Temporal Dynamics of ‘Ca. Phytoplasma mali’ Load in the Insect Vector Cacopsylla melanoneura
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
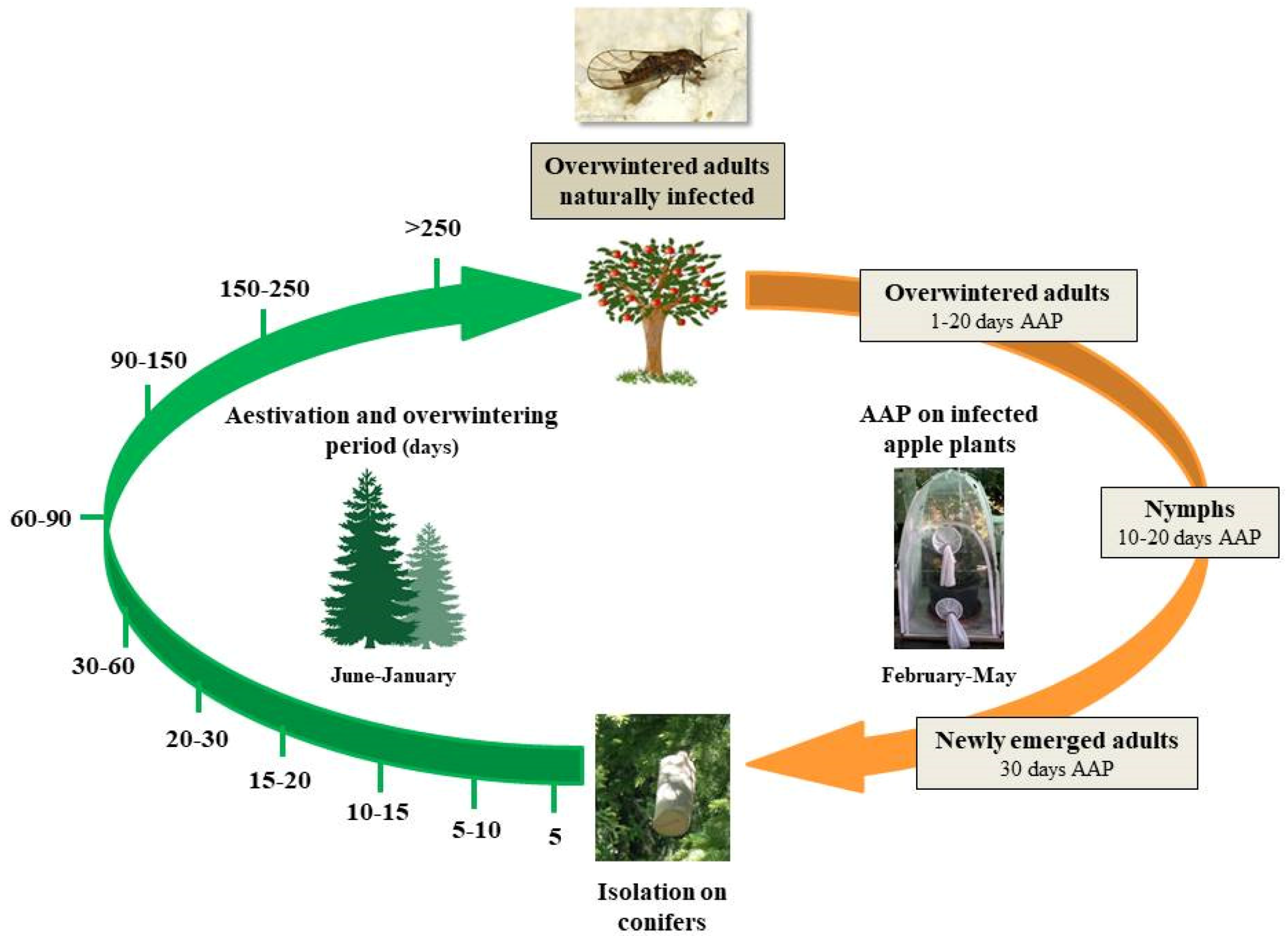
2.1. Experimental Sites and Insect Sampling
2.2. Insect Rearing and ‘Ca. Phytoplasma mali’ Acquisition Trials
2.3. Dynamics of ‘Ca. Phytoplasma mali’ Load During C. melanoneura Aestivation and Overwintering
2.4. DNA Extraction and ‘Ca. Phytoplasma mali’ Quantification via RT-qPCR
2.5. Statistical Analyses
3. Results
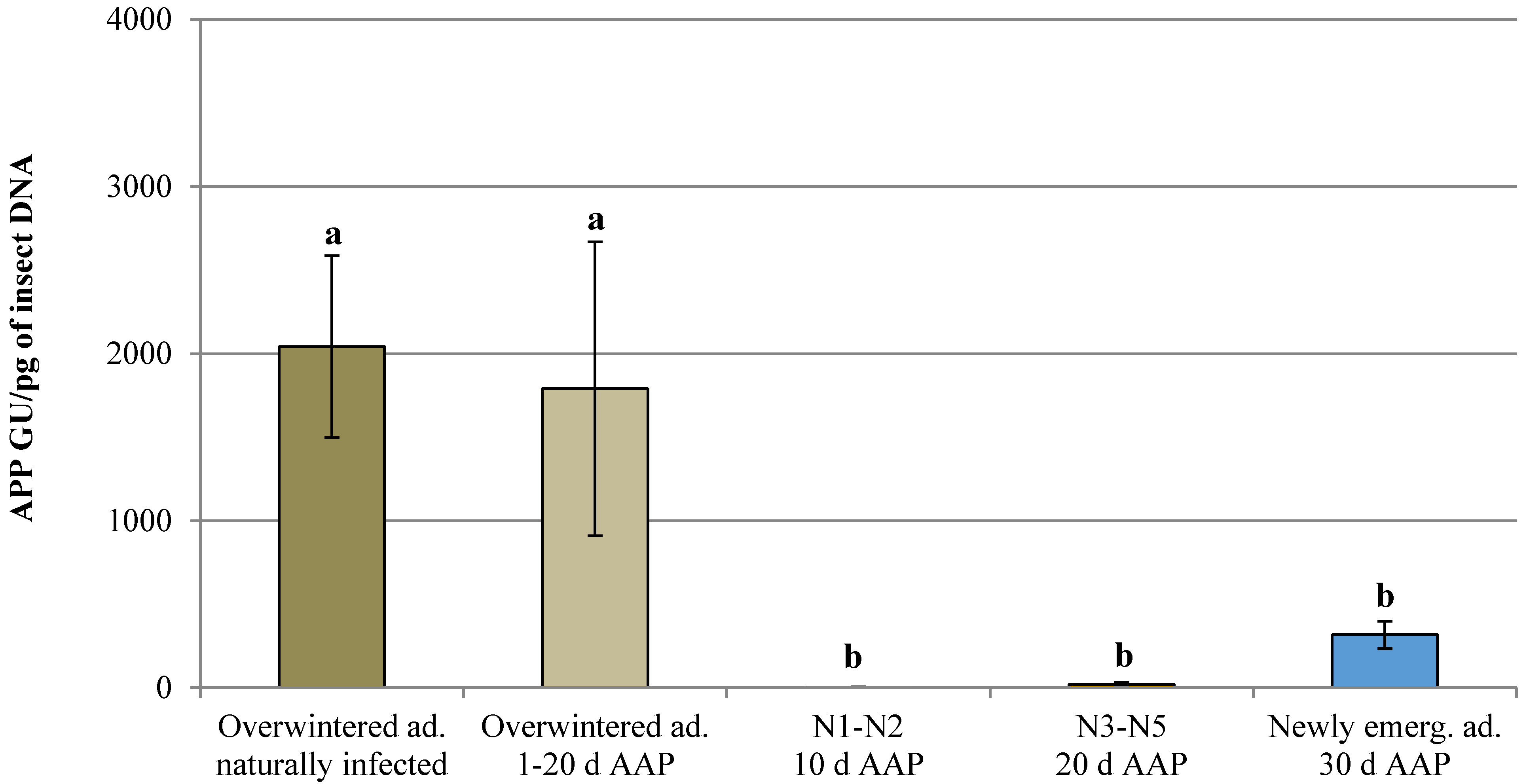
3.1. Natural Infection and Controlled Acquisition of ‘Ca. Phytoplasma mali’
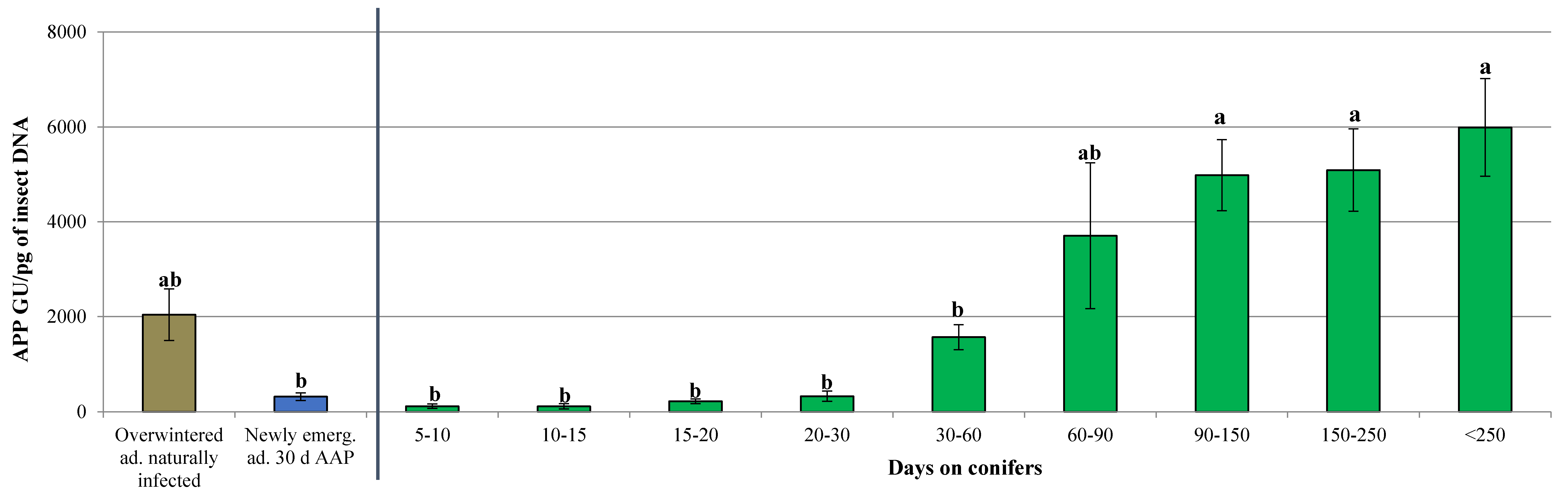
3.2. Dynamics of ‘Ca. Phytoplasma mali’ Load During the Aestivation and Overwintering Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Namba, S. Molecular and biological properties of phytoplasmas. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; D’Amelio, R. Transmission specificity and competition of multiple phytoplasmas in the insect vector. In Phytoplasmas: Genomes, Plant Hosts and Vectors; Weintraub, P.G., Jones, P., Eds.; CABI: Wallington, UK, 2010; pp. 293–308. [Google Scholar]
- Hogenhout, S.A.; Oshima, K.; Ammar, E.D.; Kakizawa, S.; Kingdom, H.N.; Namba, S. Phytoplasmas: Bacteria that manipulate plants and insects. Mol. Plant Pathol. 2008, 9, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Tedeschi, R. Insect vector transmission assays. In Phytoplasma: Methods and Protocols; Dickinson, M., Hodgetts, J., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 73–85. [Google Scholar]
- Alma, A.; Lessio, F.; Nickel, H. Insects as phytoplasma vectors: Ecological and epidemiological aspects. In Phytoplasmas: Plant Pathogenic Bacteria-II; Bertaccini, A., Weintraub, P., Rao, G., Mori, N., Eds.; Springer: Singapore, 2019; pp. 1–25. [Google Scholar]
- Bressan, A.; Spiazzi, S.; Girolami, V.; Boudon-Padieu, E. Acquisition efficiency of Flavescence dorée phytoplasma by Scaphoideus titanus Ball from infected tolerant or susceptible grapevine cultivars or experimental host plants. Vitis 2005, 44, 143–146. [Google Scholar]
- Bosco, D.; Galetto, L.; Leoncini, P.; Saracco, P.; Raccah, B.; Marzachì, C. Interrelationships between Candidatus Phytoplasma asteris and its leafhopper vectors (Homoptera: Cicadellidae). J. Econ. Entomol. 2007, 100, 1504–1511. [Google Scholar] [CrossRef]
- Galetto, L.; Marzachì, C.; Marques, R.; Graziano, C.; Bosco, D. Effects of temperature and CO2 on phytoplasma multiplication pattern in vector and plant. Bull. Insectology 2011, 64, 151–152. [Google Scholar]
- Chuche, J.; Thiéry, D. Biology and ecology of the flavescence dorée vector Scaphoideus titanus: A review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef]
- Galetto, L.; Miliordos, D.; Roggia, C.; Rashidi, M.; Sacco, D.; Marzachì, C.; Bosco, D. Acquisition capability of the grapevine flavescence dorée by the leafhopper vector Scaphoideus titanus Ball correlates with phytoplasma titre in the source plant. J. Pest. Sci. 2014, 87, 671–679. [Google Scholar] [CrossRef]
- Maggi, F.; Bosco, D.; Marzachì, C. Dynamics of acquisition and transmission of Flavescence dorée phytoplasma in grapevine. Phytopathogenic Mollicutes 2014, 4, 59–71. [Google Scholar] [CrossRef]
- Roggia, C.; Caciagli, P.; Galetto, L.; Pacifico, D.; Veratti, F.; Bosco, D.; Marzachì, C. Flavescence dorée phytoplasma titre in field-infected Barbera and Nebbiolo grapevines. Plant Pathol. 2014, 63, 31–41. [Google Scholar] [CrossRef]
- Alma, A.; Tedeschi, R.; Lessio, F.; Picciau, L.; Gonella, E.; Ferracini, C. Insect vectors of plant pathogenic mollicutes in the European-Mediterranean region. Phytopathogenic Mollicutes 2015, 5, 53–73. [Google Scholar] [CrossRef]
- Galetto, L.; Miliordos, D.; Pegoraro, M.; Sacco, D.; Veratti, F.; Marzachì, C.; Bosco, D. Acquisition of Flavescence dorée phytoplasma by Scaphoideus titanus Ball from different grapevine varieties. Int. J. Mol. Sci. 2016, 17, 1563. [Google Scholar] [CrossRef] [PubMed]
- Alma, A.; Lessio, F.; Gonella, E.; Picciau, L.; Mandrioli, M.; Tota, F. New insights in phytoplasma-vector interaction: Acquisition and inoculation of Flavescence dorée phytoplasma by Scaphoideus titanus adults in a short window of time. Ann. Appl. Biol. 2018, 173, 55–62. [Google Scholar] [CrossRef]
- Lauterer, P. Results of the investigations on Hemiptera in Moravia, made by the Moravian museum (Psylloidea 2). Acta Mus. Moraviae Sci. Biol. 1999, 84, 71–151. [Google Scholar]
- Hodkinson, I.D. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): A Global Synthesis. J. Nat. Hist. 2009, 43, 65–179. [Google Scholar] [CrossRef]
- Jarausch, B.; Jarausch, W. Establishment of a permanent rearing of Cacopsylla picta (Hemiptera: Psylloidea), the main vector of ‘Candidatus Phytoplasma mali’ in Germany. J. Pest. Sci. 2014, 87, 459–467. [Google Scholar] [CrossRef]
- Thébaud, G.; Yvon, M.; Alary, R.; Sauvion, N.; Labonne, G. Efficient transmission of ‘Candidatus Phytoplasma prunorum’ is delayed by eight months due to a long latency in its host-alternating vector. Phytopathology 2009, 99, 265–273. [Google Scholar] [CrossRef]
- Baric, S.; Berger, J.; Cainelli, C.; Kerschbamer, C.; Dalla Via, J. Molecular typing of ‘Candidatus Phytoplasma mali’ and epidemic history tracing by a combined T-RFLP/VNTR analysis approach. Eur. J. Plant Pathol. 2011, 131, 573–584. [Google Scholar] [CrossRef]
- Casati, P.; Quaglino, F.; Tedeschi, R.; Spiga, F.M.; Alma, A.; Spadone, P.; Bianco, P.A. Identification and molecular characterization of ‘Candidatus Phytoplasma mali’ isolates in North-western Italy. J. Phytopathol. 2010, 158, 81–87. [Google Scholar] [CrossRef]
- Jarausch, W.; Saillard, C.; Helliot, B.; Garnier, M.; Dosba, F. Genetic variability of apple proliferation phytoplasmas as determined by PCR-RFLP and sequencing of a non-ribosomal fragment. Mol. Cell. Probes 2000, 14, 17–24. [Google Scholar] [CrossRef]
- Cainelli, C.; Bisognin, C.; Vindimian, M.E.; Grando, M.S. Genetic variability of AP phytoplasmas detected in the apple growing area of Trentino (North Italy). Acta Hortic. 2004, 657, 425–430. [Google Scholar] [CrossRef]
- Jarausch, B.; Schwind, N.; Jarausch, W.; Krczal, G. Overwintering adults and springtime generation of Cacopsylla picta (synonym C. costalis) can transmit apple proliferation phytoplasmas. Acta Hortic. 2004, 657, 409–413. [Google Scholar] [CrossRef]
- Martini, M.; Ermacora, P.; Falginella, L.; Loi, N.; Carraro, L. Molecular differentiation of ‘Candidatus Phytoplasma mali’ and its spreading in Friuli Venezia Giulia region (North-East Italy). Acta Hortic. 2008, 781, 395–402. [Google Scholar] [CrossRef]
- Tedeschi, R.; Bosco, D.; Alma, A. Population dynamics of Cacopsylla melanoneura (Homoptera: Psyllidae), a vector of apple proliferation in northwestern Italy. J. Econ. Entomol. 2002, 95, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Pizzinat, A.; Tedeschi, R.; Alma, A. Cacopsylla melanoneura (Foerster): Aestivation and overwintering habitats in Northwest Italy. Bull. Insectology 2011, 64, 135–136. [Google Scholar]
- Tedeschi, R.; Visentin, C.; Alma, A.; Bosco, D. Epidemiology of apple proliferation (AP) in Northwestern Italy: Evaluation of the frequency of AP-positive psyllids in naturally infected populations of Cacopsylla melanoneura (Homoptera Psyllidae). Ann. Appl. Biol. 2003, 142, 285–290. [Google Scholar] [CrossRef]
- Tedeschi, R.; Alma, A. Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). J. Econ. Entomol. 2004, 97, 8–13. [Google Scholar] [CrossRef]
- Bertin, S.; Bosco, D. Molecular identification of phytoplasma vector species. In Phytoplasma: Methods and Protocols; Dickinson, M., Hodgetts, J., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 87–108. [Google Scholar]
- Monti, M.; Martini, M.; Tedeschi, R. EvaGreen real-time PCR protocol for specific ‘Candidatus Phytoplasma mali’ detection and quantification in insects. Mol. Cell. Probes 2013, 27, 129–136. [Google Scholar] [CrossRef]
- Martini, M.; Loi, N.; Ermacora, P.; Carraro, L.; Pastore, M. A real-time PCR method for detection and quantification of ‘Candidatus Phytoplasma prunorum’ in its natural hosts. Bull. Insectology 2007, 60, 251. [Google Scholar]
- Marzachì, C.; Bosco, D. Relative quantification of Chrysanthemum Yellows (16Sr l) phytoplasma in its plant and insect host using real-time polymerase chain reaction. Mol. Biotechnol. 2005, 30, 117–127. [Google Scholar] [CrossRef]
- Oppedisano, T.; Panassiti, B.; Pedrazzoli, F.; Mittelberger, C.; Bianchedi, P.L.; Angeli, G.; De Cristofaro, A.; Janik, K.; Anfora, G.; Ioriatti, C. Importance of psyllids’ life stage in the epidemiology of apple proliferation phytoplasma. J. Pest. Sci. 2020, 93, 49–61. [Google Scholar] [CrossRef]
- Galetto, L.; Bosco, D.; Balestrini, R.; Genre, A.; Fletcher, J.; Marzachì, C. The major antigenic membrane protein of “Candidatus Phytoplasma asteris” selectively interacts with ATP synthase and actin of leafhopper vectors. PLoS ONE 2011, 6, 22571. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Ferrato, V.; Rossi, J.; Alma, A. Possible phytoplasma transovarial transmission in the psyllids Cacopsylla melanoneura and Cacopsylla pruni. Plant Pathol. 2006, 55, 18–24. [Google Scholar] [CrossRef]
- Mittelberger, C.; Obkircher, L.; Oettl, S.; Oppedisano, T.; Pedrazzoli, F.; Panassiti, B.; Kerschbamer, C.; Anfora, G.; Janik, K. The insect vector Cacopsylla picta vertically transmits the bacterium ‘Candidatus Phytoplasma mali’ to its progeny. Plant Pathol. 2017, 66, 1015–1021. [Google Scholar] [CrossRef]
| Life Stage | AAP (days) | Total Tested Individuals (N.) | APP Infected Individuals (N.) | Infection Rate (%) | Phytoplasma Titer (APP GU/pg of Insect DNA) | Phytoplasma Titer Range (APP GU/pg of Insect DNA) |
|---|---|---|---|---|---|---|
| Overwintered adult naturally infected | - | 180 | 3 | 1.67 | 2.04 × 103 ± 5.44 × 102 | 1.10 × 103–2.98 × 103 |
| Overwintered adult | 1 | 77 | 0 | 0.00 | n.d. | n.d. |
| 2 | 80 | 1 | 1.25 | 4.39 × 102 ± 0.00 | 4.39 × 102 | |
| 10 | 91 | 6 | 6.59 | 2.27 × 103 ± 1.12 × 103 | 5.80 × 10–7.65 × 103 | |
| 20 | 83 | 1 | 1.20 | 2.45 × 102 ± 0.00 | 2.45 × 102 | |
| Nymph (1st–2nd instar) | 10 | 42 | 3 | 7.14 | 4.29 ± 2.21 | 0.66–8.29 |
| Nymph (3rd–5th instar) | 20 | 60 | 29 | 48.33 | 2.06 × 10 ± 1.22 × 10 | 0.01–3.52 × 102 |
| Newly emerged adult | 30 | 83 | 35 | 42.17 | 3.17 × 102 ± 8.22 × 10 | 0.09–1.74 × 103 |
| Days on Conifers | Total Tested Individuals (N.) | APP Infected Individuals (N.) | Infection Rate (%) | Phytoplasma Titer Range (APP GU/pg of Insect DNA) |
|---|---|---|---|---|
| 5 | 5 | 0 | 0.00 | n.d. |
| 5–10 | 32 | 20 | 62.50 | 0.14–9.34 × 102 |
| 10–15 | 90 | 28 | 31.11 | 0.17–1.23 × 103 |
| 15–20 | 32 | 20 | 62.50 | 5.47–5.93 × 102 |
| 20–30 | 103 | 30 | 29.13 | 0.17–2.24 × 103 |
| 30–60 | 284 | 58 | 20.42 | 0.18–9.96 × 103 |
| 60–90 | 35 | 11 | 31.43 | 5.74 × 10–1.81 × 104 |
| 90–150 | 70 | 24 | 34.29 | 3.83 × 102–1.35 × 104 |
| 150–250 | 38 | 20 | 52.63 | 4.81 × 102–1.42 × 104 |
| >250 | 65 | 43 | 66.15 | 1.13 × 102–2.42 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candian, V.; Monti, M.; Tedeschi, R. Temporal Dynamics of ‘Ca. Phytoplasma mali’ Load in the Insect Vector Cacopsylla melanoneura. Insects 2020, 11, 592. https://doi.org/10.3390/insects11090592
Candian V, Monti M, Tedeschi R. Temporal Dynamics of ‘Ca. Phytoplasma mali’ Load in the Insect Vector Cacopsylla melanoneura. Insects. 2020; 11(9):592. https://doi.org/10.3390/insects11090592
Chicago/Turabian StyleCandian, Valentina, Monia Monti, and Rosemarie Tedeschi. 2020. "Temporal Dynamics of ‘Ca. Phytoplasma mali’ Load in the Insect Vector Cacopsylla melanoneura" Insects 11, no. 9: 592. https://doi.org/10.3390/insects11090592
APA StyleCandian, V., Monti, M., & Tedeschi, R. (2020). Temporal Dynamics of ‘Ca. Phytoplasma mali’ Load in the Insect Vector Cacopsylla melanoneura. Insects, 11(9), 592. https://doi.org/10.3390/insects11090592






