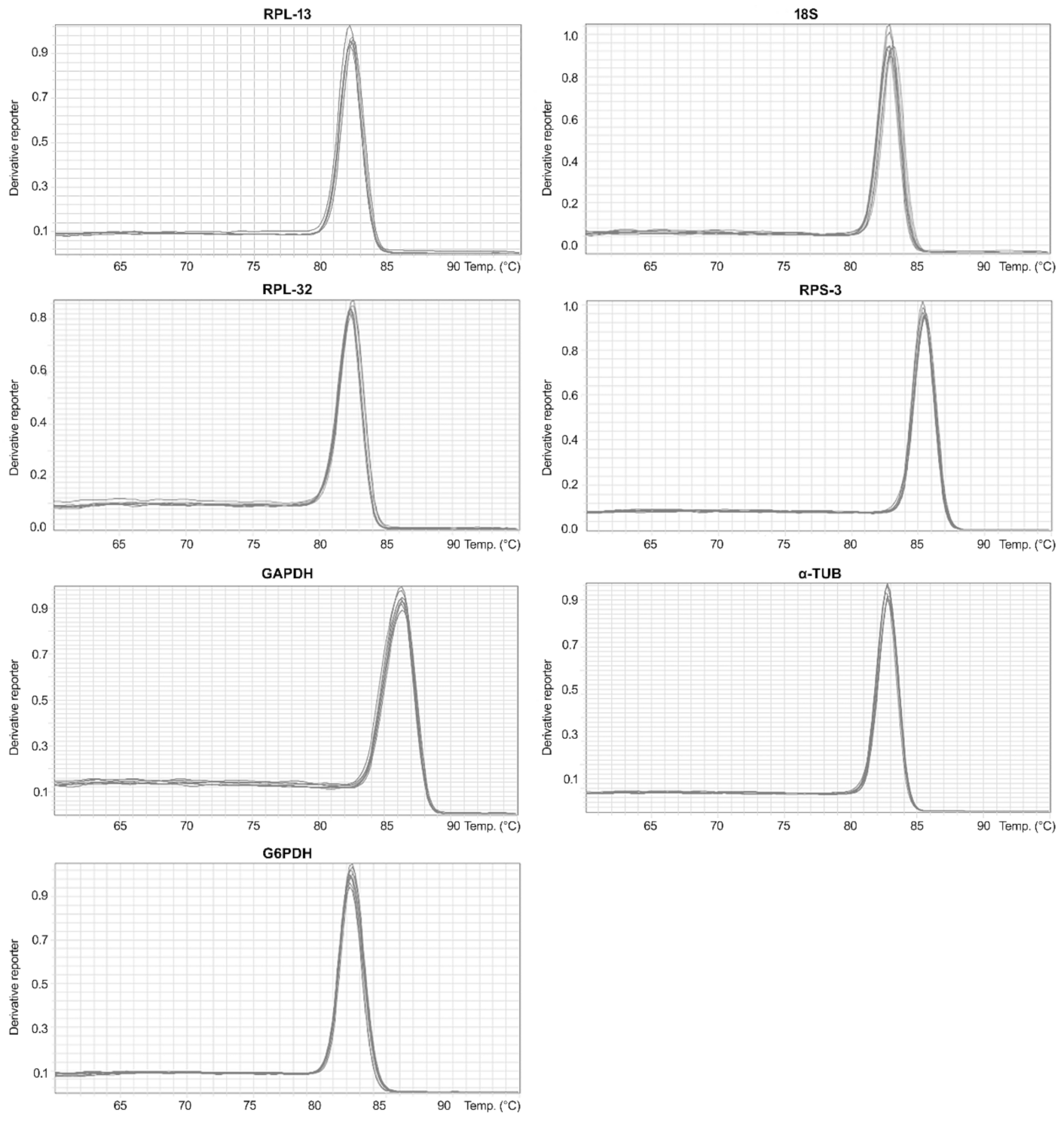
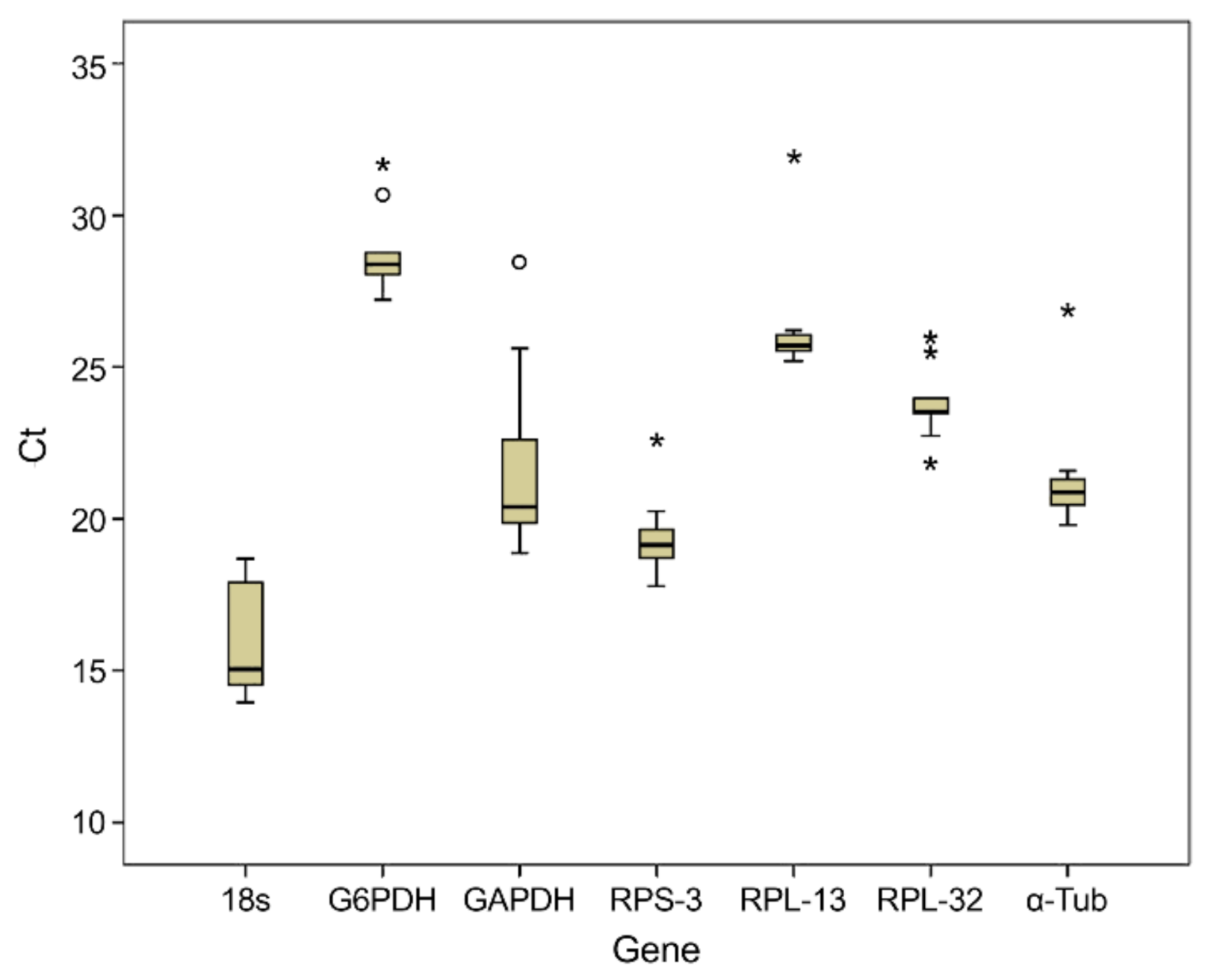
3.2. Analysis of Total RNA Quality, Primer Specificity, and Expression Stability of Reference Genes under Different Phytosanitary Treatments
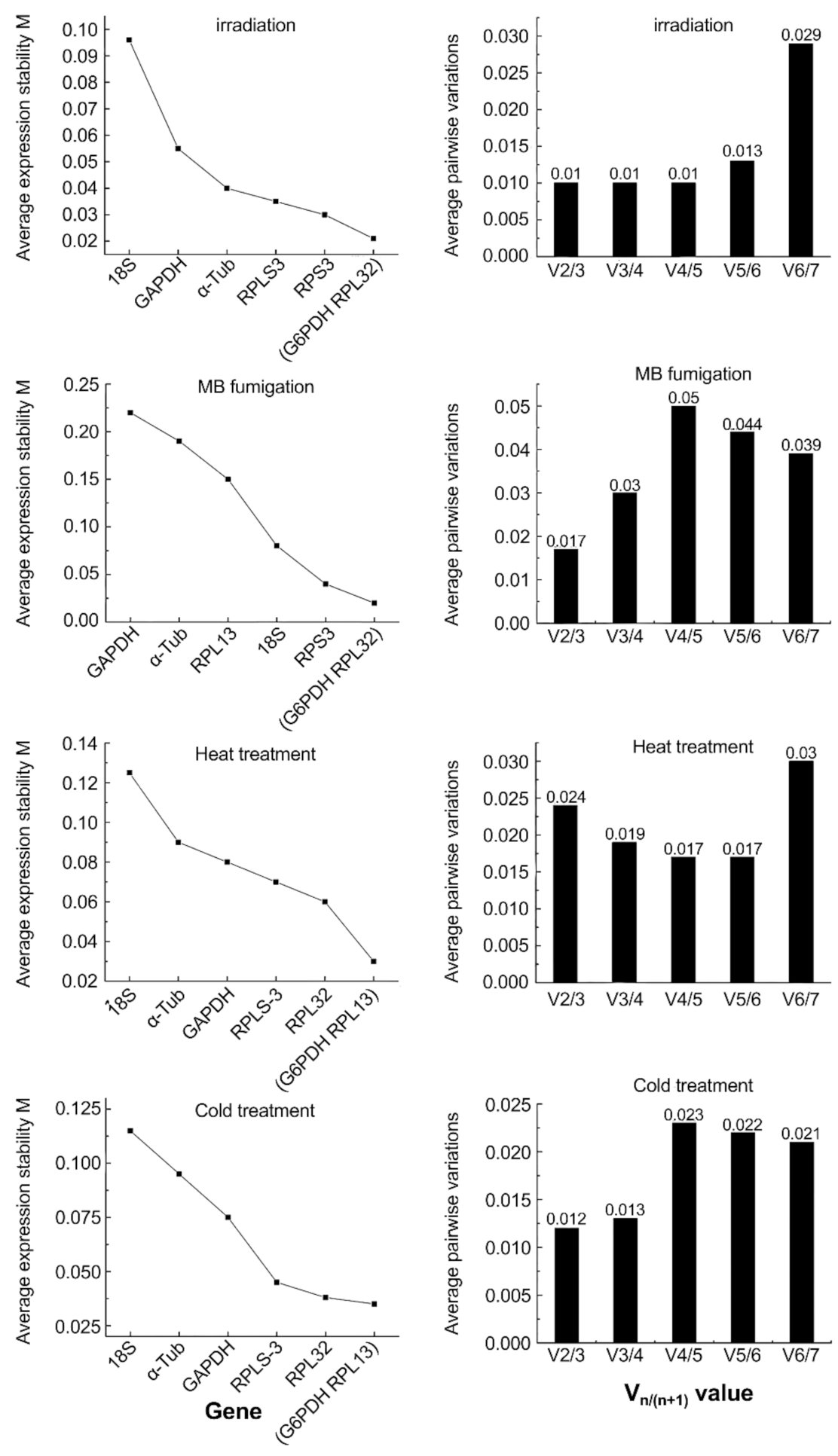
3.3. The geNorm Analysis
3.4. NormFinder Analysis
3.5. RefFinder Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.J.; Xu, L.; Nardi, F.; Li, J.G.; Zhang, R.J. The complete nucleotide sequence of the mitochondrial genome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 2007, 396, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Bateman, M.A. The ecology of fruit flies. Annu. Rev. Entomol. 1972, 17, 493–518. [Google Scholar] [CrossRef]
- Fletcher, B.S. The biology of dacine fruit flies. Annu. Rev. Entomol. 1987, 32, 115–144. [Google Scholar] [CrossRef]
- Shen, G.M.; Wang, X.N.; Dou, W.; Wang, J.J. Biochemical and molecular characterisation of acetylcholinesterase in four field populations of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest Manag. Sci. 2012, 68, 1553–1563. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, F.; Liang, G.; Hu, X. Reviews on the bionomics and control of Bactrocera dorsalis. Acta Agric. Univ. Jiangxiensis 2006, 28, 67–70. [Google Scholar]
- Li, L.; Zhang, G.; Li, B.; Yang, J.O.; Park, M.G.; Liu, T. Postharvest treatment of mandarin fruit using a combination of methyl bromide and phosphine against Bactrocera dorsalis (Diptera: Tephritidae). Pest Manag. Sci. 2020, 76, 1938–1943. [Google Scholar] [CrossRef]
- Zhao, J.P.; Hu, X.N.; Liang, F.; Liang, G.Q.; Luo, Y.Y. Study on irradiation quarantine treatment of oriental fruit fly, papaya fruit fly. Plant Quar. 2010, 24, 6–9. [Google Scholar]
- Fang, Y.; Kang, F.; Zhan, G.; Ma, C.; Li, Y.; Wang, L.; Wei, Y.; Gao, X.; Li, Z.; Wang, Y. The Effects of a Cold Disinfestation on Bactrocera dorsalis Survival and Navel Orange Quality. Insects 2019, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhan, G.; Wang, Y.; Ren, L.; Liu, B.; Li, T. Thermal Death Kinetics of Fruit Flies Bactrocera dorsalis, B. papayae and B. correcta (Diptera: Tephritidae). Acta Entomol. Sin. 2013, 56, 1404–1412. [Google Scholar]
- Dohino, T.; Hallman, G.J.; Grout, T.G.; Clarke, A.R.; Follett, P.A.; Cugala, D.R.; Minh Tu, D.; Murdita, W.; Hernandez, E.; Pereira, R.; et al. Phytosanitary Treatments Against Bactrocera dorsalis (Diptera: Tephritidae): Current Situation and Future Prospects. J. Econ. Entomol. 2017, 110, 67–79. [Google Scholar]
- Luo, M.Y.; Luo, W.G. Study on the Effect of Irradiation on Citrus Preservation. Hunan Agric. Sci. 2009, 6, 137–138. [Google Scholar]
- Liu, J. The Response of Banana (Musa spp.) Hemicellulose to Mild Chilling Stress. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2018. [Google Scholar]
- Feng, S.; Li, R.T.; Zheng, W.J.; Ye, Y.C.; Wang, J.N. Physiological reactions of three pineapple cultivars to low temperature stress. S. China Fruits 2011, 40, 16–18, 31. [Google Scholar]
- Ornelas-Paz, J.J.; Meza, M.B.; Obenland, D.; Rodríguez Friscia, K.; Jain, A.; Thornton, S.; Prakash, A. Effect of phytosanitary irradiation on the postharvest quality of the seedless kishu mandarins (Citrus kinokuni mukakukishu). Food Chem. 2017, 230, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Li, L.; Li, B.; Zhan, G.; Wang, Y. Evaluation of low-temperature phosphine fumigation for control of oriental fruit fly in loquat fruit. J. Econ. Entomol. 2018, 111, 1165–1170. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Gao, M.; Wang, Y.; Shao, B.; Liu, T. Effects of methyl bromide fumigation on the storage quality of citrus fruits. Plant Quar. 2018, 32, 46–49. [Google Scholar]
- Fang, W.Y. Organophosphorus pesticide triazophos: A new endocrine disruptor chemical of hypothalamus-pituitary-adrenal axis. Pestic. Biochem. Physiol. 2019, 159, 91–97. [Google Scholar]
- Coulon, M. Metabolisation of thiamethoxam (a neonicotinoid pesticide) and interaction with the Chronic bee paralysis virus in honeybees. Pestic. Biochem. Physiol. 2018, 144, 10–18. [Google Scholar] [CrossRef]
- Wang, F.Y.; Yang, L.; Li, L.F.; Liao, S.C.; Liao, R.Z. Selection of Reference Genes in the Bactrocera cucurbitae (Coquillett) Under Temperature Stress by RT-qPCR. J. Environ. Entomol. 2018, 40, 1097–1105. [Google Scholar]
- Ginzinger, D.G. Gene quantification using real-time quantitative PCR: An emerging technology hits the mainstream. Exp. Hematol. 2002, 30, 503–512. [Google Scholar] [CrossRef]
- Guénin, S.; Mauriat, M.; Pelloux, J.; Van Wuytswinkel, O.; Bellini, C.; Gutierrez, L. Normalization of qRT-PCR data: The necessity of adopting a systematic, experimental conditions-specific, validation of references. J. Exp. Bot. 2009, 60, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Liu, X.J.; Li, T.; Guo, Y.; Ma, E.; Zhang, J. Selection of reference genes on different days during the development of the fifth-instar nymph of Locusta migratoria with quantitative real-time PCR. Chin. J. Appl. Entomol. 2014, 51, 733–740. [Google Scholar]
- Shen, G.M.; Jiang, H.B.; Wang, X.N.; Wang, J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol. 2010, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Sagri, E.; Koskinioti, P.; Gregoriou, M.E.; Tsoumani, K.T.; Bassiakos, Y.C.; Mathiopoulos, K.D. Housekeeping in tephritid insects: The best gene choice for expression analyses in the medfly and the olive fly. Sci. Rep. 2017, 7, 45634. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR Data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Xie, F.; Sun, G.; Stiller, J.W.; Zhang, B. Genome-wide functional analysis of the cotton transcriptome by creating an integrated EST database. PLoS ONE 2011, 6, e26980. [Google Scholar] [CrossRef]
- Zhao, J.P. Gamma radiation as a phytosanitary treatment against larvae and pupae of Bactrocera dorsalis (Diptera: Tephritidae) in guava fruits. Food Control 2017, 72, 360–366. [Google Scholar] [CrossRef]
- Clare, G.K. Rearing of Bactrocera melanotus and B. Xanthodes (Diptera: T Ephritidae) for postharvest disinfestation research. N. Z. J. Zool. 1997, 24, 193–198. [Google Scholar] [CrossRef]
- Lü, Z.C.; Wang, L.H.; Dai, R.L.; Zhang, G.F.; Guo, J.Y.; Wan, F.H. Evaluation of endogenous reference genes of Bactrocera (Tetradacus) minax by gene expression profiling under various experimental conditions. Fla. Entomol. 2014, 97, 597–604. [Google Scholar] [CrossRef]
- Meng, L.W.; Yuan, G.R.; Lu, X.P.; Jing, T.X.; Zheng, L.S.; Yong, H.X.; Wang, J.J. Two delta class glutathione s-transferases involved in the detoxification of malathion in Bactrocera dorsalis (Hendel). Pest Manag. Sci. 2019, 75, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhao, J.; Ma, F.; Liu, B.; Zhong, Y.; Song, Z.; Zhao, Q.; Chen, N.; Ma, C. Radio protective effects on late third-instar Bactrocera dorsalis (Diptera: Tephritidae) larvae in low-oxygen atmospheres. Insects 2020, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, L.; Zhang, F.; Gong, S.; Li, T.; Zhan, G.; Wang, Y. Effect of low-temperature phosphine fumigation on the survival of Bactrocera correcta (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Walse, S.S.; Tebbets, J.S.; Leesch, J.G. Postharvest methyl bromide fumigation of Japanese plums to control codling moth (Lepidoptera: Tortricidae). J. Asia Pac. Entomol. 2019, 22, 807–815. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, C.; Lan, H.; Gao, S.; Liu, H.; Liu, J.; Cao, M.; Pan, G.; Rong, T.; Zhang, S. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS ONE 2014, 9, e95445. [Google Scholar] [CrossRef] [Green Version]
- Butte, A.J.; Dzau, V.J.; Glueck, S.B. Further defining housekeeping, or “maintenance,” genes focus on “a compendium of gene expression in normal human tissues”. Physiol. Genom. 2001, 7, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Lu, Y. Selection of reference genes in Phenacoccus solenopsis (Hemiptera: Pseudococcidae) under heat stress. Acta Entomol. Sin. 2014, 57, 1146–1154. [Google Scholar]
- Liu, X.; Guan, H.; Song, M.; Fu, Y.; Han, X.; Lei, M.; Ren, J.; Guo, B.; He, W.; Wei, Y. Reference gene selection for qRT-PCR assays in stellera chamaejasme subjected to abiotic stresses and hormone treatments based on transcriptome datasets. Peer J. 2018, 6, e4535. [Google Scholar] [CrossRef] [Green Version]
- Ruduś, I.; Kępczyński, J. Reference gene selection for molecular studies of dormancy in wild oat (Avena fatua L.) caryopses by RT-qPCR method. PLoS ONE 2018, 13, e0192343. [Google Scholar] [CrossRef] [Green Version]
| Gene Symbol | Gene Name | Primer Sequence (5′ 3′) | GenBank Accession Number | Fragment Length (bp) | Efficiency (%) | R2 | Reference |
|---|---|---|---|---|---|---|---|
| 18S | 18Sr-RNA | F: GCGAGAGGTGAAATTCTTGG | AF033944 | 191 | 94.9 | 0.9962 | Shen et al., 2010 [23] |
| R: CGGGTAAGCGACTGAGAGAG | |||||||
| RPL-32 | Ribosomal Protein L32 | F: CGATTTCTCCGCAGTATTCAC | —— | 147 | 107.5 | 0.9813 | Lü et al., 2014 [31] |
| R: GCCAGTACCTCATGCCTAACA | |||||||
| RPL-13 | Ribosomal Protein L13 | F: CAGTTGTACGTTGCGAGGAATT | HM236866 | 134 | 106.7 | 0.9816 | Shen et al., 2012 |
| R: TCTTGATGGAGCACGGGAG | |||||||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | F: GACGCCTACAAGCCTGACAT | GU269901 | 221 | 90.5 | 0.9896 | Shen et al., 2010 |
| R: GTTGAAGCGGGAATGATGTT | |||||||
| G6PDH | Glucose 6-phosphatedehydrogenase | F: CCTACAAACTTCTGCGGTTATGC | AB021910 | 382 | 89.1 | 0.9853 | Shen et al., 2010 |
| R: AGAGCGAGGCGAGGTGATC | |||||||
| RPS-3 | Ribosomal protein S3 | F: TGGATCACCAGAGTGGATCA | —— | 169 | 99.5 | 0.9984 | Li et al., 2019 [32] |
| R: TAAGTTGACCGGAGGTTTGG | |||||||
| α-Tub | α-Tubulin | F: CGCATTCATGGTTGATAACG | GU269902 | 184 | 108.9 | 0.9742 | Shen et al., 2010 |
| R: GGGCACCAAGTTAGTCTGGA |
| Treatment Conditions | Mortality Rate (%) | Mean ± SEM | |||
|---|---|---|---|---|---|
| Heat treatment | 47.5 °C 0 min | 2.1 | 3.4 | 1.3 | 2.27 ± 0.61 |
| 47.5 °C 2 min | 7.8 | 6.4 | 9.7 | 7.97 ± 0.96 | |
| Cold treatment | 1 °C 18 h | 4.6 | 5.8 | 2.3 | 4.23 ± 1.03 |
| 1 °C 30 h | 10.7 | 12.4 | 8.6 | 10.57 ± 1.1 | |
| MB fumigation | 6 g/m3 | 1.5 | 2.3 | 1.4 | 1.73 ± 0.28 |
| 7.5 g/m3 | 5.7 | 6.9 | 7.9 | 6.83 ± 0.64 | |
| CK (control check) | —— | 0 | 0 | 0 | —— |
| Sterility rate (%) | Mean ± SEM | ||||
| Irradiation | 40 Gy | 88 | 92.3 | 91.7 | 90.67 ± 1.34 |
| 80 Gy | 99.4 | 98 | 98.8 | 98.73 ± 0.41 | |
| Gene | Tm Value ± SEM |
|---|---|
| 18S | 83.03 ± 0.27 |
| G6PDH | 82.51 ± 0.33 |
| GAPDH | 86.29 ± 0.10 |
| RPL-13 | 82.29 ± 0.20 |
| RPS-3 | 85.41 ± 0.09 |
| RPL-32 | 82.39 ± 0.10 |
| α-Tub | 82.78 ± 0.10 |
| Gene | Minimum Value ± SEM | Maximum Value ± SEM |
|---|---|---|
| 18S | 13.96 ± 0.71 | 18.68 ± 1.33 |
| G6PDH | 18.88 ± 0.62 | 28.46 ± 0.97 |
| GAPDH | 27.21 ± 0.65 | 31.70 ± 1.67 |
| RPL-13 | 25.18 ± 0.54 | 31.97 ± 0.77 |
| RPS-3 | 17.77 ± 0.31 | 22.59 ± 2.33 |
| RPL-32 | 21.84 ± 0.46 | 25.97 ± 1.27 |
| α-Tub | 19.79 ± 1.22 | 26.88 ± 2.67 |
| Genes | Heat Treatment | Cold Treatment | Fumigation | Irradiation | ||||
|---|---|---|---|---|---|---|---|---|
| Stability and Ranking | Stability and Ranking | Stability and Ranking | Stability and Ranking | |||||
| RPL-13 | 0.010 | 1 | 0.017 | 2 | 0.081 | 3 | 0.032 | 4 |
| RPL-32 | 0.045 | 2 | 0.046 | 4 | 0.078 | 2 | 0.006 | 1 |
| RPS-3 | 0.065 | 4 | 0.070 | 5 | 0.100 | 4 | 0.008 | 2 |
| α-Tub | 0.052 | 3 | 0.011 | 1 | 0.138 | 5 | 0.044 | 6 |
| GAPDH | 0.010 | 1 | 0.031 | 3 | 0.059 | 1 | 0.037 | 5 |
| G6PDH | 0.073 | 5 | 0.099 | 7 | 0.186 | 7 | 0.030 | 3 |
| 18S | 0.142 | 6 | 0.090 | 6 | 0.156 | 6 | 0.139 | 7 |
| Heat Treatment | RPL-13 | RPL-32 | RPS-3 | 18S | G6PDH | GAPDH | α-Tub |
|---|---|---|---|---|---|---|---|
| geo Mean [Ct] | 15.83 | 21.10 | 28.32 | 20.89 | 25.68 | 23.07 | 18.89 |
| ar Mean [Ct] | 15.92 | 21.13 | 28.32 | 20.91 | 25.69 | 23.09 | 18.91 |
| min [Ct] | 13.96 | 19.88 | 28.05 | 19.84 | 25.46 | 21.84 | 17.77 |
| max [Ct] | 17.91 | 22.60 | 28.51 | 21.58 | 26.05 | 23.97 | 20.26 |
| stddev [±Ct] | 1.33 | 0.98 | 0.18 | 0.71 | 0.24 | 0.84 | 0.90 |
| CV [% Ct] | 8.33 | 4.64 | 0.63 | 3.40 | 0.95 | 3.62 | 4.76 |
| Stability rank | 2 | 4 | 6 | 7 | 1 | 5 | 3 |
| Cold treatment | RPL-13 | RPL-32 | RPS-3 | 18S | G6PDH | GAPDH | α-Tub |
| geo Mean [Ct] | 15.14 | 22.20 | 30.26 | 20.52 | 25.71 | 25.16 | 20.46 |
| ar Mean [Ct] | 15.15 | 22.31 | 30.29 | 20.53 | 25.71 | 25.17 | 20.52 |
| min [Ct] | 14.53 | 20.40 | 28.51 | 19.84 | 25.54 | 23.97 | 18.71 |
| max [Ct] | 15.88 | 25.62 | 31.70 | 21.22 | 26.06 | 25.98 | 22.59 |
| stddev [±Ct] | 0.49 | 2.20 | 1.19 | 0.46 | 0.23 | 0.81 | 1.38 |
| CV [% Ct] | 3.23 | 9.88 | 3.93 | 2.24 | 0.90 | 3.20 | 6.72 |
| Stability rank | 1 | 4 | 6 | 3 | 5 | 7 | 2 |
| Fumigation | RPL-13 | RPL-32 | RPS-3 | 18S | G6PDH | GAPDH | α-Tub |
| geo Mean [Ct] | 16.22 | 22.40 | 28.37 | 20.30 | 25.40 | 23.67 | 19.72 |
| ar Mean [Ct] | 16.27 | 22.75 | 28.37 | 20.31 | 25.40 | 23.67 | 19.72 |
| min [Ct] | 14.93 | 18.88 | 27.84 | 19.79 | 25.18 | 23.49 | 19.26 |
| max [Ct] | 18.01 | 28.46 | 28.77 | 21.31 | 25.54 | 23.97 | 20.26 |
| stddev [±Ct] | 1.16 | 3.80 | 0.36 | 0.66 | 0.14 | 0.20 | 0.36 |
| CV [% Ct] | 7.12 | 16.72 | 1.25 | 3.26 | 0.56 | 0.84 | 1.82 |
| Stability rank | 1 | 2 | 4 | 6 | 3 | 7 | 5 |
| Irradiation | RPL-13 | RPL-32 | RPS-3 | 18S | G6PDH | GAPDH | α-Tub |
| geo Mean [Ct] | 16.12 | 20.06 | 27.92 | 20.39 | 25.82 | 23.67 | 19.72 |
| ar Mean [Ct] | 16.23 | 20.08 | 27.92 | 20.39 | 25.82 | 23.67 | 19.72 |
| min [Ct] | 14.13 | 18.94 | 27.22 | 19.84 | 25.54 | 23.49 | 19.26 |
| max [Ct] | 18.68 | 20.92 | 28.51 | 20.88 | 26.21 | 23.97 | 20.26 |
| stddev [±Ct] | 1.63 | 0.76 | 0.47 | 0.37 | 0.26 | 0.20 | 0.36 |
| CV [% Ct] | 10.05 | 3.80 | 1.69 | 1.80 | 1.00 | 0.84 | 1.82 |
| Stability rank | 2 | 1 | 5 | 7 | 4 | 6 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Li, B.; Chen, N.; Yang, D.; Li, L.; Liu, T. Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments. Insects 2021, 12, 945. https://doi.org/10.3390/insects12100945
Cao Y, Li B, Chen N, Yang D, Li L, Liu T. Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments. Insects. 2021; 12(10):945. https://doi.org/10.3390/insects12100945
Chicago/Turabian StyleCao, Yue, Baishu Li, Naizhong Chen, Ding Yang, Li Li, and Tao Liu. 2021. "Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments" Insects 12, no. 10: 945. https://doi.org/10.3390/insects12100945
APA StyleCao, Y., Li, B., Chen, N., Yang, D., Li, L., & Liu, T. (2021). Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments. Insects, 12(10), 945. https://doi.org/10.3390/insects12100945







