Population Dynamics and Tree Damage of the Invasive Chestnut Gall Wasp, Dryocosmus kuriphilus, in Its Southernmost European Distributional Range
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chestnut Cultivation in the Study Area
2.2. Life-Cycle Parameters
- (a)
- The net reproduction rate (R0):
- (b)
- The intrinsic rate of increase (r):
- (c)
- The finite rate of population change (λ):
2.3. Flight Phenology
2.4. Tree Damage
2.5. Temperature Data Calculation
2.6. Statistical Analyses
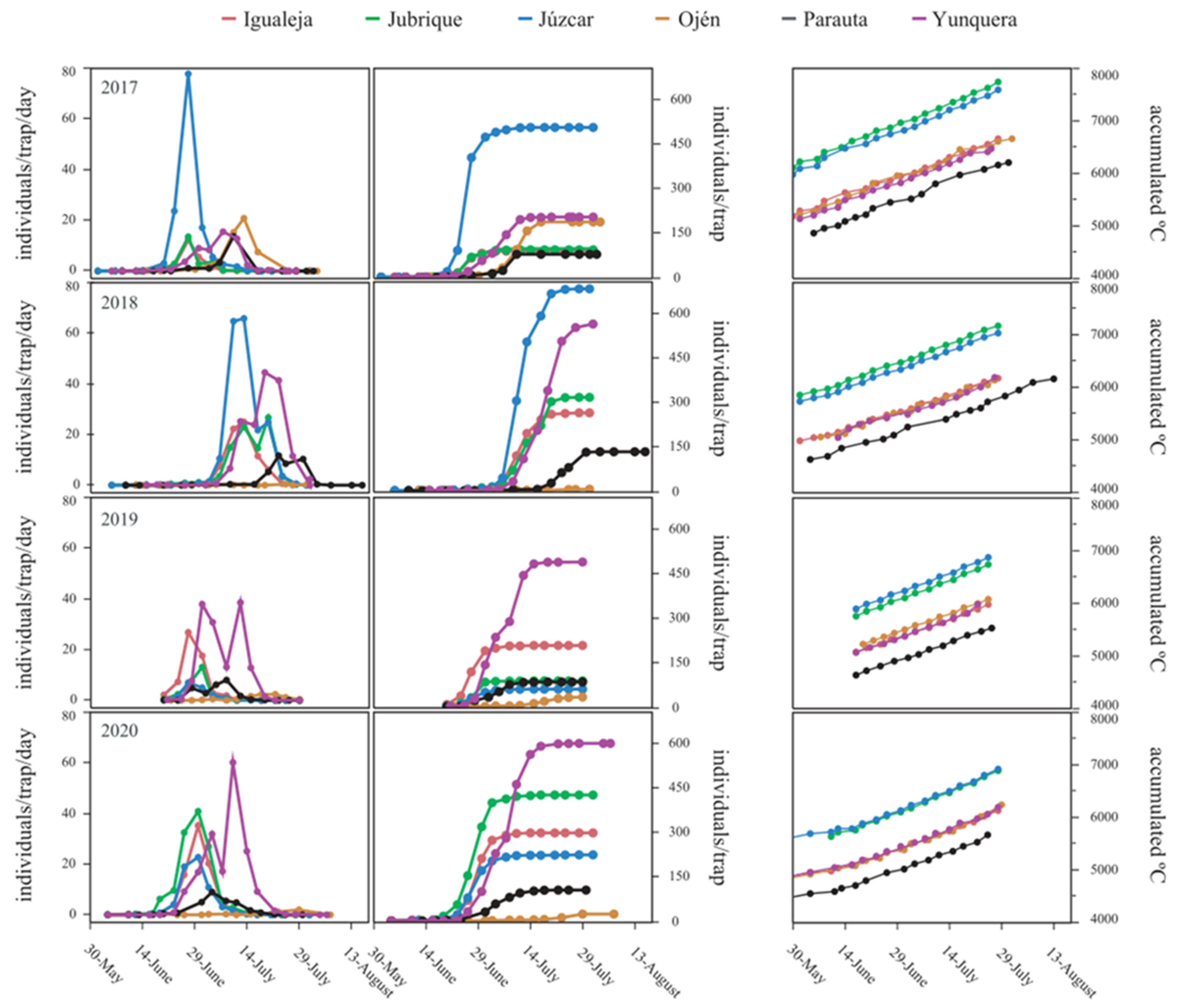
3. Results
3.1. Life-Cycle Stages and Population Trends
3.2. Factors Influencing Flight Phenology
3.3. Damage to Chestnut Trees
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, D.L.; Van Driesche, R.G. Threats posed to rare or endangered insects by invasions of nonnative species. Annu. Rev. Entomol. 2020, 55, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.A.; Ruffhead, H.J.; Wakefild, N.H.; Roberts, P.D.; Hemming, D.J.; Díaz-Soltero, H. The role played by invasive species in interactions with endangered and threatened species in the United States: A systematic review. Biodivers. Conserv. 2018, 27, 3171–3183. [Google Scholar] [CrossRef]
- Bonello, P.; Campbell, F.T.; Cipollini, D.; Conrad, A.O.; Farinas, C.; Gandhi, K.J.K.; Hain, F.P.; Parry, D.; Showalter, D.N.; Villari, C.; et al. Invasive tree pests devastate ecosystems—A proposed new response framework. Front. For. Glob. Chang. 2020, 3, 2. [Google Scholar] [CrossRef]
- Diagne, C.; Catford, J.A.; Essl, F.; Nuñez, M.A.; Courchamp, F. What are the economic costs of biological invasions? A complex topic requiring international and interdisciplinary expertise. NeoBiota 2020, 63, 25–37. [Google Scholar] [CrossRef]
- Ricciardi, A.; Cohen, J. The invasiveness of an introduced species does not predict its impact. Biol. Invasions 2007, 9, 309–315. [Google Scholar] [CrossRef]
- Kumschick, S.; Bacher, S.; Evans, T.; Markova, Z.; Pergl, J.; Pyšek, P.; Vaes-Petignat, S.; van der Veer, G.; Vilà, M.; Nentwig, W. Comparing impacts of alien plants and animals in Europe using a standard scoring system. J. Appl. Ecol. 2015, 52, 552–561. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P.; Borowiec, N.; Capdevielle, X.; Castagneyrol, B.; Maltoni, A.; Robin, C.; Selvi, F.; Halder, I.V.; Vétillard, F.; Jactel, H. Plant neighbour identity and invasive pathogen infection affect associational resistance to an invasive gall wasp. Biol. Invasions 2018, 20, 1459–1473. [Google Scholar] [CrossRef]
- Roques, A.; Auger-Rozenberg, M.A.; Blackburn, T.M.; Garnas, J.; Pyšek, P.; Rabitsch, W.; Richardson, D.M.; Wingfield, M.J.; Liebhold, A.M.; Duncan, R.P. Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biol. Invasions 2016, 18, 907–920. [Google Scholar] [CrossRef]
- Goldstein, J.; Park, J.; Haran, M.; Liebhold, A.; Bjørnstad, O.N. Quantifying spatio-temporal variation of invasion spread. Proc. Royal Soc. B 2019, 286, 20182294. [Google Scholar] [CrossRef]
- CABI. Dryocosmus kuriphilus (Oriental Chestnut Gall Wasp). Available online: https://www.cabi.org/isc/datasheet/20005#toDistributionMaps (accessed on 3 May 2021).
- Conedera, M.; Tinner, W.; Krebs, P.; de Rigo, D.; Caudullo, G. Castanea sativa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Joint Research Centre: Brussels, Belgium, 2016; pp. 78–79. [Google Scholar]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004, 13, 161–179. [Google Scholar] [CrossRef]
- Lüdders, P. Esskastanie (Castanea sativa Mill.). Erwerbs-Obstbau 2004, 46, 7–12. [Google Scholar] [CrossRef]
- Brussino, G.; Bosio, G.; Baudino, M.; Giordano, R.; Ramello, F.; Melika, G. Dangerous exotic insect for the European chestnut. Inf. Agrar. 2002, 58, 59–61. [Google Scholar]
- EFSA Panel on Plant Health. Risk assessment of the oriental chestnut gall wasp, Dryocosmus kuriphilus for the EU territory on request from the European Commission. EFSA J. 2010, 8, 1619. [Google Scholar]
- Gil-Tapetado, D.; Gómez, J.F.; Cabrero-Sañudo, F.J.; Nieves-Aldrey, J.L. Distribution and dispersal of the invasive Asian chestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), across the heterogeneous landscape of the Iberian Peninsula. Eur. J. Entomol. 2018, 115, 575–586. [Google Scholar] [CrossRef]
- Pujade-Villar, J.; Torrell, A.; Rojo, M. Nota entomològica. Primeres troballes a la península Ibèrica de Dryocosmus kuriphilus (Hym., Cynipidae), una espècie de cinípid d’origen asiàtic altament perillosa per al castanyer (Fagaceae). Orsis 2013, 27, 295–301. [Google Scholar]
- Ôtake, A. Chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae): A preliminary on trend of adult emergence and some other ecological aspect related to the final stage of its life cycle. Appl. Entomol. Zool. 1980, 15, 96–105. [Google Scholar] [CrossRef]
- Ôtake, A. Chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae): Analysis of records on cell contents inside galls and emergence of wasps and parasitoids outside galls. Appl. Entomol. Zool. 1989, 24, 193–201. [Google Scholar] [CrossRef]
- Viggiani, G.; Nugnes, F. Description of the larval stages of Dryokosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae), with notes on their phenology. J. Entomol. Acarol. Res. 2010, 2, 39–45. [Google Scholar] [CrossRef]
- Bernardo, U.; Iodice, L.; Sasso, R.; Tutore, V.A.; Cascone, P.; Guerrieri, E. Biology and monitoring of Dryocosmus kuriphilus on Castanea sativa in Southern Italy. Agric. For. Entomol. 2013, 15, 65–76. [Google Scholar] [CrossRef]
- Kato, K.; Hijii, N. Optimal clutch size of the chestnut gall-wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae). Res. Popul. Ecol. 1993, 35, 1–14. [Google Scholar] [CrossRef]
- Graziosi, I.; Rieske, L.K. Potential fecundity of a highly invasive gall maker, Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Environ. Entomol. 2014, 43, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Otero, R.; Crespo, D.; Mansilla, J.P. Dryocosmus kuriphilus Yasumatsu, 1951 (Hymenoptera: Cynipidae) in Galicia (NW Spain): Pest dispersion, associated parasitoids and first biological control attempts. Arq. Entomolóxicos 2017, 17, 439–448. [Google Scholar]
- Kato, K.; Hijii, N. Ovipositional traits of the chestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Entomol. Sci. 2001, 4, 295–299. [Google Scholar]
- Panzavolta, T.; Bracalini, M.; Croci, F.; Campani, C.; Bartoletti, T.; Miniati, G.; Benedettelli, S.; Tiberi, R. Asian chestnut gall wasp in Tuscany: Gall characteristics, egg distribution and chestnut cultivar susceptibility. Agric. For. Entomol. 2012, 14, 139–145. [Google Scholar] [CrossRef]
- Gil-Tapetado, D.; Castedo-Dorado, F.; Nieves-Aldrey, J.L.; Lombardero, M.J. Gall size of Dryocosmus kuriphilus limits down-regulation by native parasitoids. Biol. Invasions 2021, 23, 1157–1174. [Google Scholar] [CrossRef]
- Cooper, W.R.; Rieske, L.K. Gall structure affects ecological associations of Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Environ. Entomol. 2010, 39, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, A.; Mariotti, B.; Tani, A. Case study of a new method for the classification and analysis of Dryocosmus kuriphilus Yasumatsu damage to young chestnut sprouts. iForest 2012, 5, 50–59. [Google Scholar] [CrossRef]
- Sartor, C.; Dini, F.; Marinoni, D.T.; Mellano, M.G.; Beccaro, G.L.; Alma, A.; Botta, R. Impact of the Asian wasp Dryocosmus kuriphilus (Yasumatsu) on cultivated chestnut: Yield loss and cultivar susceptibility. Sci. Hortic. 2015, 197, 454–460. [Google Scholar] [CrossRef]
- Gehring, E.; Bellosi, B.; Quacchia, A.; Conedera, M. Assessing the impact of Dryocosmus kuriphilus on the chestnut tree: Branch architecture matters. J. Pest Sci. 2018, 91, 189–202. [Google Scholar] [CrossRef]
- Jara-Chiquito, J.L.; Pujade-Villar, J.; Garcia Ferreira, B.; Álvarez, R. Histological changes induced by the cynipid wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in leaves of the chestnut Castanea sativa (Fagaceae): Mechanisms of galling impact on host vigor. Arthropod Plant Interac. 2021, 15, 223–233. [Google Scholar] [CrossRef]
- Marcolin, E.; Pividori, M.; Colombari, F.; Manetti, M.C.; Pelleri, F.; Conedera, M.; Gehring, E. Impact of the Asian gall wasp Dryocosmus kuriphilus on the radial growth of the European chestnut Castanea sativa. J. Appl. Ecol. 2021, 58, 1212–1224. [Google Scholar] [CrossRef]
- Kato, K.; Hijii, N. Effects of gall formation by Dryocosmus kuriphilus Yasumatsu (Hym., Cynipidae) on the growth of chestnut trees. J. Appl. Entomol. 1997, 121, 9–15. [Google Scholar] [CrossRef]
- Battisti, A.; Benvegnú, I.; Colombari, F.; Robert, A.; Haack, R.A. Invasion by the chestnut gall wasp in Italy causes significant yield loss in Castanea sativa nut production. Agric. For. Entomol. 2013, 16, 75–79. [Google Scholar] [CrossRef]
- Gehring, E.; Bellosi, B.; Reynaud, N.; Conedera, M. Chestnut tree damage evolution due to Dryocosmus kuriphilus attacks. J. Pest Sci. 2020, 93, 103–115. [Google Scholar] [CrossRef]
- Bonsignore, C.P.; Bernardo, U. Effects of environmental parameters on the chestnut gall wasp and its complex of indigenous parasitoids. Sci. Nat. 2018, 105, 20. [Google Scholar] [CrossRef] [PubMed]
- Contarini, M.; Rossini, L.; Caccia, R.; Morelli, S.; Beritognolo, I.; Gaudet, M.; Villani, F.; Paparatti, B.; Speranza, S. Do Castanea sativa wild provenances influence Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) infestations? Turk. J. Zool. 2021, 45, 206–215. [Google Scholar] [CrossRef]
- Bonsignore, C.P.; Vono, G.; Bernardo, U. Environmental thermal levels affect the phenological relationships between the chestnut gall wasp and its parasitoids. Physiol. Entomol. 2019, 44, 87–98. [Google Scholar] [CrossRef]
- Gil-Tapetado, D.; Cabrero-Sañudo, F.J.; Polidori, C.; Gómez, J.F.; Nieves-Aldrey, J.L. Climate as a possible driver of gall morphology in the chestnut pest Dryocosmus kuriphilus across Spanish invaded areas. Bull. Entomol. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Price, P.W.; Fernandes, G.W.; Waring, G.L. Adaptive nature of insect gall. Environ. Entomol. 1987, 16, 15–24. [Google Scholar] [CrossRef]
- Crespi, B.J.; Carmean, D.A.; Chapman, T.W. Ecology and evolution of galling thrips and their allies. Annu. Rev. Entomol. 1997, 42, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.G.I.I.I.; Ivey, C.T.; Shedd, J.D. Support for the microenvironment hypothesis for adaptive value of gall induction in the California gall wasp, Andricus quercuscalifornicus. Entomol. Exp. Appl. 2009, 132, 126–133. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Castedo-Dorado, F.; Ayres, M.P. Extreme climatic events affect populations of Asian chestnut gall wasps, Dryocosmus kuriphilus, but do not stop the spread. Agric. For. Entomol. 2021. [Google Scholar] [CrossRef]
- Tamura, M. Studies on the chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Part 2). On the life history. J. Agric. Sci. Tokyo Nogyo Daigaku 1960, 6, 13–26. [Google Scholar]
- Wong, M.E.; Quinto, J.; Boyero, J.R.; Sánchez-Callado, F.M.; Álvarez-Castro, F.; Aguirrebengoa, M. Estado actual de la plaga de la avispilla del castaño Dryocosmus kuriphilus y su control biológico en Andalucía. Phytoma 2021, 326, 28–34. [Google Scholar]
- Bombi, P.; Fedi, C.; Zapparoli, M.; Cammarano, M.; Guidolotti, G.; Pallozzi, E.; Gaudet, M.; Mattioni, C.; Cherubini, M.; Beritognolo, I.; et al. Infestation potential of Dryocosmus kuriphilus Yasumatsu, 1951 (Hymenoptera: Cynipidae) in different natural populations of Castanea sativa Miller: An experimental ex situ test. Int. J. Pest Manag. 2018, 65, 147–153. [Google Scholar] [CrossRef]
- CAGPDS. Anuario de Estadísticas Agrarias y Pesqueras de Andalucía. Superficies y Producciones de Cultivos. Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible. Junta de Andalucía. Available online: https://juntadeandalucia.es/organismos/agriculturaganaderiapescaydesarrollosostenible/servicios/estadistica-cartografia/anuarios.html (accessed on 3 May 2021).
- Rubio, A. 9260 Bosques de Castanea sativa. In Bases Ecológicas Preliminares Para la Conservación de los Tipos de Hábitat de Interés Comunitario en España; Secretaría General Técnica; Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2009; 64p. [Google Scholar]
- Avila, A.; Wong, M.E.; Aguirrebengoa, M.; Carbonero, M.D. Enfermedades Ligadas a la Seca del Castaño en Huelva; Instituto de Investigación y Formación Agraria y Pesquera, Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible, Junta de Andalucía: Hinojosa del Duque, Spain, 2021; pp. 1–27.
- Dorado, F.J.; Pujade-Villar, J.; Muñoz-Adalia, E.J.; Vinagrero, J.C.; Diez-Casero, J.J.; Fernández-Fernández, M.M. Characterization of native parasitoid community associated with the invasive pest Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Cantabria (northern Spain). Scand. J. For. Res. 2020, 35, 334–340. [Google Scholar] [CrossRef]
- Chi, H. Life-Table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Atlas Climático Ibérico. Agencia Estatal de Meteorología—AEMET. Available online: http://agroclimap.aemet.es/ (accessed on 7 June 2021).
- AEMET. Agencia Estatal de Meteorología—AEMET. Available online: https://opendata.aemet.es/centrodedescargas/inicio (accessed on 7 June 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 March 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://CRAN.R-project.org/package=nlme (accessed on 14 March 2021).
- Lefcheck, J.S. PiecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.42.1. Available online: https://cran.r-project.org/package=MuMIn (accessed on 14 March 2021).
- Gil-Tapetado, D.; Castedo-Dorado, F.; Lombardero, M.J.; Martel, J.; Álvarez-Álvarez, P. Spatial propagation and patterns of abundance of Dryocosmus kuriphilus throughout an invaded region. J. Appl. Entomol. 2020, 145, 10–25. [Google Scholar] [CrossRef]
- Bonsignore, C.P.; Vizzari, G.; Vono, G.; Bernardo, U. Short-term cold stress affects parasitism on the Asian chestnut gall wasp Dryocosmus kuriphilus. Insects 2020, 11, 841. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Melika, G.; Matošević, D.; Coyle, D.R. The Asian chestnut gall wasp Dryocosmus kuriphilus: A global invader and a successful case of classical biological control. J. Pest Sci. 2018, 92, 107–115. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, E.; Pontini, M.; Saladini, M.; Alma, A. Effectiveness of Torymus sinensis: A successful long-term control of the Asian chestnut gall wasp in Italy. J. Pest Sci. 2019, 92, 353–359. [Google Scholar] [CrossRef]
- Nieves-Aldrey, J.L.; Gil-Tapetado, D.; Gavira, O.N.; Boyero, J.R.; Polidori, C.; Lombardero, M.J.; Blanco, D.; Rey del Castillo, C.; Rodríguez-Rojo, P.; Vela, J.M.; et al. Torymus sinensis Kamijo, a biocontrol agent against the invasive chestnut gall wasp Dryocosmus kuriphilus Yasumatsu in Spain: Its natural dispersal from France and first data on establishment after experimental releases. For. Syst. 2019, 28, e001. [Google Scholar] [CrossRef]
- Borowiec, N.; Thaon, M.; Brancaccio, L.; Warot, S.; Vercken, E.; Fauvergue, X.; Ris, N.; Malausa, J.C. Classical biological control against the chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera, Cynipidae) in France. Plant Prot. Q. 2014, 29, 7–10. [Google Scholar]
- Paparella, F.; Ferracini, C.; Portaluri, A.; Manzo, A.; Alma, A. Biological control of the chestnut gall wasp with T. sinensis: A mathematical model. Ecol. Model. 2016, 338, 17–36. [Google Scholar] [CrossRef]
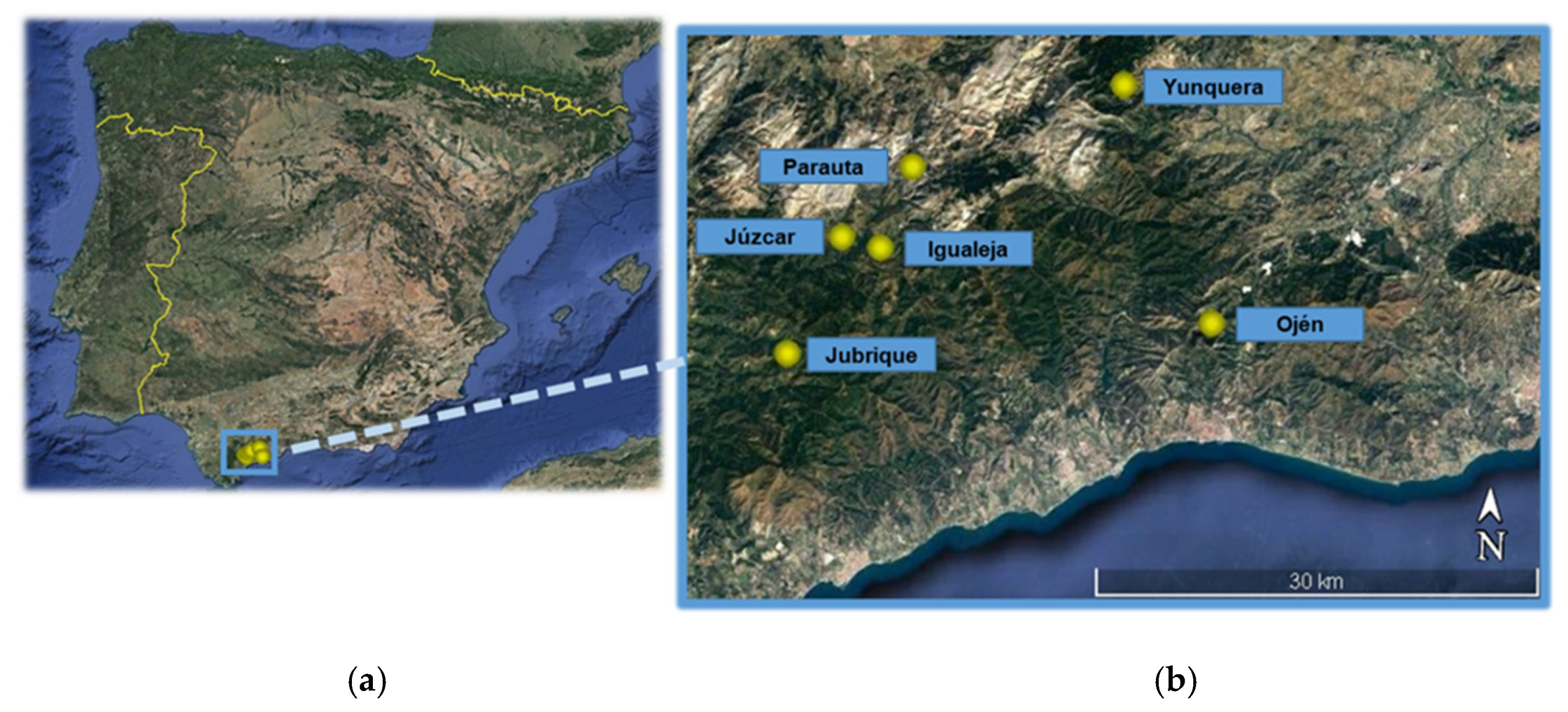



| Locality | Latitude | Longitude | Elevation | Historical Annual Mean Temperature | Life-Cycle Parameters | Flight Phenology | Tree Damage |
|---|---|---|---|---|---|---|---|
| Igualeja | 36.617647 | −5.135904 | 740 | 13.6 | ✓ | ✓ | ✓ |
| Jubrique | 36.552279 | −5.204078 | 789 | 15.7 | ✓ | ||
| Júzcar | 36.623390 | −5.165245 | 719 | 14.7 | ✓ | ✓ | ✓ |
| Ojén | 36.577863 | −4.884878 | 812 | 14.2 | ✓ | ✓ | ✓ |
| Parauta | 36.667257 | −5.113545 | 1049 | 13.1 | ✓ | ||
| Yunquera | 36.720823 | −4.954187 | 940 | 13.7 | ✓ | ✓ | ✓ |
| Explanatory Variable | Individuals Across Developmental Stages and Per Bud | |||
|---|---|---|---|---|
| df | F | p | R2 | |
| 2016–2017 | ||||
| Sampling date | 1, 48 | 24.14 | <0.001 | 0.394 |
| Locality | 3, 48 | 1.64 | 0.19 | |
| Sampling date × Locality | 3, 48 | 0.72 | 0.54 | |
| Cumulative °C | 1, 48 | 39.38 | <0.001 | 0.493 |
| Locality | 3, 48 | 5.76 | 0.002 | |
| Cumulative °C × Locality | 3, 48 | 0.49 | 0.68 | |
| 2017–2018 | ||||
| Sampling date | 1, 36 | 60.32 | <0.001 | 0.709 |
| Locality | 3, 36 | 3.31 | 0.030 | |
| Sampling date × Locality | 3, 36 | 5.91 | 0.002 | |
| Cumulative °C | 1, 36 | 72.12 | <0.001 | 0.745 |
| Locality | 3, 36 | 3.01 | 0.042 | |
| Cumulative °C × Locality | 3, 36 | 7.28 | <0.001 | |
| Explanatory Variable | Individuals/Trap/Day | |||
|---|---|---|---|---|
| df | F | p | R2 | |
| Year | 3, 314 | 1.61 | 0.18 | 0.207 |
| Locality | 5, 314 | 5.39 | <0.001 | |
| Date | 1, 314 | 0.77 | 0.37 | |
| Year × Locality | 15, 314 | 1.48 | 0.10 | |
| Year × Date | 3, 314 | 4.92 | 0.002 | |
| Locality × Date | 5, 314 | 0.52 | 0.75 | |
| Year × Locality × Date | 15, 314 | 0.85 | 0.62 | |
| Year | 3, 314 | 1.80 | 0.14 | 0.205 |
| Locality | 5, 314 | 4.88 | <0.001 | |
| Cumulative °C | 1, 314 | 0.32 | 0.57 | |
| Year × Locality | 15, 314 | 1.30 | 0.19 | |
| Year × Cumulative °C | 3, 314 | 5.14 | 0.002 | |
| Locality × Cumulative °C | 5, 314 | 0.46 | 0.80 | |
| Year × Locality ×Cumulative °C | 15, 314 | 0.81 | 0.66 | |
| Individuals/Trap/Day | ||||
| df | F | p | R2 | |
| Year | 3, 6 | 2.68 | 0.14 | 0.894 |
| Locality | 5, 6 | 5.23 | 0.033 | |
| Cumulative °C | 1, 6 | 0.00 | 0.99 | |
| Year × Cumulative °C | 3, 6 | 1.65 | 0.27 | |
| Locality ×Cumulative °C | 5, 6 | 1.95 | 0.21 | |
| Explanatory Variable | Sprouts With Galls | Galls Per Sprout | ||||||
|---|---|---|---|---|---|---|---|---|
| df | F | p | R2 | df | F | p | R2 | |
| Year | 5, 182 | 14.80 | <0.001 | 0.433 | 5, 182 | 18.50 | <0.001 | 0.433 |
| Locality | 3, 182 | 7.35 | 0.001 | 3, 182 | 8.14 | <0.001 | ||
| Year × Locality | 9, 182 | 3.17 | 0.001 | 9, 182 | 1.36 | 0.20 | ||
| Year | 5, 186 | 13.56 | <0.001 | 0.371 | 5, 186 | 17.36 | <0.001 | 0.405 |
| Locality | 3, 186 | 7.50 | <0.001 | 3, 186 | 8.84 | <0.001 | ||
| Annual mean temperature | 1, 186 | 0.10 | 0.75 | 1, 186 | 0.72 | 0.39 | ||
| Year × Annual mean temperature | 1, 186 | 1.70 | 0.19 | 1, 186 | 1.10 | 0.29 | ||
| Locality × Annual mean temperature | 1, 186 | 0.60 | 0.40 | 1, 186 | 2.68 | 0.10 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinto, J.; Wong, M.E.; Boyero, J.R.; Vela, J.M.; Aguirrebengoa, M. Population Dynamics and Tree Damage of the Invasive Chestnut Gall Wasp, Dryocosmus kuriphilus, in Its Southernmost European Distributional Range. Insects 2021, 12, 900. https://doi.org/10.3390/insects12100900
Quinto J, Wong ME, Boyero JR, Vela JM, Aguirrebengoa M. Population Dynamics and Tree Damage of the Invasive Chestnut Gall Wasp, Dryocosmus kuriphilus, in Its Southernmost European Distributional Range. Insects. 2021; 12(10):900. https://doi.org/10.3390/insects12100900
Chicago/Turabian StyleQuinto, Javier, María Eva Wong, Juan Ramón Boyero, José Miguel Vela, and Martin Aguirrebengoa. 2021. "Population Dynamics and Tree Damage of the Invasive Chestnut Gall Wasp, Dryocosmus kuriphilus, in Its Southernmost European Distributional Range" Insects 12, no. 10: 900. https://doi.org/10.3390/insects12100900
APA StyleQuinto, J., Wong, M. E., Boyero, J. R., Vela, J. M., & Aguirrebengoa, M. (2021). Population Dynamics and Tree Damage of the Invasive Chestnut Gall Wasp, Dryocosmus kuriphilus, in Its Southernmost European Distributional Range. Insects, 12(10), 900. https://doi.org/10.3390/insects12100900





