Successful Eradication of the Asian Longhorn Beetle, Anoplophora glabripennis, from North-Eastern Italy: Protocol, Techniques and Results
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Tree Database
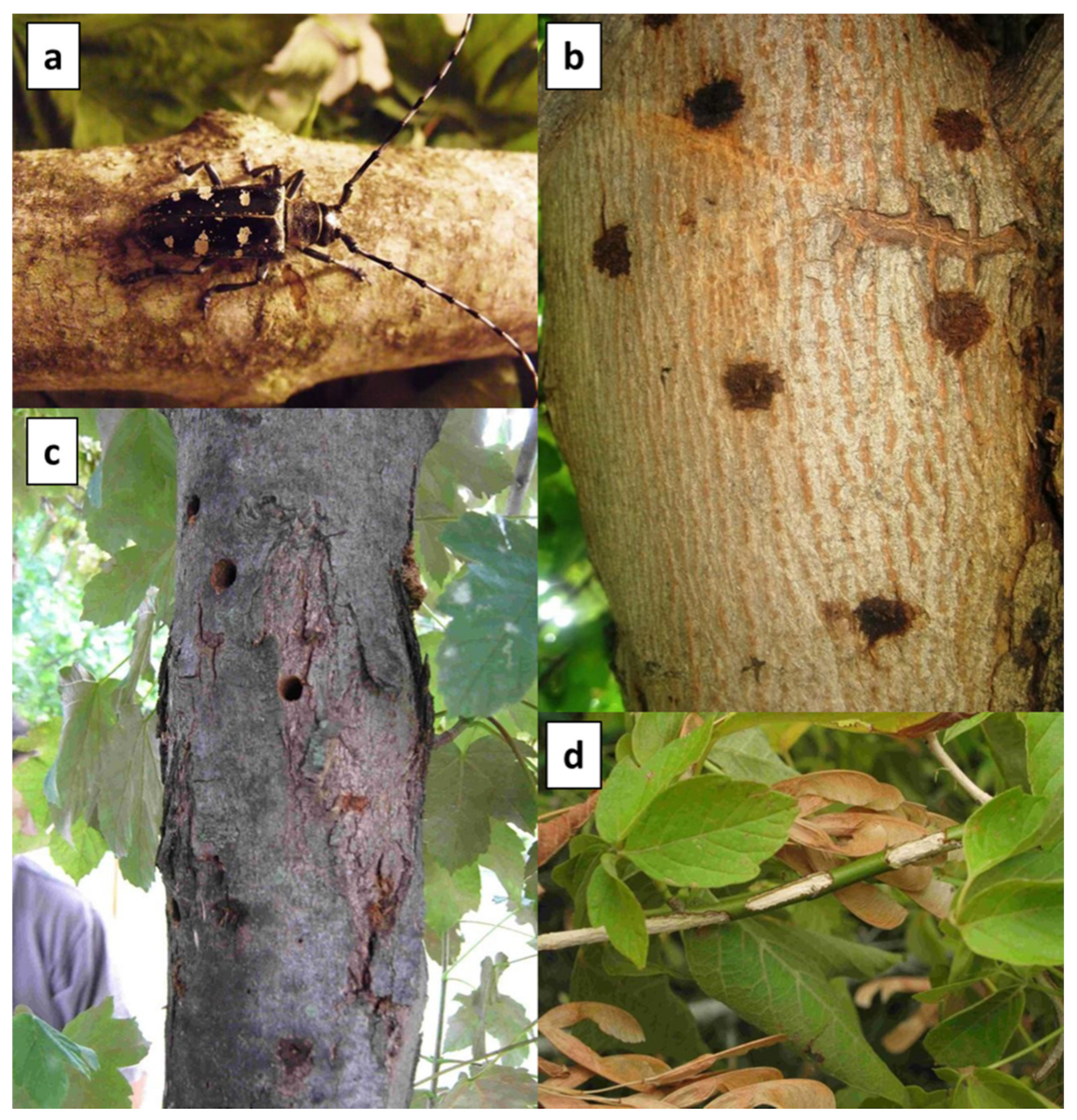
2.3. Visual Tree Survey
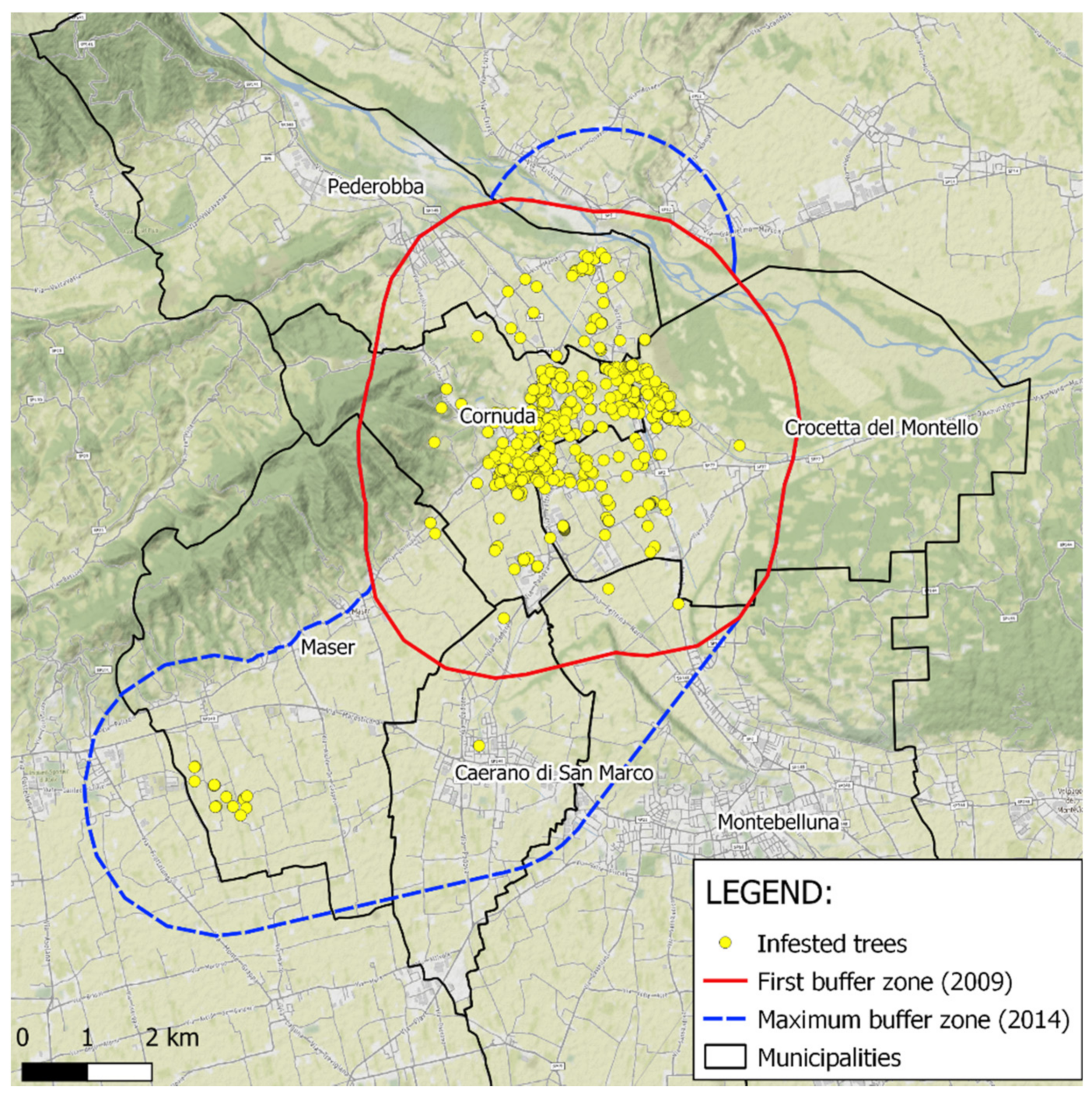
2.4. Zone Delimitation
2.5. Pheromone Traps
2.6. Sanitation Felling and Tree Destruction
2.7. Mitigation Plan
2.8. Communication
3. Results
3.1. Visual Tree Survey
3.2. Zone Delimitation
3.3. Pheromone Traps
3.4. Sanitation Felling, Tree Destruction, and Mitigation Plan
- €380,000 used by the Regional Plant Protection Organization for the annual tree survey (twice per year).
- €520,000 used by Regional Forest Services for felling and chipping trees.
- €20,000 used by the University of Padua for scientific support and research activities.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lingafelter, S.W.; Hoebeke, E.R. Revision of the Genus Anoplophora (Coleoptera: Cerambycidae); Entomological Society of Washington: Washington, DC, USA, 2002. [Google Scholar]
- Wang, Z.G. Study on the Occurrence Dynamics of Anoplophora glabripennis (Coleoptera: Cerambycidae) and its Control Measures. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2004. [Google Scholar]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Angeli, S.; Schuetz, S.; Luo, Y.; Hajek, A.E. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agric. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Dodds, K.J.; Orwig, D.A. An invasive urban forest pest invades natural environments—Asian longhorned beetle in northeastern US hardwood forests. Can. J. For. Res. 2011, 41, 1729–1742. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Huang, J. Adult behavior of Anoplophora glabripennis. Acta Entomol. Sin. 1993, 36, 51–55. [Google Scholar]
- Gao, R.; Xixiang, Q.; Dejun, C.; Weipei, C. A Study on the damage of poplar caused by Anoplophora glabripennis. For. Res. 1993, 6, 189–193. [Google Scholar]
- Haugen, D.A. Update on Asian longhorned beetle infestations in the US. Newsl. Mich. Entomol. Soc. 2000, 45, 2–3. [Google Scholar]
- Nowak, D.J.; Pasek, J.E.; Sequeira, R.A.; Crane, D.E.; Mastro, V.C. Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. J. Econ. Entomol. 2001, 94, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Cavey, J.F.; Hoebeke, E.R.; Passoa, S.; Lingafelter, S.W. A new exotic threat to North American hardwood forests: An Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae). I. Larval description and diagnosis. Proc. Entomol. Soc. Washingt. 1998, 100, 373–381. [Google Scholar]
- Haack, R.A.; Cavey, J.F.; Hoebeke, E.R.; Law, K.R. Anoplophora glabripennis: A new tree-infesting exotic cerambycid invades New York. Newsl. Mich. Entomol. Soc. 1996, 41, 1–3. [Google Scholar]
- Shatz, A.J.; Rogan, J.; Sangermano, F.; Ogneva-Himmelberger, Y.; Chen, H. Characterizing the potential distribution of the invasive Asian longhorned beetle (Anoplophora glabripennis) in Worcester County, Massachusetts. Appl. Geogr. 2013, 45, 259–268. [Google Scholar] [CrossRef]
- Poland, T. Chicago joins New York in battle with the Asian longhorned beetle. Newsl. Mich. Entomol. 1998, 43, 15–17. [Google Scholar]
- Haack, R.A. Exotics, exotics, exotics: Recently detected bark-and wood-boring insects in the US. Newsl. Mich. Entomol. Soc. 2003, 48, 16–17. [Google Scholar]
- Coyle, D.R.; Trotter, R.T.; Bean, M.S.; Pfister, S.E. First recorded Asian Longhorned Beetle (Coleoptera: Cerambycidae) infestation in the Southern United States. J. Integr. Pest Manag. 2021, 12, 10–11. [Google Scholar] [CrossRef]
- Pedlar, J.H.; McKenney, D.W.; Yemshanov, D.; Hope, E.S. Potential economic impacts of the Asian Longhorned Beetle (Coleoptera: Cerambycidae) in Eastern Canada. J. Econ. Entomol. 2020, 113, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Tomiczek, C.; Krehan, H.; Menschhorn, P. Dangerous Asiatic longicorn beetle found in Austria: New danger for our trees. Allg. Forst Z. Waldwirtsch. Umweltvorsorge 2002, 57, 52–54. [Google Scholar]
- Hérard, F.; Ciampitti, M.; Maspero, M.; Krehan, H.; Benker, U.; Boegel, C.; Schrage, R.; Bouhot-Delduc, L.; Bialooki, P. Anoplophora species in Europe: Infestations and management processes. EPPO Bull. 2006, 36, 470–474. [Google Scholar] [CrossRef]
- Benker, U.; Bogel, C.; Blaschke, M. Tree fellings due to black longicorn beetle: The asiatic longicorn beetle has been found in the district of Passau [Bavaria, Germany]. Allg. Forst Z. Waldwirtsch. Umweltvorsorge 2004, 59, 1112. [Google Scholar]
- EPPO. First record of Anoplophora glabripennis in Italy. EPPO Report. Serv. 2007, 9, 2. [Google Scholar]
- EPPO. First record of Anoplophora glabripennis in the Netherlands. EPPO Report. Serv. 2010, 11, 2. [Google Scholar]
- EPPO. First report of Anoplophora glabripennis in Switzerland. EPPO Report. Serv. 2011, 9, 3–4. [Google Scholar]
- EPPO. First outbreak of Anoplophora glabripennis in the United Kingdom. EPPO Report. Serv. 2012, 4, 2. [Google Scholar]
- EPPO. Anoplophora glabripennis found in Corse (FR). EPPO Report. Serv. 2013, 7, 2–3. [Google Scholar]
- EPPO. First report of Anoplophora glabripennis in Finland. EPPO Report. Serv. 2015, 10, 4–5. [Google Scholar]
- EPPO. First report of Anoplophora glabripennis in Montenegro. EPPO Report. Serv. 2017, 1, 3–4. [Google Scholar]
- EPPO. EPPO Global Database. Available online: https://gd.eppo.int/taxon/ANOLGL/distribution (accessed on 2 September 2021).
- EPPO. Anoplophora glabripennis detected in the Veneto region, Italy. EPPO Report. Serv. 2009, 8, 4. [Google Scholar]
- EPPO. Anoplophora glabripennis found in Piemonte, Italy. EPPO Report. Serv. 2018, 10, 6–7. [Google Scholar]
- EPPO. Anoplophora glabripennis found for the first time in Marche region, Italy. EPPO Report. Serv. 2013, 9, 4. [Google Scholar]
- Faccoli, M.; Favaro, R.; Smith, M.T.; Wu, J. Life history of the Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern Europe. Agric. For. Entomol. 2015, 17, 188–196. [Google Scholar] [CrossRef]
- Faccoli, M.; Favaro, R. Host preference and host colonization of the Asian long-horned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae), in Southern Europe. Bull. Entomol. Res. 2016, 106, 359–367. [Google Scholar] [CrossRef]
- Faccoli, M.; Vettorazzo, M.; Zampini, M.; Zanini, G.; Coppe, M.; Battisti, A. An outbreak of Anoplophora glabripennis (Coleoptera: Cerambycidae) in NE Italy: First results of pest management and eradication attempt. Proc. IUFRO Work. Party 2011, 7, 3–5. [Google Scholar]
- EPPO. PM 9/15 (1) Anoplophora glabripennis: Procedures for official control. EPPO Bull. 2013, 43, 510–517. [Google Scholar] [CrossRef]
- EPPO. Update on the situation of Anoplophora glabripennis in Italy. EPPO Report. Serv. 2020, 10, 12–13. [Google Scholar]
- Williams, D.W.; Lee, H.-P.; Kim, I.-K. Distribution and abundance of Anoplophora glabripennis (Coleoptera: Cerambycidae) in natural acer stands in South Korea. Environ. Entomol. 2004, 33, 540–545. [Google Scholar] [CrossRef]
- Myers, J.H.; Savoie, A.; van Randen, E. Eradication and pest management. Annu. Rev. Entomol. 1998, 43, 471–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebhold, A.M.; Tobin, P.C. Population ecology of insect invasions and their management. Annu. Rev. Entomol. 2008, 53, 387–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockerhoff, E.G.; Liebhold, A.M.; Richardson, B.; Suckling, D.M. Eradication of invasive forest insects: Concepts, methods, costs and benefits. N. Z. J. For. Sci. 2010, 40, 117–135. [Google Scholar]
- Tobin, P.C.; Kean, J.M.; Suckling, D.M.; McCullough, D.G.; Herms, D.A.; Stringer, L.D. Determinants of successful arthropod eradication programs. Biol. Invasions 2014, 16, 401–414. [Google Scholar] [CrossRef]
- Pluess, T.; Jarošík, V.; Pyšek, P.; Cannon, R.; Pergl, J.; Breukers, A.; Bacher, S. Which factors affect the success or failure of eradication campaigns against alien species? PLoS ONE 2012, 7, e48157. [Google Scholar] [CrossRef] [PubMed]
- Rejmánek, M.; Pitcairn, M. When is eradication of exotic pest plants a realistic goal. In Turning the Tide: The Eradication of Invasive Species; Veitch, C., Clout, M., Eds.; IUCN: Gland, Switzerland, 2002; pp. 249–253. [Google Scholar]
- Eyre, D.; Barbrook, J. The eradication of Asian longhorned beetle at Paddock Wood, UK. CABI Agric. Biosci. 2021, 2, 1–17. [Google Scholar] [CrossRef]
- Straw, N.A.; Fielding, N.J.; Tilbury, C.; Williams, D.T.; Inward, D. Host plant selection and resource utilisation by Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern England. Forestry 2015, 88, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.; Bond, S. USDA Announces 2021 Plans for Asian Longhorned Beetle Eradication Efforts in Massachusetts, New York, Ohio and South Carolina. Available online: https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2021/alb-eradication-efforts (accessed on 2 September 2021).
- Trotter, R.T.; Hull-Sanders, H.M. Quantifying dispersal of the Asian longhorned beetle (Anoplophora glabripennis, Coleoptera) with incomplete data and behavioral knowledge. Biol. Invasions 2015, 17, 3359–3369. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Suckling, D.M.; Wearing, C.H.; Byers, J.A. Potential of mass trapping for long-term pest management and eradication of invasive species. J. Econ. Entomol. 2006, 99, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Suckling, D.M.; Stringer, L.D.; Stephens, A.E.; Woods, B.; Williams, D.G.; Baker, G.; El-Sayed, A.M. From integrated pest management to integrated pest eradication: Technologies and future needs. Pest Manag. Sci. 2014, 70, 179–189. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Suckling, D.M.; Byers, J.A.; Jang, E.B.; Wearing, C.H. Potential of “Lure and Kill” in long-term pest management and eradication of invasive species. J. Econ. Entomol. 2009, 102, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Husillos, E.; Etxebeste, I.; Pajares, J. Effectiveness of mass trapping in the reduction of Monochamus galloprovincialis Olivier (Col.: Cerambycidae) populations. J. Appl. Entomol. 2015, 139, 747–758. [Google Scholar] [CrossRef]
- Zhang, A.; Oliver, J.E.; Chauhan, K.; Zhao, B.; Xia, L.; Xu, Z. Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Naturwissenschaften 2003, 90, 410–413. [Google Scholar] [CrossRef]
- Zhang, A.; Oliver, J.E.; Aldrich, J.R.; Wang, B.; Mastro, V.C. Stimulatory beetle volatiles for the Asian Longhorned Beetle, Anoplophora glabripennis (Motschulsky). Z. Naturforsch. C 2002, 57, 553–558. [Google Scholar] [CrossRef]
- Nehme, M.E.; Keena, M.A.; Zhang, A.; Baker, T.C.; Xu, Z.; Hoover, K. Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. Environ. Entomol. 2010, 39, 169–176. [Google Scholar] [CrossRef]
- Nehme, M.E.; Keena, M.A.; Zhang, A.; Baker, T.C.; Hoover, K. Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. Environ. Entomol. 2009, 38, 1745–1755. [Google Scholar] [CrossRef]
- Nehme, M.E.; Trotter, R.T.; Keena, M.A.; McFarland, C.; Coop, J.; Hull-Sanders, H.M.; Meng, P.; De Moraes, C.M.; Mescher, M.C.; Hoover, K. Development and evaluation of a trapping system for Anoplophora glabripennis (Coleoptera: Cerambycidae) in the United States. Environ. Entomol. 2014, 43, 1034–1044. [Google Scholar] [CrossRef]
- Hoppe, B.; Schrader, G.; Kinkar, M.; Vos, S. Pest survey card on Anoplophora glabripennis. EFSA Support. Publ. 2019, 16, 1750E. [Google Scholar]
- Branco, S.; Faccoli, M.; Brockerhoff, E.G.; Roux, G.; Jactel, H.; Desneux, N.; Gachet, E.; Mouttet, R.; Streito, J.-C.; Branco, M. Preventing invasions of Asian longhorn beetle and citrus longhorn beetle: Are we on the right track? J. Pest Sci. 2021. [Google Scholar] [CrossRef]
- Keena, M.A. Anoplophora glabripennis (Coleoptera: Cerambycidae) fecundity and longevity under laboratory conditions: Comparison of populations from New York and Illinois on Acer saccharum. Environ. Entomol. 2002, 31, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Keena, M.A. Effects of temperature on Anoplophora glabripennis (Coleoptera: Cerambycidae) adult survival, reproduction, and egg hatch. Environ. Entomol. 2006, 35, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.T.; Tobin, P.C.; Bancroft, J.; Li, G.; Gao, R. Dispersal and spatiotemporal dynamics of Asian Longhorned Beetle (Coleoptera: Cerambycidae) in China. Environ. Entomol. 2004, 33, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Favaro, R.; Wichmann, L.; Ravn, H.P.; Faccoli, M. Spatial spread and infestation risk assessment in the Asian longhorned beetle, Anoplophora glabripennis. Entomol. Exp. Appl. 2015, 155, 95–101. [Google Scholar] [CrossRef]
- Kappel, A.P.; Trotter, R.T.; Keena, M.A.; Rogan, J.; Williams, C.A. Mapping of the Asian longhorned beetle’s time to maturity and risk to invasion at contiguous United States extent. Biol. Invasions 2017, 19, 1999–2013. [Google Scholar] [CrossRef]
- ARPAV (Agenzia Regionale per la Prevenzione e Protezione Ambientale Veneto). Dati Meteorologici Ultimi Anni. Available online: https://www.arpa.veneto.it/bollettini/storico/ (accessed on 2 September 2021).
- Straw, N.A.; Tilbury, C.; Fielding, N.J.; Williams, D.T.; Cull, T. Timing and duration of the life cycle of Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern England. Agric. For. Entomol. 2015, 17, 400–411. [Google Scholar] [CrossRef]
- Keena, M.A. Factors that influence flight propensity in Anoplophora glabripennis (Coleoptera: Cerambycidae). Environ. Entomol. 2018, 47, 1233–1241. [Google Scholar] [CrossRef]
- Straw, N.A.; Fielding, N.J.; Tilbury, C.; Williams, D.T.; Cull, T. History and development of an isolated outbreak of Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern England. Agric. For. Entomol. 2016, 18, 280–293. [Google Scholar] [CrossRef]
- Haack, R.; Bauer, L.; Gao, R.-T.; McCarthy, J.; Miller, D.; Petrice, T.; Poland, T. Anoplophora Glabripennis within-tree distribution, seasonal development, and host suitability in China and Chicago. Gt. Lakes Entomol. 2006, 39, 169–183. [Google Scholar]
- Yin, W.L.; Lu, W. Review of Tree Selection and Afforestation for Control of Asian Longhorned Beetle in North China; Forest Health & Biosecurity Working Paper FBS/7E; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Faccoli, M.; Gatto, P. Analysis of costs and benefits of Asian longhorned beetle eradication in Italy. Forestry 2016, 89, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Mastro, V.C.; McLane, W.H. Impacts of chipping on surrogates for the longhorned beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in logs. J. Econ. Entomol. 2000, 93, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Porth, E.F.; Dandy, N.; Marzano, M. “My garden is the one with no trees”: Residential lived experiences of the 2012 Asian Longhorn Beetle eradication programme in Kent, England. Hum. Ecol. 2015, 43, 669–679. [Google Scholar] [CrossRef]
| Year | Trap Type | Trap Color | Trap Number | Lure |
|---|---|---|---|---|
| 2011 | Cross-vane, long | Black | 2 | CHEMTICA |
| Cross-vane, long | Transparent | 1 | ||
| Cross-vane, short | Black | 1 | ||
| Cross-vane, short | Transparent | 3 | ||
| Multi-funnel, long | Black | 3 | ||
| Multi-funnel, short | White | 4 | ||
| 2012 | Cross-vane, long | Black | 3 | CHEMTICA |
| 4 | RUSSIAN (I) | |||
| 4 | RUSSIAN (II) | |||
| 4 | RUSSIAN (III) | |||
| 4 | RUSSIAN (IV) | |||
| 4 | RUSSIAN (V) | |||
| 4 | RUSSIAN (VI) | |||
| 2013 | Cross-vane, long | Black | 3 | CONTROL |
| 3 | CHEMTICA | |||
| 3 | RUSSIAN (VII) | |||
| 3 | RUSSIAN (VIII) | |||
| Multi-funnel, long | Black | 3 | CONTROL | |
| 3 | CHEMTICA | |||
| 3 | RUSSIAN (VII) | |||
| 3 | RUSSIAN (VIII) | |||
| 2019 | Cross-vane, long | Black | 10 | CHEMTICA |
| Blends legend: | ||||
| CHEMTICA = 1:1 ratio of 4-(n-heptyloxy)butanal (0.3 mg) and 4-(n-heptyloxy)butan-1-ol (0.3 mg) + (−)-linalool (3 mg) + trans-caryophyllene (3 mg) + (Z)-3-hexen-1-ol (3 mg) | ||||
| RUSSIAN (I) = 1:1 ratio of 4-(n-heptyloxy)butanal (5 μL) and 4-(n-heptyloxy)butan-1-ol (5 μL) | ||||
| RUSSIAN (II) = 1:1 ratio of 4-(n-heptyloxy)butanal (50 μL) and 4-(n-heptyloxy)butan-1-ol (50 μL) | ||||
| RUSSIAN (III) = 1:1 ratio of 4-(n-heptyloxy)butanal (500 μL) and 4-(n-heptyloxy)butan-1-ol (500 μL) | ||||
| RUSSIAN (IV) = 1:1 ratio of 4-(n-heptyloxy)butanal (5 μL) and 4-(n-heptyloxy)butan-1-ol (5 μL) + (Z)-3-hexen-1-ol (100 μL) | ||||
| RUSSIAN (V) = 1:1 ratio of 4-(n-heptyloxy)butanal (50 μL) and 4-(n-heptyloxy)butan-1-ol (50 μL) + (Z)-3-hexen-1-ol (1000 μL) | ||||
| RUSSIAN (VI) = 1:1 ratio of 4-(n-heptyloxy)butanal (500 μL) and 4-(n-heptyloxy)butan-1-ol (500 μL) + (Z)-3-hexen-1-ol (3000 μL) | ||||
| RUSSIAN (VII) = 1:1 ratio of 4-(n-heptyloxy)butanal (0.3 mg) and 4-(n-heptyloxy)butan-1-ol (0.3 mg) + (−)-linalool (3 mg) | ||||
| RUSSIAN (VIII) = 1:1 ratio of 4-(n-heptyloxy)butanal (0.6 mg) and 4-(n-heptyloxy)butan-1-ol (0.6 mg) + (−)-linalool (6 mg) | ||||
| Tree Genus | Monitored Plants (n) | Infested Plants (n) | Ratio (%) |
|---|---|---|---|
| Cercidiphyllum | 11 | 2 | 18.18 |
| Aesculus | 147 | 17 | 11.56 |
| Betula | 2067 | 210 | 10.16 |
| Ulmus | 6227 | 337 | 5.41 |
| Acer | 10,277 | 431 | 4.19 |
| Salix | 5271 | 149 | 2.83 |
| Prunus | 1361 | 9 | 0.66 |
| Populus | 1709 | 2 | 0.12 |
| Alnus | 59 | 0 | - |
| Carpinus | 4837 | 0 | - |
| Corylus | 1238 | 0 | - |
| Fagus | 486 | 0 | - |
| Fraxinus | 680 | 0 | - |
| Ostrya | 43 | 0 | - |
| Platanus | 908 | 0 | - |
| Tilia | 1040 | 0 | - |
| Total | 36,361 | 1157 | 3.18 |
| Year | Buffer Zone (ha) | Monitored Plants (n) | Infested Plants (n) | Susceptible Plants (Clear-Cut) (n) | Total Felled Trees (n) | Clear-Cut Radius (m) |
|---|---|---|---|---|---|---|
| 2009 | 4105 | 12,816 | 576 | 54 | 630 | - |
| 2010 | 5625 | 20,366 | 327 | 198 | 525 | - |
| 2011 | 7214 | 24,292 | 163 | 82 | 245 | - |
| 2012 | 7214 | 24,292 | 67 | 679 | 746 | 50 |
| 2013 | 7594 | 25,223 | 15 | 83 | 98 | 50 |
| 2014 | 7594 | 30,990 | 5 | 52 | 57 | 100 |
| 2015 | 7594 | 36,361 | 4 | 56 | 60 | 100 |
| 2016 | 4555 | 24,511 | 0 | 0 | 0 | 100 |
| 2017 | 4555 | 24,511 | 0 | 0 | 0 | 100 |
| 2018 | 1843 | 13,041 | 0 | 0 | 0 | 100 |
| 2019 | 1843 | 13,041 | 0 | 0 | 0 | 100 |
| Total (n) | 36,361 * | 1157 | 1204 | 2361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchioro, M.; Faccoli, M. Successful Eradication of the Asian Longhorn Beetle, Anoplophora glabripennis, from North-Eastern Italy: Protocol, Techniques and Results. Insects 2021, 12, 877. https://doi.org/10.3390/insects12100877
Marchioro M, Faccoli M. Successful Eradication of the Asian Longhorn Beetle, Anoplophora glabripennis, from North-Eastern Italy: Protocol, Techniques and Results. Insects. 2021; 12(10):877. https://doi.org/10.3390/insects12100877
Chicago/Turabian StyleMarchioro, Matteo, and Massimo Faccoli. 2021. "Successful Eradication of the Asian Longhorn Beetle, Anoplophora glabripennis, from North-Eastern Italy: Protocol, Techniques and Results" Insects 12, no. 10: 877. https://doi.org/10.3390/insects12100877
APA StyleMarchioro, M., & Faccoli, M. (2021). Successful Eradication of the Asian Longhorn Beetle, Anoplophora glabripennis, from North-Eastern Italy: Protocol, Techniques and Results. Insects, 12(10), 877. https://doi.org/10.3390/insects12100877






