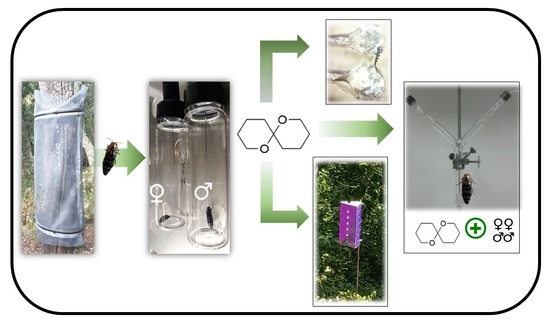
Olean (1,7-dioxaspiro[5.5]undecane): A Novel Intraspecific Chemical Cue in Coraebus undatus (F.) (Coleoptera: Buprestidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Headspace Solid-Phase Microextraction (HS-SPME)
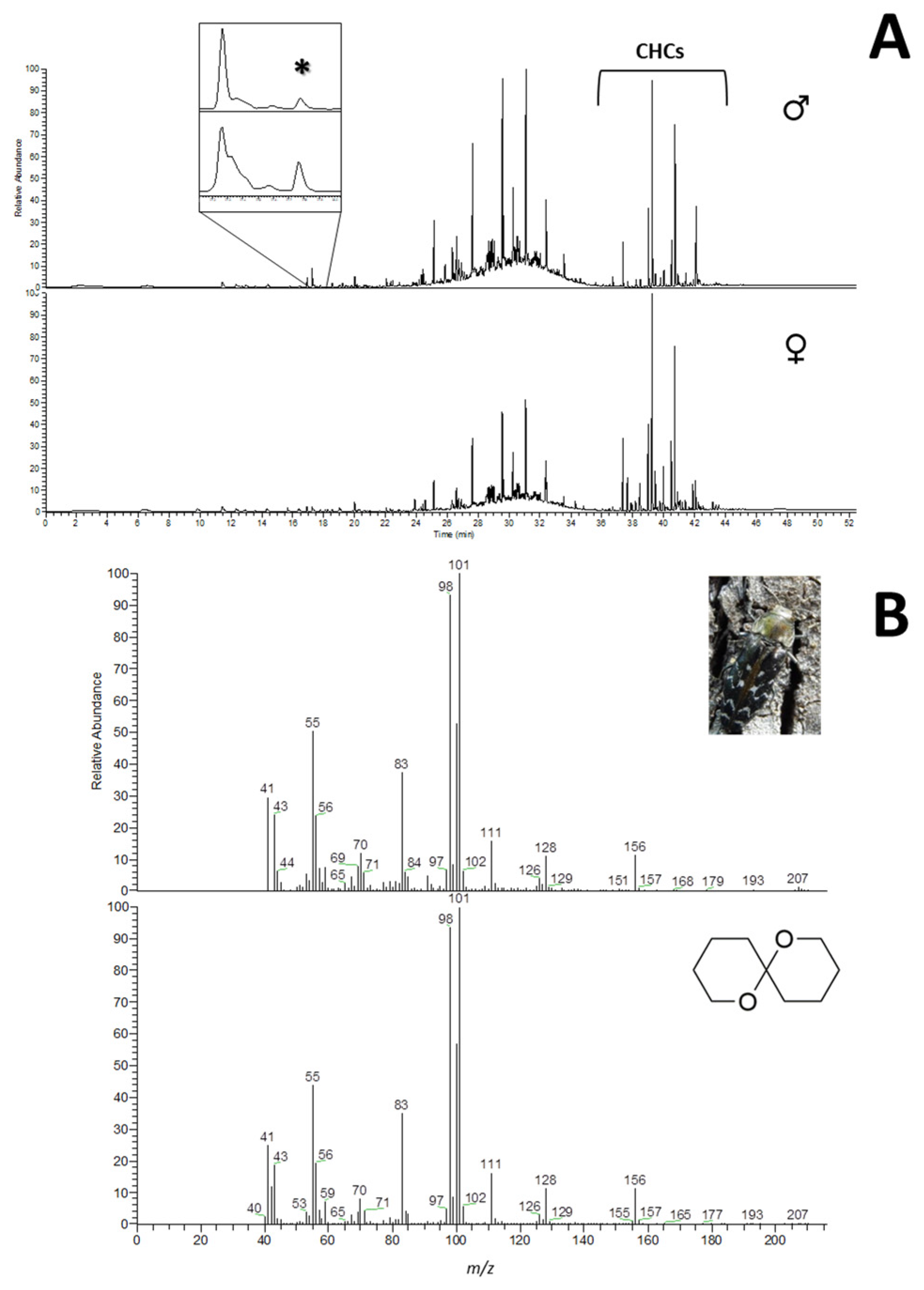
2.4. Chemical Analysis
2.5. Electroantennographic (EAG) Response
2.6. Behavioral Bioassays
2.7. Field Tests
2.8. Statistical Analysis
3. Results
3.1. Headspace Solid-Phase Microextraction
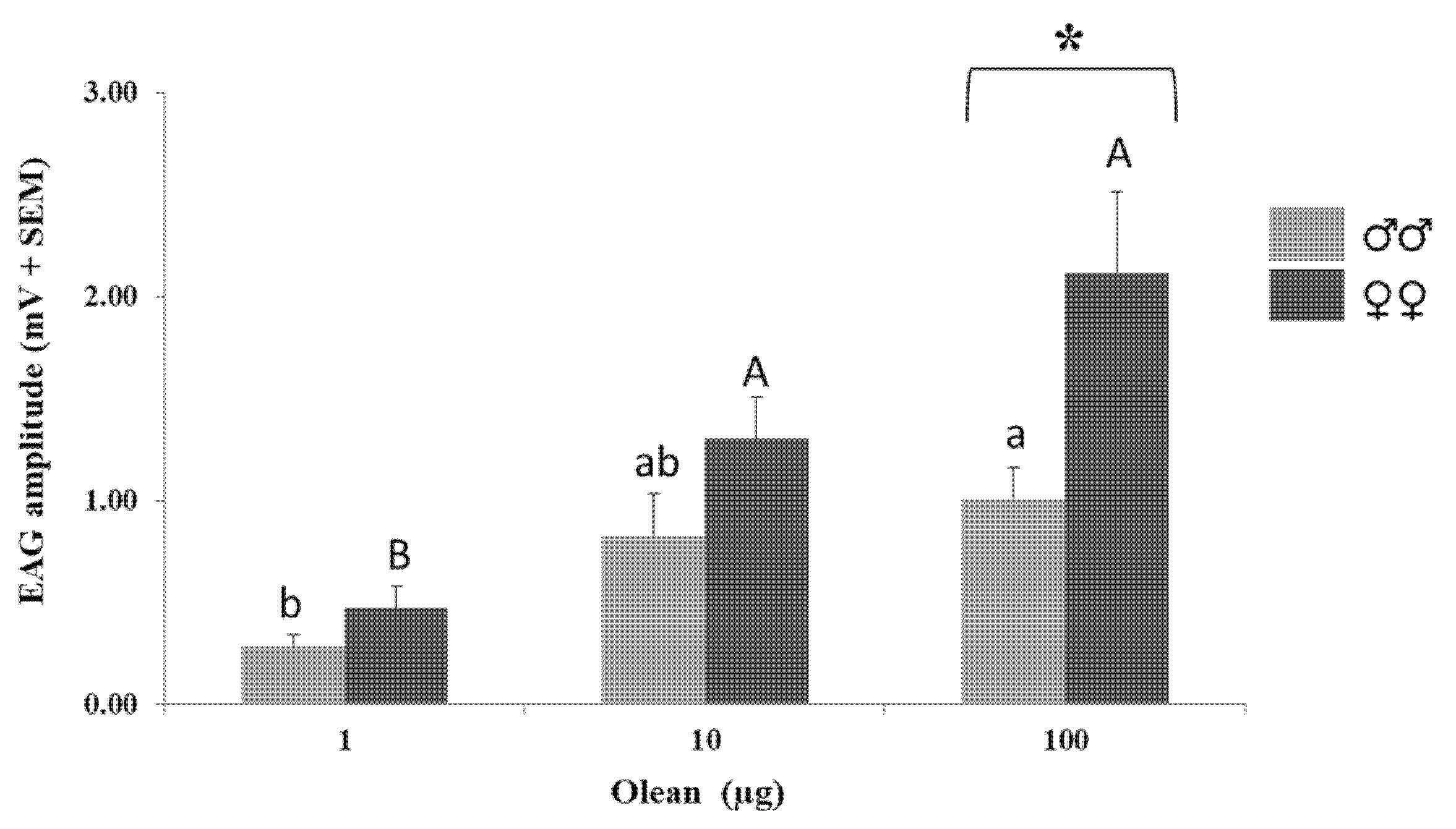
3.2. Electroantennographic Response
3.3. Behavioral Assays
3.4. Field Assays
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Lierop, P.; Lindquist, E.; Sathyapala, S.; Franceschini, G. Global forest area disturbance from fire, insect pests, diseases and severe weather events. For. Ecol. Manag. 2015, 352, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.L. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu. Rev. Entomol. 1982, 27, 411–446. [Google Scholar] [CrossRef]
- Evans, H.F.; Moraal, L.G.; Pajares, J.A. Biology, ecology and economic importance of buprestidae and cerambycidae. In Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis; Lieutier, F., Day, K.R., Battisti, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 447–474. [Google Scholar]
- Linnakoski, R.; Forbes, K.M. Pathogens—The hidden face of forest invasions by wood-boring insect pests. Front. Plant. Sci. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingfield, M.J.; Garnas, J.R.; Hajek, A.; Hurley, B.P.; de Beer, Z.W.; Taerum, S.J. Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biol. Invasions 2016, 18, 1045–1056. [Google Scholar] [CrossRef]
- Haack, R.A.; Benjamin, D.M. The biology and ecology of the twolined chestnut borer, Agrilus bilineatus (Coleoptera: Buprestidae), on oaks, Quercus spp. in Wisconsin. Can. Entomol. 1982, 114, 385–396. [Google Scholar] [CrossRef]
- Tluczek, A.R.; McCullough, D.G.; Poland, T.M. Influence of host stress on emerald ash borer (Coleoptera: Buprestidae) adult density, development, and distribution in Fraxinus pennsylvanica trees. Environ. Entomol. 2011, 40, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Svihra, P.; Koehler, C.S. Flatheaded borer in white alder landscape trees. J. Arboric. 1993, 19, 260–265. [Google Scholar]
- Barter, G.W. Studies of the bronze birch borer, Agrilus anxius Gory, in New Brunswick. Can. Entomol. 1957, 89, 12–36. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Michigan Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Kovacs, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Hope, E.; Sun, L.; Mckenney, D.; Bogdanski, B.; Pedlar, J.; Macaulay, L.; Macdonald, H.; Lawrence, K. Emerald Ash Borer, Agrilus planipennis: An. Economic Analysis of Regulations in Canada; Canadian Forest Service Pacific Forestry Centre Information Report BC-X-454; Natural Resources Canada: Victoria, BC, Canada, 2020; ISBN 9780660358666.
- Silk, P.; Mayo, P.; Ryall, K.; Roscoe, L. Semiochemical and communication ecology of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Insects 2019, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, A.; Jiménez, A.; Antonietty, C.A.; Villagrán, M.; Ocete, M.E.; Soria, F.J. Forecasting infestation by Coraebus undatus (Coleoptera, Buprestidae) in cork oak forests. Int. J. Pest. Manag. 2012, 58, 275–280. [Google Scholar] [CrossRef]
- Codina, A. Nota sobre el corc del suro Coraebus undatus F. (Col. Buprestidae). Bull. Inst. Cat. Hist. Nat. 1926, 2, 107–109. [Google Scholar]
- Wachtendorf, W. Beiträge zur Kenntnis der Eichenprachtkäfer Agrilus biguttatus Fabr. und Coraebus undatus Fabr. (Col. Bupr.). Z. für Angew. Entomol. 1955, 37, 327–339. [Google Scholar] [CrossRef]
- Soria, F.J.; Villagrán, M.; Ocete, M.E. Estudios poblacionales sobre Coroebus undatus (Fabricius) (Coleoptera, Buprestidae) en alcornocales de Andalucía occidental. II: Aspectos ecológicos de la larva. Bol. San. Veg. Plagas 1992, 18, 385–394. [Google Scholar]
- Fürstenau, B.; Quero, C.; Riba, J.M.; Rosell, G.; Guerrero, A. Field trapping of the flathead oak borer Coroebus undatus (Coleoptera: Buprestidae) with different traps and volatile lures. Insect Sci. 2015, 22, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, B.; Rosell, G.; Guerrero, A.; Quero, C. Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. J. Chem. Ecol. 2012, 38, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubáň, V.; Majer, K.; Kolibáč, J. Classification of the tribe Coraebini Bedel, 1921 (Coleoptera, Buprestidae, Agrilinae). Acta Mus. Morav. Sci. Biol. 2000, 85, 185–287. [Google Scholar]
- Meglič, A.; Ilić, M.; Quero, C.; Arikawa, K.; Belušič, G. Two chiral types of randomly rotated ommatidia are distributed across the retina of the flathead oak borer Coraebus undatus (Coleoptera: Buprestidae). J. Exp. Biol. 2020, 223, jeb225920. [Google Scholar] [CrossRef]
- Francke, W.; Hindorf, G.; Reith, W. Mass-spectrometric fragmentation of alkyl-1,6-dioxaspiro[4.5]decanes. Naturwissenschaften 1979, 66, 619–620. [Google Scholar] [CrossRef]
- Lelito, J.P.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Böröczky, K.; Baker, T.C. Visually mediated “paratrooper copulations” in the mating behavior of Agrilus planipennis (Coleoptera: Buprestidae), a highly destructive invasive pest of North American ash trees. J. Insect Behav. 2007, 20, 537–552. [Google Scholar] [CrossRef] [Green Version]
- Crook, D.J.; Khrimian, A.; Francese, J.A.; Fraser, I.; Poland, T.M.; Sawyer, A.J.; Mastro, V.C. Development of a host-based semiochemical lure for trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Environ. Entomol. 2008, 37, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pureswaran, D.S.; Poland, T.M. The role of olfactory cues in short-range mate finding by the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). J. Insect Behav. 2009, 22, 205–216. [Google Scholar] [CrossRef]
- Lelito, J.P.; Domingue, M.J.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Field investigation of mating behaviour of Agrilus cyanescens and Agrilus subcinctus. Can. Entomol. 2011, 143, 370–379. [Google Scholar] [CrossRef]
- Vuts, J.; Woodcock, C.M.; Sumner, M.E.; Caulfield, J.C.; Reed, K.; Inward, D.J.; Leather, S.R.; Pickett, J.A.; Birkett, M.A.; Denman, S. Responses of the two-spotted oak buprestid, Agrilus biguttatus (Coleoptera: Buprestidae), to host tree volatiles. Pest. Manag. Sci. 2016, 72, 845–851. [Google Scholar] [CrossRef] [Green Version]
- De Groot, P.; Grant, G.G.; Poland, T.M.; Scharbach, R.; Buchan, L.; Nott, R.W.; MacDonald, L.; Pitt, D. Electrophysiological response and attraction of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage. J. Chem. Ecol. 2008, 34, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Bari, G.; Scala, A.; Garzone, V.; Salvia, R.; Yalcin, C.; Vernile, P.; Aresta, A.M.; Facini, O.; Baraldi, R.; Bufo, S.A.; et al. Chemical ecology of Capnodis tenebrionis (L.) (Coleoptera: Buprestidae): Behavioral and biochemical strategies for intraspecific and host interactions. Front. Physiol. 2019, 10, 604. [Google Scholar] [CrossRef]
- Coleman, T.W.; Chen, Y.; Graves, A.D.; Hishinuma, S.M.; Grulke, N.E.; Flint, M.L.; Seybold, S.L. Developing monitoring techniques for the invasive goldspotted oak borer (Coleoptera: Buprestidae) in California. Environ. Entomol. 2014, 43, 729–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Fraser, I. Antennally active macrolide from the emerald ash borer Agrilus planipennis emitted predominantly by females. J. Chem. Ecol. 2007, 33, 1299–1302. [Google Scholar] [CrossRef]
- Silk, P.J.; Ryall, K.; Mayo, P.; Lemay, M.A.; Grant, G.; Crook, D.; Cossé, A.; Fraser, I.; Sweeney, J.D.; Lyons, D.B.; et al. Evidence for a volatile pheromone in Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) that increases attraction to a host foliar volatile. Environ. Entomol. 2011, 40, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.P.; Potter, D.A. Evidence for sexual attraction by the twolined chestnut borer Agrilus bilineatus (Weber) (Coleoptera: Buprestidae). Can. Entomol. 1988, 120, 1037–1039. [Google Scholar] [CrossRef]
- Lelito, J.P.; Böröczky, K.; Jones, T.H.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Behavioral evidence for a contact sex pheromone component of the emerald ash borer, Agrilus planipennis Fairmaire. J. Chem. Ecol. 2009, 35, 104–110. [Google Scholar] [CrossRef]
- Silk, P.J.; Ryall, K.; Barry Lyons, D.; Sweeney, J.; Wu, J. A contact sex pheromone component of the emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Naturwissenschaften 2009, 96, 601–608. [Google Scholar] [CrossRef]
- Francke, W.; Kitching, W. Spiroacetals in Insects. Curr. Org. Chem. 2005, 5, 233–251. [Google Scholar] [CrossRef]
- Noushini, S.; Park, S.J.; Jamie, I.; Jamie, J.; Taylor, P. Rectal gland exudates and emissions of Bactrocera bryoniae: Chemical identification, electrophysiological and pheromonal functions. Chemoecology 2021, 31, 137–148. [Google Scholar] [CrossRef]
- Helms, A.M.; De Moraes, C.M.; Tröger, A.; Alborn, H.T.; Francke, W.; Tooker, J.F.; Mescher, M.C. Identification of an insect-produced olfactory cue that primes plant defenses. Nat. Commun. 2017, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Francke, W.; Heemann, V.; Gerken, B.; Renwick, J.A.A.; Vité, J.P. 2-Ethyl-1,6-dioxaspiro[4.4]nonane, principal aggregation pheromone of Pityogenes chalcographus (L.). Naturwissenschaften 1977, 64, 590–591. [Google Scholar] [CrossRef]
- Byers, J.A.; Birgersson, G.; Francke, W. Aggregation pheromones of bark beetles, Pityogenes quadridens and P. bidentatus, colonizing Scotch pine: Olfactory avoidance of interspecific mating and competition. Chemoecology 2013, 23, 251–261. [Google Scholar] [CrossRef]
- Birgersson, G.; Dalusky, M.J.; Berisford, C.W. Identification of an aggregation pheromone for Pityogenes hopkinsi (Coleoptera: Scolytidae). Can. Entomol. 2000, 132, 951–963. [Google Scholar] [CrossRef]
- Dallara, P.L.; Seybold, S.J.; Meyer, H.; Tolasch, T.; Francke, W.; Wood, D.L. Semiochemicals from three species of Pityophthorus (Coleoptera: Scolytidae): Identification and field response. Can. Entomol. 2000, 132, 889–906. [Google Scholar] [CrossRef]
- Långström, B. Life Cycles and Shoot-Feeding of the Pine Shoot Beetles; Studia Forestalia Suecica: Stockholm, Sweden, 1983; ISBN 9157612463. [Google Scholar]
- Haack, R.A. Feeding biology of cerambycids. In Cerambycidae of the World; Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 105–124. [Google Scholar]
- Ryall, K.L.; Dutkiewicz, D.; Silk, P.J.; Antunes, P.M.; Ochoa, I. Ovarian development of Agrilus planipennis: Effects of age and mating status and influence on attraction to host volatiles. Entomol. Exp. Appl. 2013, 149, 77–84. [Google Scholar] [CrossRef]
- Krohn, S.; Fletcher, M.T.; Kitching, W.; Drew, R.A.I.; Moore, C.J.; Francke, W. Chemistry of fruit flies: Nature of glandular secretion and volatile emission of Bactrocera (Bactrocera) cacuminatus (Héring). J. Chem. Ecol. 1991, 17, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Haniotakis, G.; Francke, W.; Mori, K.; Redlich, H.; Schurig, V. Sex-specific activity of (R)-(-)- and (S)- (+)-1,7-dioxaspiro[5.5]undecane, the major pheromone of Dacus oleae. J. Chem. Ecol. 1986, 12, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Levi-Zada, A.; Nestel, D.; Fefer, D.; Nemni-Lavy, E.; Deloya-Kahane, I.; David, M. Analyzing diurnal and age-related pheromone emission of the olive fruit fly, Bactrocera oleae by sequential SPME-GCMS analysis. J. Chem. Ecol. 2012, 38, 1036–1041. [Google Scholar] [CrossRef]
- Singh, A.A.; Rowley, J.A.; Schwartz, B.D.; Kitching, W.; De Voss, J.J. Oxidative carbon–carbon bond cleavage is a key step in spiroacetal biosynthesis in the fruit fly Bactrocera cacuminata. J. Org. Chem. 2014, 79, 7799–7821. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.A.; Borden, J.H. Bark reflectance spectra of conifers and angiosperms: Implications for host discrimination by coniferophagous bark and timber beetles. Can. Entomol. 2005, 137, 719–722. [Google Scholar] [CrossRef]
- Strom, B.L.; Goyer, R.A. Effect of silhouette color on trap catches of Dendroctonus frontalis (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 2001, 94, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Crook, D.J.; Francese, J.A.; Zylstra, K.E.; Fraser, I.; Sawyer, A.J.; Bartels, D.W.; Lance, D.R.; Mastro, V.C. Laboratory and field response of the emerald ash borer (Coleoptera: Buprestidae), to selected regions of the electromagnetic spectrum. J. Econ. Entomol. 2009, 102, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Domingue, M.J.; Lelito, J.P.; Myrick, A.J.; Csóka, G.; Szőcs, L.; Imrei, Z.; Baker, T.C. Differences in spectral selectivity between stages of visually-guided mating approaches in a buprestid beetle. J. Exp. Biol. 2016, 219, 2837–2843. [Google Scholar] [CrossRef] [Green Version]
- Imrei, Z.; Lohonyai, Z.; Csóka, G.; Muskovits, J.; Szanyi, S.; Vétek, G.; Fail, J.; Tóth, M.; Domingue, M.J. Improving trapping methods for buprestid beetles to enhance monitoring of native and invasive species. For. An. Int. J. For. Res. 2020, 93, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Rassati, D.; Marini, L.; Marchioro, M.; Rapuzzi, P.; Magnani, G.; Poloni, R.; Di Giovanni, F.; Mayo, P.; Sweeney, J. Developing trapping protocols for wood-boring beetles associated with broadleaf trees. J. Pest. Sci. 2019, 92, 267–279. [Google Scholar] [CrossRef]
- Poland, T.M.; Petrice, T.R.; Ciaramitaro, T.M. Trap Designs, colors, and lures for emerald ash borer detection. Front. For. Glob. Chang. 2019, 2, 80. [Google Scholar] [CrossRef] [Green Version]
- Silk, P.J.; Ryall, K.L.; Grant, G.; Roscoe, L.E.; Mayo, P.; Williams, M.; LeClair, G.; Kimoto, T.; Williams, D.; Rutledge, C. Tree girdling and host tree volatiles provides a useful trap for bronze birch borer Agrilus anxius Gory (Coleoptera: Buprestidae). For. An. Int. J. For. Res. 2019, 93, 265–272. [Google Scholar] [CrossRef]
- Cavaletto, G.; Faccoli, M.; Marini, L.; Spaethe, J.; Magnani, G.; Rassati, D. Effect of trap color on captures of bark- and wood-boring beetles (Coleoptera; Buprestidae and Scolytinae) and associated predators. Insects 2020, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Petrice, T.R.; Haack, R.A.; Poland, T.M. Attraction of Agrilus planipennis (Coleoptera: Buprestidae) and other buprestids to sticky traps of various colors and shapes. Great Lakes Entomol. 2013, 46, 13–30. [Google Scholar]
- Brown, N.; Jeger, M.; Kirk, S.; Williams, D.; Xu, X.; Pautasso, M.; Denman, S. Acute oak decline and Agrilus biguttatus: The co-occurrence of stem bleeding and D-shaped emergence holes in Great Britain. Forests 2017, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Francese, J.A.; Crook, D.J.; Fraser, I.; Lance, D.R.; Sawyer, A.J.; Mastro, V.C. Optimization of trap color for emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2010, 103, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Domingue, M.J.; Imrei, Z.; Lelito, J.P.; Muskovits, J.; Janik, G.; Csóka, G.; Mastro, V.C.; Baker, T.C. Trapping of European buprestid beetles in oak forests using visual and olfactory cues. Entomol. Exp. Appl. 2013, 148, 116–129. [Google Scholar] [CrossRef]
- Ryall, K.L.; Silk, P.J.; Mayo, P.; Crook, D.; Khrimian, A.; Cossé, A.A.; Sweeney, J.; Scarr, T. Attraction of Agrilus planipennis (Coleoptera: Buprestidae) to a volatile pheromone: Effects of release rate, host volatile, and trap placement. Environ. Entomol. 2012, 41, 648–656. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Miller, J.R.; Poland, T.M.; Kuhn, T.M.; Otis, G.W.; Turk, T.; Ward, D.L. Behaviors of adult Agrilus planipennis (Coleoptera: Buprestidae). Great Lakes Entomol. 2007, 40, 1. [Google Scholar]
- Schlyter, F.; Zhang, Q.-H.; Liu, G.-T.; Ji, L.-Z. A successful case of pheromone mass trapping of the bark beetle Ips duplicatus in a forest island, analysed by 20-year time-series data. Integr. Pest. Manag. Rev. 2001, 6, 185–196. [Google Scholar] [CrossRef]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of western North American bark beetles with semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef]
- Pajares, J.A.; Álvarez, G.; Hall, D.R.; Ibarra, N.; Hoch, G.; Halbig, P.; Cocoş, D.; Johansson, H.; Schroeder, M. Attractants for management of the pine sawyer beetle Monochamus sutor, a potential vector of Bursaphelenchus xylophilus. J. Appl. Entomol. 2017, 141, 97–111. [Google Scholar] [CrossRef]
- Álvarez, G.; Gallego, D.; Hall, D.R.; Jactel, H.; Pajares, J.A. Combining pheromone and kairomones for effective trapping of the pine sawyer beetle Monochamus galloprovincialis. J. Appl. Entomol. 2016, 140, 58–71. [Google Scholar] [CrossRef]
- Chen, G.; Song, Y.; Wang, P.; Chen, J.; Zhang, Z.; Wang, S.; Huang, X.; Zhang, Q.-H. Semiochemistry of Dendroctonus armandi Tsai and Li (Coleoptera: Curculionidae: Scolytinae): Both female-produced aggregation pheromone and host tree kairomone are critically important. Chemoecology 2015, 25, 135–145. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.P.; Guerrero, A. Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. J. Agric. Food Chem. 2000, 48, 6025–6029. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C.; Jang, E.B.; Light, D.M.; Alford, A.R. Enhancement of insect pheromone responses by green leaf volatiles. Naturwissenschaften 1990, 77, 29–31. [Google Scholar] [CrossRef]
- Savoie, A.; Borden, J.H.; Pierce, H.D.; Gries, R.; Gries, G. Aggregation pheromone of Pityogenes knechteli and semiochemical-based interactions with three other bark beetles. J. Chem. Ecol. 1998, 24, 321–337. [Google Scholar] [CrossRef]
- Deglow, E.K.; Borden, J.H. Green leaf volatiles disrupt and enhance response to aggregation pheromones by the ambrosia beetle, Gnathotrichus sulcatus (Coleoptera: Scolytidae). Can. J. For. Res. 1998, 28, 1697–1705. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Álvarez-Calero, J.M.; Riba-Flinch, J.M.; Coca-Abia, M.M.; Torrell, A.; Quero, C. Olean (1,7-dioxaspiro[5.5]undecane): A Novel Intraspecific Chemical Cue in Coraebus undatus (F.) (Coleoptera: Buprestidae). Insects 2021, 12, 1085. https://doi.org/10.3390/insects12121085
López S, Álvarez-Calero JM, Riba-Flinch JM, Coca-Abia MM, Torrell A, Quero C. Olean (1,7-dioxaspiro[5.5]undecane): A Novel Intraspecific Chemical Cue in Coraebus undatus (F.) (Coleoptera: Buprestidae). Insects. 2021; 12(12):1085. https://doi.org/10.3390/insects12121085
Chicago/Turabian StyleLópez, Sergio, José María Álvarez-Calero, Josep Maria Riba-Flinch, María Milagro Coca-Abia, Antoni Torrell, and Carmen Quero. 2021. "Olean (1,7-dioxaspiro[5.5]undecane): A Novel Intraspecific Chemical Cue in Coraebus undatus (F.) (Coleoptera: Buprestidae)" Insects 12, no. 12: 1085. https://doi.org/10.3390/insects12121085
APA StyleLópez, S., Álvarez-Calero, J. M., Riba-Flinch, J. M., Coca-Abia, M. M., Torrell, A., & Quero, C. (2021). Olean (1,7-dioxaspiro[5.5]undecane): A Novel Intraspecific Chemical Cue in Coraebus undatus (F.) (Coleoptera: Buprestidae). Insects, 12(12), 1085. https://doi.org/10.3390/insects12121085