What Are the Most Important Factors Influencing Springtail Tetrodontophora bielanensis?
Abstract
:Simple Summary
Abstract
1. Introduction
Main Aims
- How do tree characteristics and deadwood presence influence the species at the landscape scale?
- How is the distribution of the species influenced by forest tree factors at the site level?
2. Materials and Methods
2.1. Trapping Method
2.2. Study Areas
2.2.1. Krkonoše—Landscape Level
2.2.2. Orlické Hory—Site Level
2.3. Environmental Variables
2.3.1. Krkonoše
2.3.2. Orlické Hory
2.4. Statistical Analyses
3. Results
3.1. Landscape Scale—Krkonoše
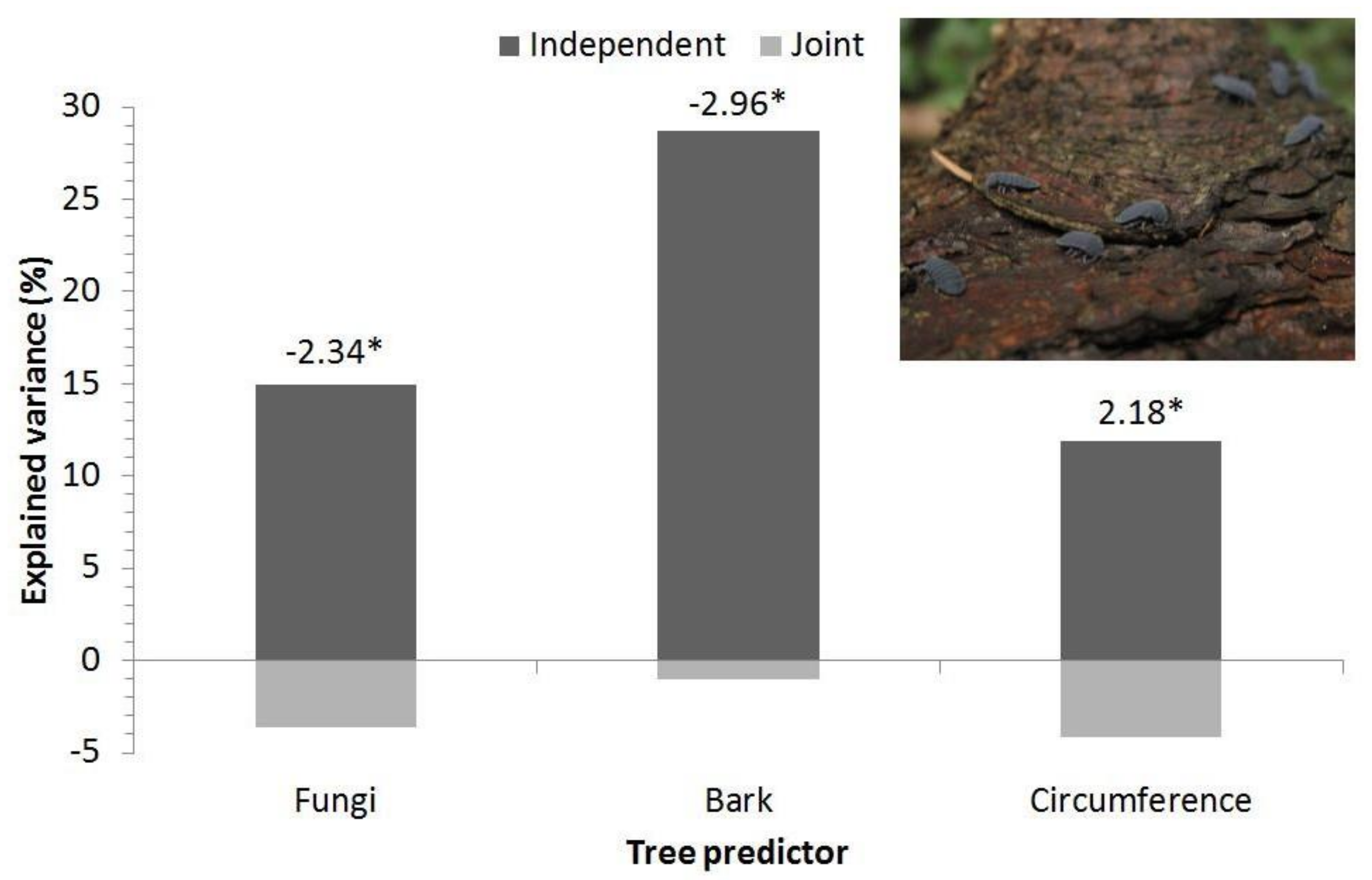
3.2. Site Level—Orlické Hory
4. Discussion
4.1. Effects at the Landscape Scale
4.2. Meso-Scale Overview
4.3. Springtails and Dead Wood Habitats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Ozanne, C.M.P. Arthropod communities of coniferous forest trees. Selbyana 1996, 17, 43–49. [Google Scholar]
- Chen, B.; Wise, D.H. Bottom-up limitation of predaceous arthropods in a detritus-based terrestrial food web. Ecology 1999, 80, 761–772. [Google Scholar] [CrossRef]
- Raschmanová, N.; Kováč, L.; Miklisová, D. The effect of mesoclimate on Collembola diversity in the Zádiel Valley, Slovak Karst (Slovakia). Eur. J. Soil Biol. 2008, 44, 463–472. [Google Scholar] [CrossRef]
- Dettner, K.; Scheuerlein, A.; Fabian, P.; Schultz, S.; Francke, W. Chemical defense of giant springtail Tetrodontophora bielanenesis (Waga) (Insecta: Collembola). J. Chem. Ecol. 1996, 22, 1051–1074. [Google Scholar] [CrossRef] [PubMed]
- Materna, J. Larvěnka obrovská—Obr mezi chvostoskoky. Krkonoše—Jizerské Hory 2006, 10–11. [Google Scholar]
- Rusek, J. Tetrodontophora bielanensis (Collembola: Onychiuridae), its distribution and ecological requirements. Pedobiologia 1997, 41, 74–79. [Google Scholar]
- Grear, J.S.; Schmitz, O.J. Effects of grouping behavior and predators on the spatial distribution of a forest floor arthropod. Ecology 2005, 86, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Kontschán, J.; Murányi, D.; Traser, G. Data to the distribution of Tetrodontophora bielanensis (Waga, 1842) (Collembola: Onychiuridae). Ann. Hist. Nat. Mus. Nat. Hung. 2003, 95, 107–121. [Google Scholar]
- Hopkin, S.P. Biology of the Springtails; Oxford University Press: Oxford, UK, 1997; p. 340. [Google Scholar]
- Addison, J.A.; Trofymow, J.A.; Marshall, V.G. Functional role of Collembola in successional coastal temperate forests on Vancouver Island. Appl. Soil Ecol. 2003, 24, 247–261. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Leinaas, H.P.; Bengtsson, J.; Birokemoe, T. Age and level of self-organization affect the small-scale distribution of springtails (Collembola). Ecosphere 2018, 9, e02058. [Google Scholar] [CrossRef]
- Horák, J.; Vodka, Š.; Pavlíček, J.; Boža, P. Unexpected visitors: Flightless beetles in window traps. J. Insect Conserv. 2013, 17, 441–449. [Google Scholar] [CrossRef]
- Mazzei, A.; Bonacci, T.; Horák, J.; Brandmayr, P. The role of topography, stand and habitat features for management and biodiversity of a prominent forest hotspot of the Mediterranean Basin: Saproxylic beetles as possible indicators. Forest Ecol. Manag. 2018, 410, 66–75. [Google Scholar] [CrossRef]
- Bogusch, P.; Horák, J. Saproxylic bees and wasps. In Saproxylic Insects; Springer: Cham, Germany, 2018; pp. 217–235. [Google Scholar]
- Hájek, J.; Kučera, J. Orlické hory. In Chráněná Území ČR; Agentura Ochrany Přírody a Krajiny ČR a Ekocentrum Brno: Praha, Czech Republic, 2002. [Google Scholar]
- Horák, J.; Materna, J.; Halda, J.P.; Mladenović, S.; Bogusch, P.; Pech, P. Biodiversity in remnants of natural mountain forests under conservation-oriented management. Sci. Rep. 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horák, J.; Pavliček, J. Tree level indicators of species composition of saproxylic beetles in old-growth mountainous spruce-beech forest through variation partitioning. J. Insect Conserv. 2013, 17, 1003–1009. [Google Scholar] [CrossRef]
- Frutos, A.; Olea, P.P.; Vera, R. Analyzing and modelling spatial distribution of summering lesser kestrel: The role of spatial autocorrelation. Ecol. Model. 2007, 200, 33–44. [Google Scholar] [CrossRef]
- Hanzák, J.; Halík, L.; Mikulová, M.; Pospíšil, A. Světem Zvířat-Bezobratlí 1; Albatros: Praha, Czech Republic, 1973; p. 322. [Google Scholar]
- Negri, I. Spatial distribution of Collembola in presence and absence of a predator. Pedobiologia 2004, 48, 585–588. [Google Scholar] [CrossRef]
- Flousek, J.; Hartmanová, O.; Štursa, J.; Potocki, J. Krkonoše, Příroda, Historie, Život; Miloš Uhlíř—Baset: Praha, Czech Republic, 2007; p. 864. [Google Scholar]
- Gilbertson, R.L. Relationships between insects and wood-rotting basidiomycetes. In Fungus-Insect Relationships, Perspectives in Ecology and Evolution; Columbia University Press: New York, NY, USA, 1984. [Google Scholar]
- Wermelinger, B. Development and distribution of predators and parasitoids during two consecutive years of an Ips typographus (Col., Scolytidae) infestation. J. Appl. Entomol. 2002, 126, 521–527. [Google Scholar] [CrossRef]
- Matic, R.; Koledin, D. Preference and feeding specificity of Tetrodontophora bielanensis (Collembola, Insecta) under laboratory conditions. Rev. Ecol. Biol. Sol. 1985, 22, 121–129. [Google Scholar]
- Anslan, S.; Bahram, M.; Tedersoo, L. Temporal changes in fungal communities associated with guts and appendages of Collembola as based on culturing and high-throughput sequencing. Soil Biol. Biochem. 2016, 96, 152–159. [Google Scholar] [CrossRef]
- De Ruiter, P.C.; Van Veen, J.A.; Moore, J.C.; Brussaard, L.; Hunt, H.W. Calculation of nitrogen mineralization in soil food webs. Plant Soil 1993, 157, 263–273. [Google Scholar] [CrossRef]
- Chahartaghi, M.; Langel, R.; Scheu, S.; Ruess, L. Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 2005, 37, 1718–1725. [Google Scholar] [CrossRef]
- Garrick, R.C.; Sands, C.J.; Rowell, D.M.; Tait, N.N.; Greenslade, P.; Sunnucks, P. Phylogeography recapitulates topography: Very fine-scale local endemism of a saproxylic ‘giant’ springtail at Tallaganda in the great dividing range of South–East Australia. Mol. Ecol. 2004, 13, 3329–3344. [Google Scholar] [CrossRef]
- Smolis, A.; Kadej, M. A new saproxylic Paleonurini (Collembola, Neanuridae) species from North America with the first record of Galanura agnieskae Smolis, 2000 from the continent. Fla. Entomol. 2014, 97, 1386–1395. [Google Scholar] [CrossRef]
- Smolis, A.; Deharveng, L. Diversity of Paranura Axelson, 1902 (Collembola: Neanuridae) in pacific region of Russia and United States. Zootaxa 2015, 4033, 203–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiera, C. Application of stable isotopes and lipid analysis to understand trophic interactions in springtails. North-West. J. Zool. 2014, 10, 227–235. [Google Scholar]
- Anderson, R.V.; Coleman, D.C.; Cole, C.V. Effects of saprotrophic grazing on net mineralization—Role of soil fauna. Terrestrial nitrogen cycles: Processes, ecosystem strategies and management impacts. Ecol. Bull. 1981, 33, 201–216. [Google Scholar]
- Seibold, S.; Bässler, C.; Baldrian, P.; Reinhard, L.; Thorn, S.; Ulyshen, M.D.; Müller, J. Dead-wood addition promotes non-saproxylic epigeal arthropods but effects are mediated by canopy openness. Biol. Conserv. 2016, 204, 181–188. [Google Scholar] [CrossRef]
- Setälä, H.; Marshall, V.G.; Trofymow, J.A. Influence of micro- and macro- habitat factors on collembolan communities in Douglas-fir stumps during forest succession. Appl. Soil Ecol. 1995, 2, 227–242. [Google Scholar] [CrossRef]
- Mladenović, S.; Loskotová, T.; Boháč, J.; Pavlíček, J.; Brestovanský, J.; Horák, J. The effects of within stand disturbance in plantation forests indicate complex and contrasting responses among and within beetle families. Bull. Entomol. Res. 2018, 108, 750–764. [Google Scholar] [CrossRef]
- Grove, S.J. The influence of forest management history on the integrity of the saproxylic beetle fauna in an Australian lowland tropical rainforest. Biol. Conserv. 2002, 104, 149–171. [Google Scholar] [CrossRef]
- Raymond-Léonard, L.J.; Bouchard, M.; Handa, I.T. Dead wood provides habitat for springtails across a latitudinal gradient of forests in Quebec, Canada. Forest Ecol. Manag. 2020, 472, 118237. [Google Scholar] [CrossRef]


| Level | Variable | Mean | Standard Error |
|---|---|---|---|
| Landscape | Altitude (m a.s.l.) | 800.8 | 12.32 |
| Circumference (cm) | 154.92 | 3.46 | |
| Dead wood (m3) | 2.17 | 0.31 | |
| Canopy openness (%) | 16.11 | 0.53 | |
| Site | Bark coverage (%) | 74.71 | 5.89 |
| Circumference (cm) | 161.59 | 10.14 |
| Environmental Variable | t | p |
|---|---|---|
| Altitude | 1.18 | 0.24 |
| Tree species (spruce) | 2.61 | 0.011 |
| Tree circumference | −1.73 | 0.09 |
| Dead wood | −0.99 | 0.33 |
| Canopy openness | 0.23 | 0.82 |
| Year | 1.23 | 0.22 |
| Autocovariate | −4.24 | <0.001 |
| Environmental Variable | t | p |
|---|---|---|
| Tree species (spruce) | −0.92 | 0.38 |
| Tree decay stage | 0.28 | 0.78 |
| Fungi | −2.26 | 0.045 |
| Bark | −2.54 | 0.028 |
| Tree circumference | 1.82 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, S.; Materna, J.; Brestovanská, T.; Horák, J. What Are the Most Important Factors Influencing Springtail Tetrodontophora bielanensis? Insects 2021, 12, 858. https://doi.org/10.3390/insects12100858
Mladenović S, Materna J, Brestovanská T, Horák J. What Are the Most Important Factors Influencing Springtail Tetrodontophora bielanensis? Insects. 2021; 12(10):858. https://doi.org/10.3390/insects12100858
Chicago/Turabian StyleMladenović, Strahinja, Jan Materna, Tereza Brestovanská, and Jakub Horák. 2021. "What Are the Most Important Factors Influencing Springtail Tetrodontophora bielanensis?" Insects 12, no. 10: 858. https://doi.org/10.3390/insects12100858
APA StyleMladenović, S., Materna, J., Brestovanská, T., & Horák, J. (2021). What Are the Most Important Factors Influencing Springtail Tetrodontophora bielanensis? Insects, 12(10), 858. https://doi.org/10.3390/insects12100858






