Sub-Lethal Effects of Partially Purified Protein Extracted from Beauveria bassiana (Balsamo) and Its Presumptive Role in Tomato (Lycopersicon esculentum L.) Defense against Whitefly (Bemisia tabaci Genn.)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Rearing of B. tabaci
2.2. Culture of B. bassiana
2.3. Preparation of Crude Protein and Its Partial Purification
2.4. Bioassay of Protein Activity against B. tabaci
2.5. RNA Extraction and cDNA Synthesis
2.6. Real-Time Quantitative PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
3.1. Crude Protein-Induced Necrosis in Tobacco Leaves
3.2. Effect of B. bassiana-Derived Protein on B. tabaci Survival
3.3. Effect of B. bassiana-Derived Protein on B. tabaci Fecundity
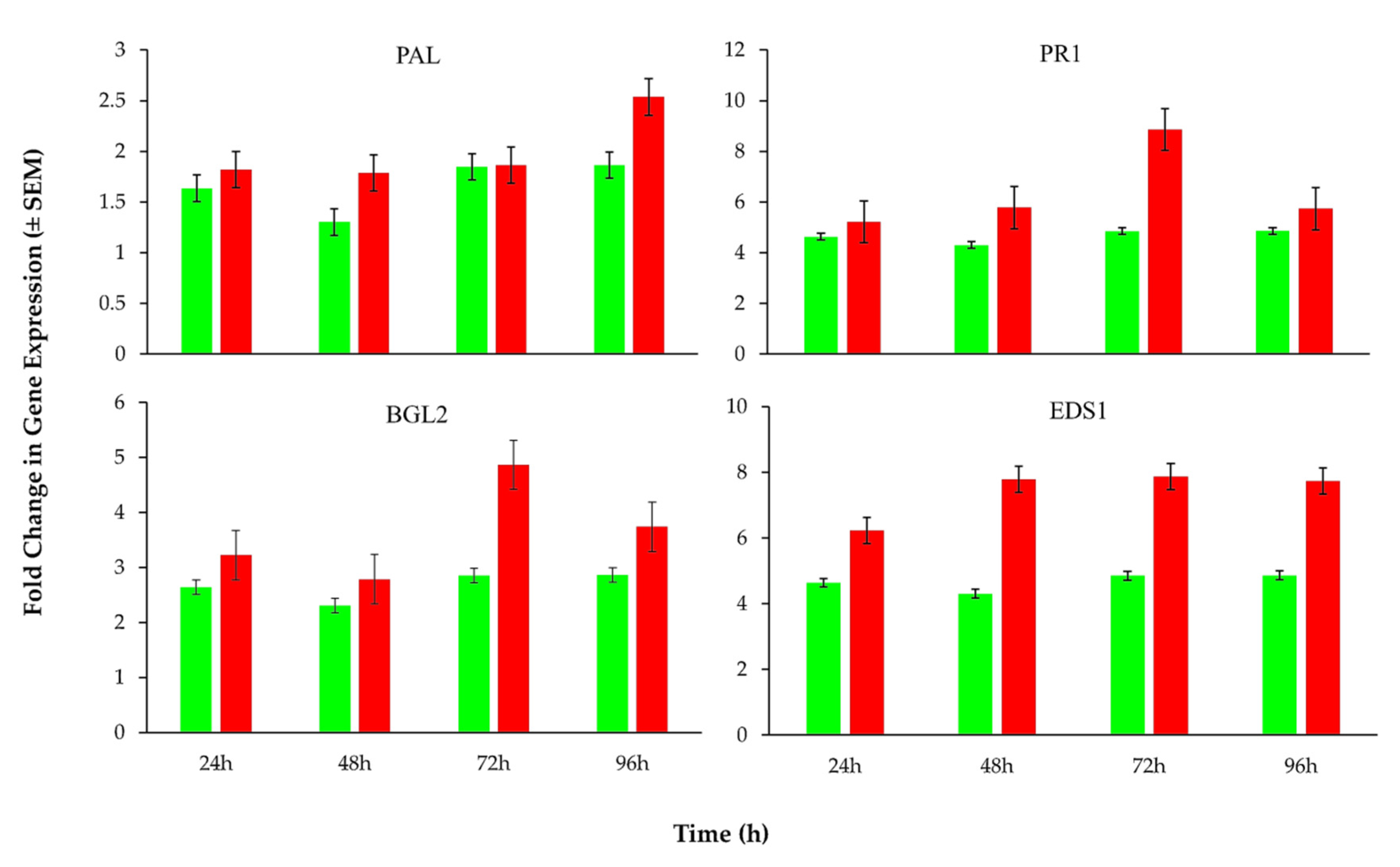
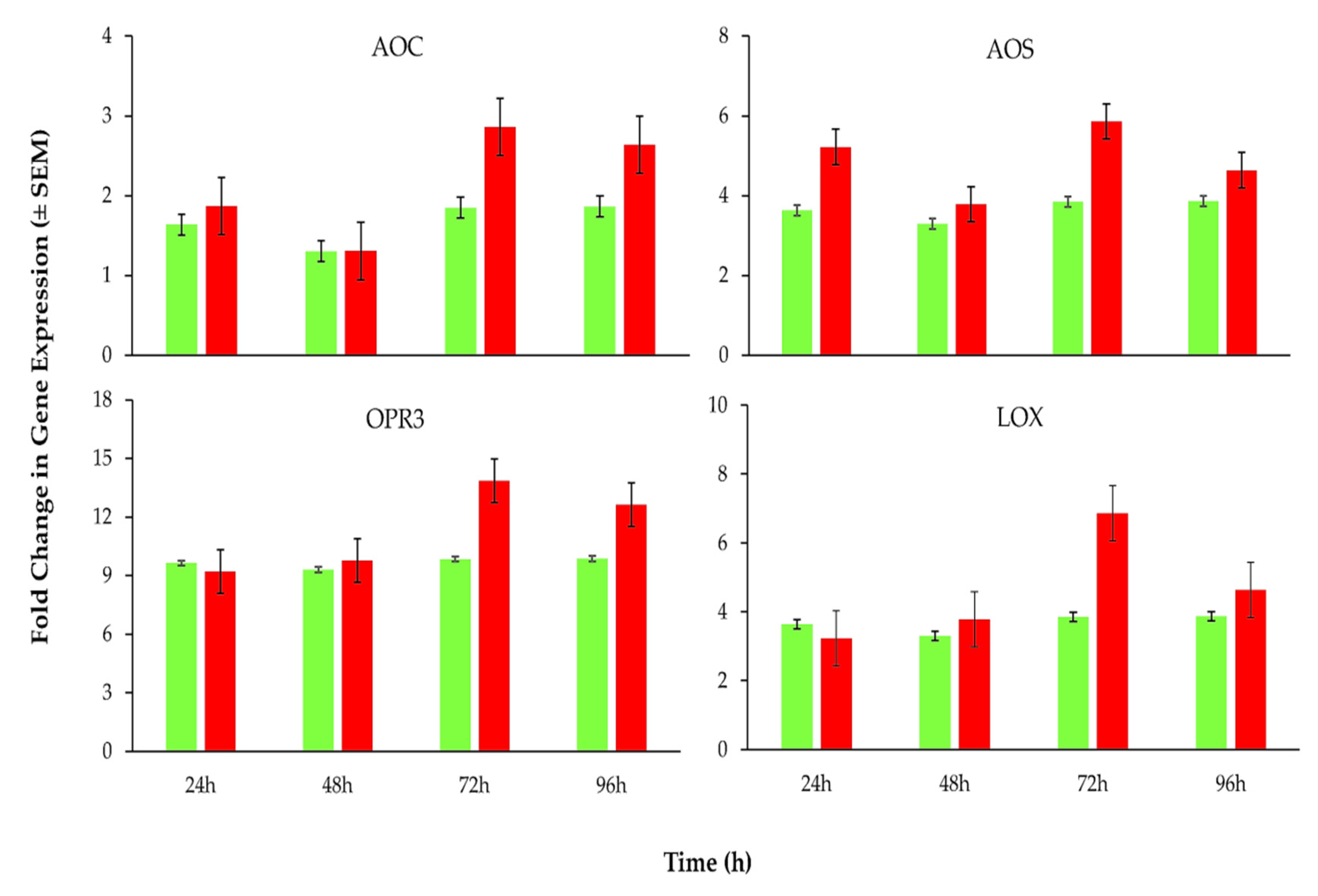
3.4. Expression Levels of Plant Defense Related Genes in Response to B. basiana-Derived Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fortes, I.M.; Sánchez-Campos, S.; Fiallo-Olivé, E.; Díaz-Pendón, J.A.; Navas-Castillo, J.; Moriones, E. A novel strain of tomato leaf curl New Delhi virus has spread to the Mediterranean basin. Viruses 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Abrahamian, P.; Sobh, H.; Seblani, R.; Abou-Jawdah, Y. Co-infection of two criniviruses and a begomovirus enhances the disease severity in cucumber. Eur. J. Plant Pathol. 2015, 142, 521–530. [Google Scholar] [CrossRef]
- Qiu, B.L.; Ren, S.X.; Sun, T.X.; Lin, L.; Kuang, Z.B. Preliminary report on the host plants of Bemisia tabaci in Guangzhou. J. South China Agric. Univ. 2001, 22, 43–47. [Google Scholar]
- Cahill, M.; Denholm, I.; Ross, G.; Gorman, K.; Johnston, D. Relationship between bioassay data and the simulated field performance of insecticides against susceptible and resistant adult Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 1996, 86, 109–116. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Eyre, D.P.; Cannon, R.J.C.; Miller, J.; Northing, P. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 2011, 18, 1–10. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Ishaaya, I. Dynamics of resistance to the neonicotinoids acetamiprid and thiamethoxam in Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2004, 97, 2051–2056. [Google Scholar] [CrossRef]
- Silva, L.D.; Omoto, C.; Bleicher, E.; Dourado, P.M. Monitoring the susceptibility to insecticides in Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations from Brazil. Neotrop. Entomol. 2009, 38, 862–871. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2005, 58, 216–225. [Google Scholar]
- Goolsby, J.A.; Ciomperlik, M.A.; Legaspi, B.C.; Legaspi, J.C.; Wendel, L.E. Laboratory and field evaluation of exotic parasitoids of Bemisia tabaci (Biotype ‘B’) in the Lower Rio Grande Valley of Texas. In Biological Control; Citeseer: State College, PA, USA, 1998; pp. 127–135. [Google Scholar]
- Wan, F.-H.; Yang, N.-W. Invasion and management of agricultural alien insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, H.E.; Jones, W.A. Pathogenicity of Isaria sp. (Hypocreales: Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot. 2009, 28, 333–337. [Google Scholar] [CrossRef]
- Majeed, M.Z.; Fiaz, M.; Ma, C.-S.; Afzal, M. Entomopathogenicity of three muscardine fungi, Beauveria bassiana, Isaria fumosorosea and Metarhizium anisopliae, against the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Egypt. J. Biol. Pest Control 2017, 27, 211–215. [Google Scholar]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzon, A.; Ownley, B.H. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Wraight, S.P.; Carruthers, R.I. Production, delivery, and use of mycoinsecticides for control of insect pests on field crops. In Biopesticides: Use and Delivery; Springer: New York, NY, USA, 1999; pp. 233–269. [Google Scholar]
- Vey, A.; Hoagland, R.E.; Butt, T.M. 12 Toxic Metabolites of Fungal Biocontrol Agents. Fungi as Biocontrol Agents; Springer: New York, NY, USA, 2001; p. 311. [Google Scholar]
- Bandani, A.R.; Khambay, B.P.S.; Faull, J.L.; Newton, R.; Deadman, M.; Butt, T.M. Production of efrapeptins by Tolypocladium species and evaluation of their insecticidal and antimicrobial properties. Mycol. Res. 2000, 104, 537–544. [Google Scholar] [CrossRef]
- Kim, J.; Yeon, S.; Kim, H.; Ahn, Y. Larvicidal activity against Plutella xylostella of cordycepin from the fruiting body of Cordyceps militaris. Pest Manag. Sci. Former. Pestic. Sci. 2002, 58, 713–717. [Google Scholar] [CrossRef]
- Farooq, M.; Freed, S. Insecticidal activity of toxic crude proteins secreted by entomopathogenic fungi against Musca domestica L. (Diptera: Muscidae). Kuwait J. Sci. 2018, 45, 64–74. [Google Scholar]
- Quesada-Moraga, E.; Carrasco-Díaz, J.; Santiago-Álvarez, C. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae). J. Appl. Entomol. 2006, 130, 442–452. [Google Scholar] [CrossRef]
- Nazir, T.; Basit, A.; Hanan, A.; Majeed, M.; Qiu, D. In vitro pathogenicity of some entomopathogenic fungal strains against green peach aphid Myzus persicae (Homoptera: Aphididae). Agronomy 2019, 9, 7. [Google Scholar] [CrossRef]
- Hanan, A.; Nazir, T.; Basit, A.; Ahmad, S.; Qiu, D. Potential of Lecanicillium lecanii (Zimm.) as a microbial control agent for green peach aphid, Myzus persicae (Sulzer)(Hemiptera: Aphididae). Pak. J. Zool. 2020, 52, 131. [Google Scholar] [CrossRef]
- Basit, A.; Hanan, A.; Nazir, T.; Majeed, M.Z.; Qiu, D. Molecular and functional characterization of elicitor PeBC1 extracted from Botrytis cinerea involved in the induction of resistance against green peach aphid (Myzus persicae) in common beans (Phaseolus vulgaris L.). Insects 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Arimura, G.-I.; Mithöfer, A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012, 29, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Rowe, H.C. Ecological costs of biotrophic versus necrotrophic pathogen resistance, the hypersensitive response and signal transduction. Plant Sci. 2008, 174, 551–556. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef]
- Kabaluk, J.T.; Ericsson, J.D. Metarhizium anisopliae seed treatment increases yield of field corn when applied for wireworm control. Agron. J. 2007, 99, 1377–1381. [Google Scholar] [CrossRef]
- Puthoff, D.P.; Holzer, F.M.; Perring, T.M.; Walling, L.L. Tomato pathogenesis-related protein genes are expressed in response to Trialeurodes vaporariorum and Bemisia tabaci biotype B feeding. J. Chem. Ecol. 2010, 36, 1271–1285. [Google Scholar] [CrossRef]
- Thaler, J.S.; Agrawal, A.A.; Halitschke, R. Salicylate-mediated interactions between pathogens and herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant. Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J.; Cheng, Y.; Cassell, J.L.; Thompson, G.A. Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2002, 51, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Luan, J.; Qi, J.; Huang, C.; Li, M.; Zhou, X.; Liu, S. Begomovirus–whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol. Ecol. 2012, 21, 1294–1304. [Google Scholar] [CrossRef]
- Hanan, A.; Basit, A.; Nazir, T.; Majeed, M.Z.; Qiu, D. Anti-insect activity of a partially purified protein derived from the entomopathogenic fungus Lecanicillium lecanii (Zimmermann) and its putative role in a tomato defense mechanism against green peach aphid. J. Invertebr. Pathol. 2020, 170, 107282. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Walling, L.L. Adaptive defense responses to pathogens and insects. Adv. Bot. Res. 2009, 51, 551–612. [Google Scholar]
- Chen, M.; Zhang, C.; Zi, Q.; Qiu, D.; Liu, W.; Zeng, H. A novel elicitor identified from Magnaporthe oryzae triggers defense responses in tobacco and rice. Plant Cell Rep. 2014, 33, 1865–1879. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop Prot. 2011, 30, 349–353. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Tomson, M. Impact of two pathogenic fungal crude metabolites on mortality, biology and enzymes of Dysdercus cingulatus (Fab.) (Hemiptera: Pyrrhocoridae). J. Biopestic. 2010, 3, 163–167. [Google Scholar]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996, 22, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Hogg, D.B.; Gratton, C. Methyl salicylate attracts natural enemies and reduces populations of soybean aphids (Hemiptera: Aphididae) in soybean agroecosystems. J. Econ. Entomol. 2011, 104, 115–124. [Google Scholar] [CrossRef]
- Nazir, T.; Hanan, A.; Basit, A.; Majeed, M.Z.; Anwar, T.; Nawaz, I.; Qiu, D. Putative role of a yet uncharacterized protein elicitor PeBb1 derived from Beauveria bassiana ARSEF 2860 strain against Myzus persicae (Homoptera: Aphididae) in Brassica rapa ssp. pekinensis. Pathogens 2020, 9, 111. [Google Scholar] [CrossRef]
- Farmer, E.E.; Johnson, R.R.; Ryan, C.A. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 1992, 98, 995–1002. [Google Scholar] [CrossRef]




| SOV | DF | Type III SS | MS | F-Value | p-Value * |
|---|---|---|---|---|---|
| Concentration | 3 | 32,206.9 | 10,735.6 | 37.99 | <0.001 |
| Time | 3 | 4486.9 | 1496.5 | 5.29 | <0.001 |
| Concentration × Time | 9 | 385.6 | 42.8 | 0.15 | 0.9980 |
| Error | 144 | 40,690.0 | 282.6 | ||
| Total | 159 | 77,769.4 |
| SOV | DF | Type III SS | MS | F-Value | p-Value * |
|---|---|---|---|---|---|
| Concentration | 3 | 59.261 | 19.7538 | 7.52 | <0.001 |
| Time | 7 | 14.162 | 2.0231 | 0.77 | 0.6130 |
| Concentration × Time | 21 | 2.882 | 0.1373 | 0.05 | 1.0000 |
| Error | 288 | 776.115 | 2.6278 | ||
| Total | 391 | 850.966 | |||
| GM/CV | 3.02/53.75 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keerio, A.U.; Nazir, T.; Anwar, T.; Zeeshan Majeed, M.; Abdulle, Y.A.; Jatoi, G.H.; Gadhi, M.A.; Qiu, D. Sub-Lethal Effects of Partially Purified Protein Extracted from Beauveria bassiana (Balsamo) and Its Presumptive Role in Tomato (Lycopersicon esculentum L.) Defense against Whitefly (Bemisia tabaci Genn.). Insects 2020, 11, 574. https://doi.org/10.3390/insects11090574
Keerio AU, Nazir T, Anwar T, Zeeshan Majeed M, Abdulle YA, Jatoi GH, Gadhi MA, Qiu D. Sub-Lethal Effects of Partially Purified Protein Extracted from Beauveria bassiana (Balsamo) and Its Presumptive Role in Tomato (Lycopersicon esculentum L.) Defense against Whitefly (Bemisia tabaci Genn.). Insects. 2020; 11(9):574. https://doi.org/10.3390/insects11090574
Chicago/Turabian StyleKeerio, Azhar Uddin, Talha Nazir, Tauqir Anwar, Muhammad Zeeshan Majeed, Yusuf Ali Abdulle, Ghulam Hussain Jatoi, Muswar Ali Gadhi, and Dewen Qiu. 2020. "Sub-Lethal Effects of Partially Purified Protein Extracted from Beauveria bassiana (Balsamo) and Its Presumptive Role in Tomato (Lycopersicon esculentum L.) Defense against Whitefly (Bemisia tabaci Genn.)" Insects 11, no. 9: 574. https://doi.org/10.3390/insects11090574
APA StyleKeerio, A. U., Nazir, T., Anwar, T., Zeeshan Majeed, M., Abdulle, Y. A., Jatoi, G. H., Gadhi, M. A., & Qiu, D. (2020). Sub-Lethal Effects of Partially Purified Protein Extracted from Beauveria bassiana (Balsamo) and Its Presumptive Role in Tomato (Lycopersicon esculentum L.) Defense against Whitefly (Bemisia tabaci Genn.). Insects, 11(9), 574. https://doi.org/10.3390/insects11090574










