Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Observation Hive Experiment
2.1.1. Hive Setup
2.1.2. Temperature Recording
2.1.3. Experimental Periods
2.2. Regression Analyses
2.3. Cage Experiment
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Statistical Analysis
3. Results
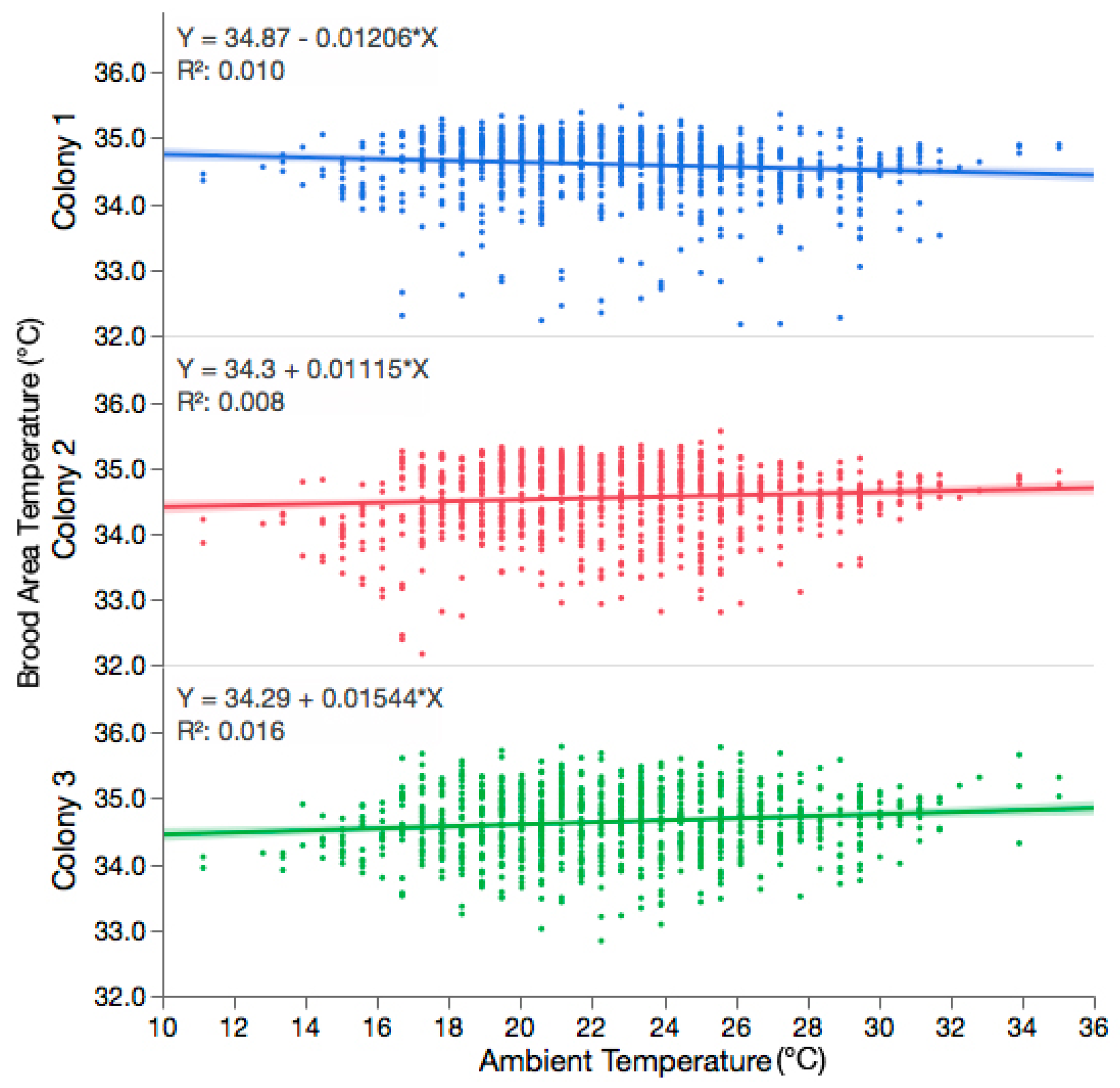
3.1. The Variability in Observed Brood Temperature Was Not Influenced by the Ambient Temperature
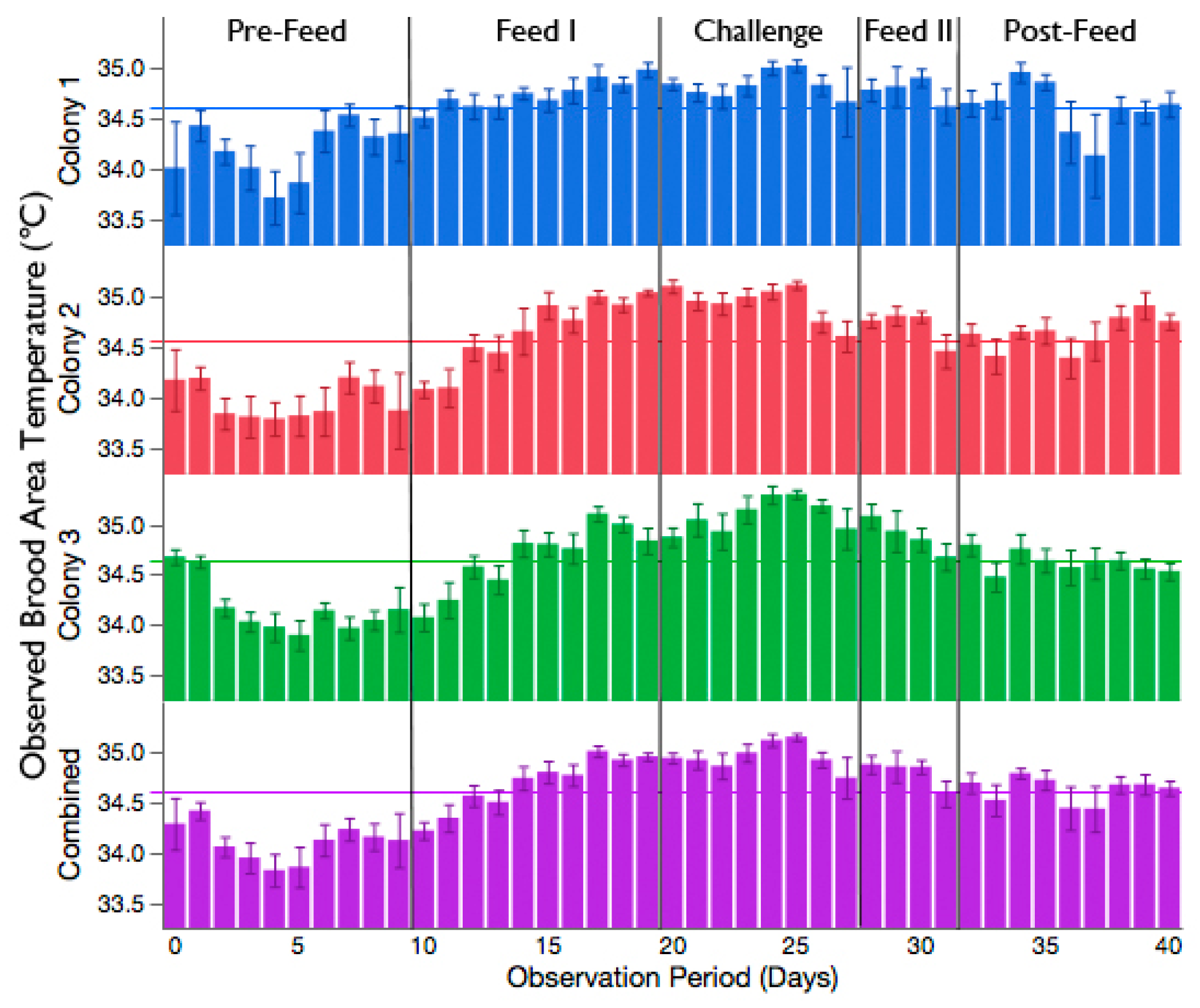
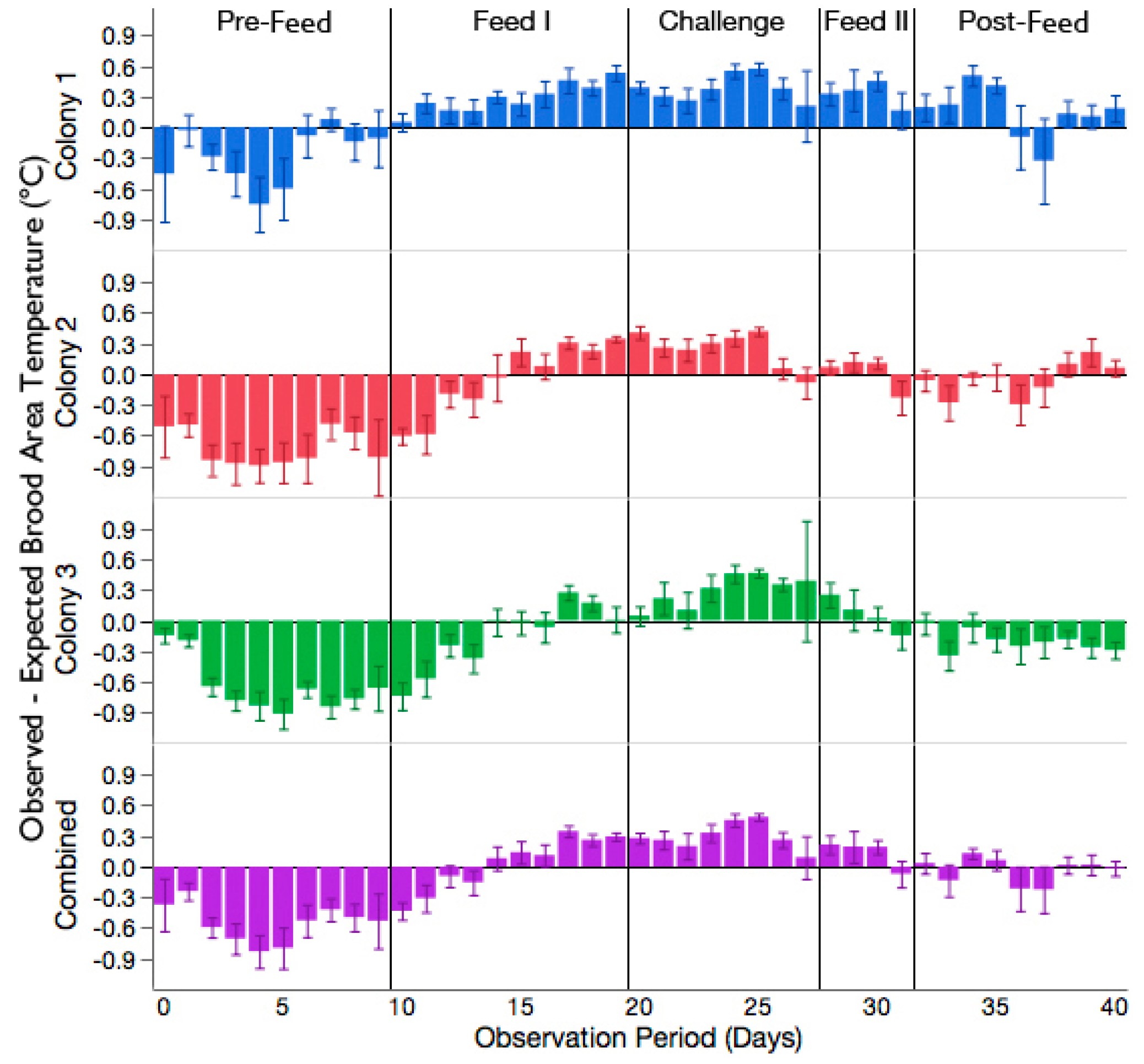
3.2. Increased Brood Nest Temperature but No Reduction in Signs of Chalkbrood Disease after Challenge with A. apis
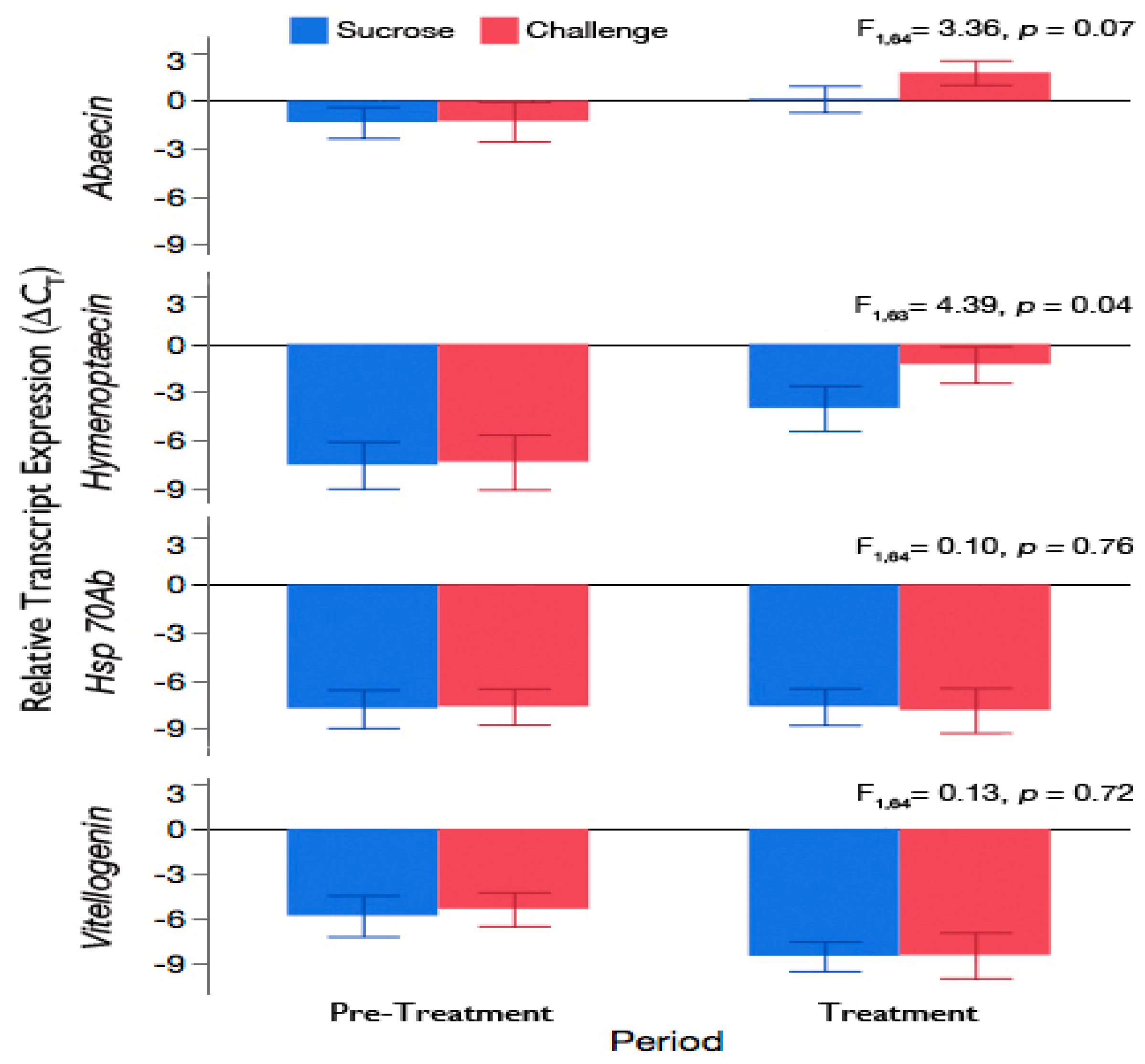
3.3. Challenge of Adult Workers with the Brood Pathogen, A. apis, Leads to an Increase in Expression of Antimicrobial Peptides
3.4. Exposure of Adult Workers to A. apis Was Not Sufficient to Induce Hsp 70Ab-Like or Accelerate Downregulation of Vitellogenin Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simpson, J. Nest climate regulation in honey bee colonies. Science 1961, 133, 1327–1333. [Google Scholar] [CrossRef]
- Bujok, B.; Kleinhenz, M.; Fuchs, S.; Tautz, J. Hot spots in the bee hive. Naturwissenschaften 2002, 89, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Kleinhenz, M.; Bujok, B.; Fuchs, S.; Tautz, J. Hot bees in empty broodnest cells: Heating from within. J. Exp. Biol. 2003, 206, 4217–4231. [Google Scholar] [CrossRef] [PubMed]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. Honeybee Ecology. A Study of Adaptation in Social Life; Princeton University Press: Princeton, NJ, USA, 1985; p. 214. [Google Scholar]
- Tautz, J.; Maier, S.; Groh, C.; Rossler, W.; Brockmann, A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef]
- Groh, C.; Tautz, J.; Rössler, W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl. Acad. Sci. USA 2004, 101, 4268–4273. [Google Scholar] [CrossRef]
- Jones, J.C.; Helliwell, P.; Beekman, M.; Maleszka, R.; Oldroyd, B.P. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. 2005, 191, 1121–1129. [Google Scholar] [CrossRef]
- Medina, R.G.; Paxton, R.J.; De Luna, E.; Fleites-Ayil, F.A.; Medina, L.A.; Quezada-Euán, J.J.G. Developmental stability, age at onset of foraging and longevity of Africanized honey bees (Apis mellifera L.) under heat stress (Hymenoptera: Apidae). J. Therm. Biol. 2018, 74, 214–225. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, X.; Zhou, S.; Wang, Q.; Chen, L.; Hao, Z.; Zhou, B. Low temperature exposure (20 °C) during the sealed brood stage induces abnormal venation of honey bee wings. J. Apic. Res. 2018, 57, 458–465. [Google Scholar] [CrossRef]
- Becher, M.A.; Scharpenberg, H.; Moritz, R.F.A. Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L.). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. 2009, 195, 673–679. [Google Scholar] [CrossRef]
- Medrzycki, P.; Sgolastra, F.; Bortolotti, L.; Bogo, G.; Tosi, S.; Padovani, E.; Porrini, C.; Sabatini, A.G. Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. J. Apic. Res. 2010, 49, 52–59. [Google Scholar] [CrossRef]
- Czekońska, K.; Chuda-Mickiewicz, B.; Chorbiński, P. The effect of brood incubation temperature on the reproductive value of honey bee (Apis mellifera) drones. J. Apic. Res. 2013, 52, 96–100. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.; Zhu, X.; Chen, L.; Zhou, S.; Huang, Z.-Y.; Zheng, B. Low-temperature stress during capped brood stage increases pupal mortality, misorientation and adult mortality in honey bees. PLoS ONE 2016, 11, e0154547. [Google Scholar] [CrossRef]
- Bailey, L. The effect of temperature on the pathogenicity of the fungus, Ascosphaera apis, for larvae of the honey bee, Apis mellifera. In Insect Pathology and Microbial Control; Van der Laan, P.A., Ed.; North Holland Publishing Co.: Amsterdam, The Netherlands, 1966; pp. 162–167. [Google Scholar]
- Gilliam, M.; Vandenberg, J.D. Fungi. In Honey Bee Pests, Predators and Diseases; Morse, R.A., Flottum, K., Eds.; AI Root Company: Medina, OH, USA, 1997; pp. 79–110. [Google Scholar]
- Aronstein, K.A.; Murray, K.D. Chalkbrood disease in honey bees. J. Invertebr. Pathol. 2010, 103 (Suppl. 1), 20–29. [Google Scholar] [CrossRef]
- Flores, J.M.; Ruiz, J.A.; Ruz, J.M.; Puerta, F.; Bustos, M.; Padilla, F.; Campano, F. Effect of temperature and humidity of sealed brood on chalkbrood development under controlled conditions. Apidologie 1996, 27, 185–192. [Google Scholar] [CrossRef]
- Vojvodic, S.; Jensen, A.B.; James, R.R.; Boomsma, J.J.; Eilenberg, J. Temperature dependent virulence of obligate and facultative fungal pathogens of honeybee brood. Vet. Microbiol. 2011, 149, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Starks, P.T.; Blackie, C.A.; Seeley, T.D. Fever in honeybee colonies. Naturwissenschaften 2000, 87, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Bonoan, R.E.; Iglesias Feliciano, P.M.; Chang, J.; Starks, P.T. Social benefits require a community: The influence of colony size on behavioral immunity in honey bees. Apidologie 2020, 27, 185–192. [Google Scholar] [CrossRef]
- De Roode, J.C.; Lefèvre, T. Behavioral immunity in insects. Insects 2012, 3, 789–820. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, 693–702. [Google Scholar] [CrossRef]
- Cotter, S.C.; Kilner, R.M. Personal immunity versus social immunity. Behav. Ecol. 2010, 21, 663–668. [Google Scholar] [CrossRef]
- Meunier, J. Social immunity and the evolution of group living in insects. Philos. Trans. R. Soc. B 2015, 370, 20140102. [Google Scholar] [CrossRef] [PubMed]
- Cremer, S. Social immunity in insects. Curr. Biol. 2019, 29, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Foo, B.; Tarpy, D.R.; Starks, P.T. Impact of food availability, pathogen exposure, and genetic diversity on thermoregulation in honey bees (Apis mellifera). J. Insect Behav. 2017, 27, 527–539. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Grodzicki, P.; Piechowicz, B.; Caputa, M. Effect of the own vs. foreign colony odor on daily shifts in olfactory and thermal preference and metabolic rate of the honey bee (Apis mellifera) workers. J. Apic. Res. 2020, 59, 691–702. [Google Scholar] [CrossRef]
- Doublet, V.; Poeschl, Y.; Gogo-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.F.; Bull, J.C.; et al. Unity in defence: Honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom. 2017, 18, 207. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Ferguson, L.V.; Salehipour-Shirazi, G.; MacMillan, H.A. Cross-tolerance and cross-talk in the cold: Relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 2013, 53, 545–556. [Google Scholar] [CrossRef]
- Shafeeq, T.; Ahmed, S.; Kim, Y. Toll immune signal activates cellular immune response via eicosanoids. Dev. Comp. Immunol. 2018, 84, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Bundey, S.; Raymond, S.; Dean, P.; Roberts, S.K.; Dillon, R.J.; Charnley, A.K. Eicosanoid involvement in the regulation of behavioral fever in the desert locust, Schistocerca gregaria. Arch. Insect Biochem. Physiol. 2003, 52, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I. Temperature stress and insect immunity. J. Therm. Biol. 2017, 68, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef]
- McKinstry, M.; Chung, C.; Truong, H.; Johnston, B.A.; Snow, J.W. The heat shock response and humoral immune response are mutually antagonistic in honey bees. Sci. Rep. 2017, 7, 8850. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Daughenbaugh, K.F.; Flenniken, M.L. The heat shock response in the Western honey bee (Apis Mellifera) is antiviral. Viruses 2020, 12, 245. [Google Scholar] [CrossRef]
- Campbell, J.; Kessler, B.; Mayack, C.; Naug, D. Behavioural fever in infected honeybees: Parasitic manipulation or coincidental benefit? Parasitology 2010, 137, 1487–1491. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Meana, A.; García-Palencia, P.; Marín, P.; Botías, C.; Garrido-Bailón, E.; Barrios, L.; Higes, M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009, 75, 2554–2557. [Google Scholar] [CrossRef]
- Stabentheiner, A. Effect of foraging distance on the thermal behaviour of honeybees during dancing, walking and trophallaxis. Ethology 1996, 102, 360–370. [Google Scholar] [CrossRef]
- Farina, W.M.; Wainselboim, A.J. Changes in the thoracic temperature of honeybees while receiving nectar from foragers collecting at different reward rates. J. Exp. Biol. 2001, 204, 1653–1658. [Google Scholar]
- Moffatt, L.; Nunez, J.A. Oxygen consumption in the foraging honeybee depends on the reward rate at the food source. J. Comp. Physiol. B 1997, 167, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nieh, J.C.; León, A.; Cameron, S.; Vandame, R. Hot bumble bees at good food: Thoracic temperature of feeding Bombus wilmattae foragers is tuned to sugar concentration. J. Exp. Biol. 2006, 209, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Heller, C. Colonial thermoregulation in honey bees (Apis mellifera). J. Comp. Physiol. 1982, 2148, 65–76. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goblirsch, M.; Warner, J.F.; Sommerfeldt, B.A.; Spivak, M. Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees. Insects 2020, 11, 528. https://doi.org/10.3390/insects11080528
Goblirsch M, Warner JF, Sommerfeldt BA, Spivak M. Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees. Insects. 2020; 11(8):528. https://doi.org/10.3390/insects11080528
Chicago/Turabian StyleGoblirsch, Michael, Jenny F. Warner, Brooke A. Sommerfeldt, and Marla Spivak. 2020. "Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees" Insects 11, no. 8: 528. https://doi.org/10.3390/insects11080528
APA StyleGoblirsch, M., Warner, J. F., Sommerfeldt, B. A., & Spivak, M. (2020). Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees. Insects, 11(8), 528. https://doi.org/10.3390/insects11080528




