Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Handling of Larvae
2.3. Radiation Treatments
2.4. Data Analyses
3. Results
3.1. Effects of Radiation Dose and Oxygen Level
3.2. Estimating Doses for the Prevention of Adult Emergence
3.2.1. Linear Regression
3.2.2. Probit Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crop Protection Compendium. Bactrocera dorsalis (Oriental fruit fly). Available online: https://www.cabi.org/ISC/datasheet/17685 (accessed on 24 January 2020).
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef]
- Fang, Y.; Kang, F.F.; Zhan, G.P.; Ma, C.; Li, Y.G.; Wang, L.; Wei, Y.D.; Gao, X.W.; Li, Z.H.; Wang, Y.J. The effects of a cold disinfestation on Bactrocera dorsalis survival and navel orange quality. Insects 2019, 10, 452. [Google Scholar] [CrossRef]
- Zhao, J.P.; Ma, J.; Wu, M.T.; Jiao, X.G.; Wang, Z.G.; Liang, F.; Zhan, G.P. Gamma radiation as a phytosanitary treatment against larvae and pupae of Bactrocera dorsalis (Diptera: Tephritidae) in guava fruits. Food Control 2017, 72, 360–366. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, D.J.; Xu, Y.J.; Wang, L.; Cheng, D.F.; Qi, Y.X.; Zeng, L.; Lu, Y.Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Integr. Agric. 2019, 18, 771–787. [Google Scholar] [CrossRef]
- Hallman, G.J. Process control in phytosanitary irradiation of fresh fruits and vegetables as a model for other phytosanitary treatment processes. Food Control 2017, 72, 372–377. [Google Scholar] [CrossRef]
- Hallman, G.J. Phytosanitary applications of irradiation. Compr. Rev. Food Sci. Food Saf. 2011, 10, 143–151. [Google Scholar] [CrossRef]
- Hallman, G.J.; Blackburn, C.M. Phytosanitary Irradiation. Foods 2016, 5, 8. [Google Scholar] [CrossRef]
- International Irradiation Association, Thailand Institute of Nuclear Technology, and the Joint FAO/IAEA Programme. The 8th Annual Chapman University Phytosanitary Forum. June, 2018. Bangkok, Thailand. Available online: https://iiaglobal.com/news/8th-chapman-annual-forum-phytosanitary/ (accessed on 24 January 2019).
- Follett, P.A.; Armstrong, J.W. Revised irradiation doses to control melon fly, Mediterranean fruit fly, and oriental fruit fly (Diptera: Tephritidae) and a generic dose for tephritid fruit flies. J. Econ. Entomol. 2004, 97, 1254–1262. [Google Scholar] [CrossRef]
- Srimartpirom, M.; Burikam, I.; Limohpasmanee, W.; Kongratarporn, T.; Thannarin, T.; Bunsiri, A.; Follett, P.A. Low-Dose Irradiation with modified atmosphere packaging for mango against the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 135–140. [Google Scholar] [CrossRef]
- Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.S.; Parker, A.G.; Caravantes, L.A.; Rivera, C.; Araújo, A.S.; Maxwell, F.; et al. Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies. Insects 2020, 11. [Google Scholar] [CrossRef]
- Follett, P.A.; Wall, M.M.; Bailey, W. Influence of modified atmosphere packaging on radiation tolerance in the phytosanitary pest melon fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.P.; Ren, L.L.; Shao, Y.; Wang, Q.L.; Yu, D.J.; Wang, Y.J.; Li, T.X. Gamma irradiation as a phytosanitary treatment of Bactrocera tau (Diptera: Tephritidae) in pumpkin fruits. J. Econ. Entomol. 2015, 108, 88–94. [Google Scholar]
- IPPC (International Plant Protection Convention). List of Topics for IPPC Standards. Available online: https://www.ippc.int/en/core-activities/standards-setting/list-topics-ippc-standards/list) (accessed on 19 February 2020).
- Wall, M. Quality of postharvest horticultural crops after irradiation treatment. Stewart Postharvest Rev. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Follett, P.A.; Wall, M.M. Phytosanitary irradiation for export of fresh produce: Commercial adoption in Hawaii and current issues. J. Radioanal. Nucl. Chem. 2013, 296, 517–522. [Google Scholar] [CrossRef]
- Lacroix, M.; Follett, P.A. Combination irradiation treatments for food safety and phytosanitary uses. Stewart Postharvest Rev. 2015, 11, 1–10. [Google Scholar]
- Neven, L.G.; Hansen, L.D. Effects of temperature and controlled atmospheres on codling moth metabolism. Ann. Entomol. Soc. Am. 2010, 103, 418–423. [Google Scholar] [CrossRef]
- IPPC (International Plant Protection Convention) ISPM 28. Annex 1 PT 11: Irradiation Treatment for Grapholita Molesta; FAO: Rome, Italy, 2010. [Google Scholar]
- Hallman, G.J.; Levang-Brilz, N.M.; Zettler, J.L.; Winborne, I.C. Factors affecting ionizing radiation phytosanitary treatments, and implications for research and generic treatments. J. Econ. Entomol. 2010, 103, 1950–1963. [Google Scholar] [CrossRef]
- Condon, C.H.; White, S.; Meagher, R.L.; Jeffers, L.A.; Bailey, W.D.; Hahn, D.A. Effects of low-oxygen environments on the radiation tolerance of the cabbage looper moth (Lepidoptera: Noctuidae). J. Econ. Entomol. 2017, 110, 80–86. [Google Scholar] [CrossRef]
- Chen, C.; Condon, C.H.; Boardman, L.; Meagher, R.L.; Jeffers, L.A.; Beam, A.; Bailey, W.D.; Hahn, D.A. Critical PO2 as a diagnostic biomarker for the effects of low-oxygen modified and controlled atmospheres on phytosanitary irradiation treatments in the cabbage looper Trichoplusia ni (Hübner). Pest Manag. Sci. 2020, 76, 76. [Google Scholar] [CrossRef]
- Liu, B.; Li, B.S.; Zhan, G.P.; Zha, T.; Wang, Y.J.; Ma, C. Forced hot-air treatment against Bactrocera papayae (Diptera: Tephritidae) in papaya. Appl. Entomol. Zool. 2017, 52, 531–541. [Google Scholar] [CrossRef]
- Gueorguiev, G. Irradiation System and Method Using X-Ray and Gamma-Ray Reflector. U.S. Patent 6,389,099, 14 May 2002. [Google Scholar]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- DPS (Data Processing System). User’s Guide; Version 13.5; Hangzhou RuiFeng Information Technology Co., Lt.: Hangzhou, China, 2010; Available online: http://www.chinadps.net/files/dps2nd.pdf (accessed on 25 January 2020).
- Robertson, J.L.; Preisler, K.H.; Russell, R.M. A user’s guide to probit or logit analysis. In PoloPlus Package Version 2.0; LeOra Software: Berkeley, CA, USA, 2007. [Google Scholar]
- Myers, S.W.; Cancio-Martinez, E.; Hallman, G.Y.; Fontenot, E.A.; Vreysen, M.J.B. Relative tolerance of six Bactrocera (Diptera: Tephritidae) to phytosanitary cold treatment. J. Econ. Entomol. 2016, 109, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.W.; Robert, M.; Park, R.M.; Bailer, J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- IPPC (International Plant Protection Convention), ISPM 18. Guidelines for the Use of Irradiation as a Phytosanitary Measure; FAO: Rome, Italy, 2003. [Google Scholar]
- IPPC (International Plant Protection Convention), ISPM 28. Phytosanitary Treatments for Regulated Pests; FAO: Rome, Italy, 2007. [Google Scholar]
- López-Martínez, G.; Hahn, D.A. Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. J. Exp. Biol. 2012, 215, 2150–2161. [Google Scholar] [CrossRef]
- Nestel, D.; Nemny-Lavy, E.; Islam, A.; Wornoayporn, V.; Cáceres, C. Effects of pre-irradiation conditioning of medfly pupae (Diptera: Tephritidae): Hypoxia and quality of sterile males. Fla. Entomol. 2007, 90, 80–87. [Google Scholar] [CrossRef]
- Follett, P.A.; Swedman, A.; Mackey, B. Effect of low oxygen conditions created by modified atmosphere packaging on radiation tolerance in Drosophila suzukii (Diptera: Drosophilidae) in sweet cherries. J. Econ. Entomol. 2018, 111, 141–145. [Google Scholar] [CrossRef]
- Hallman, G.J. Ionizing irradiation quarantine treatment against oriental fruit moth (Lepidoptera: Tortricidae) in ambient and hypoxic atmospheres. J. Econ. Entomol. 2004, 97, 824–827. [Google Scholar] [CrossRef]
- Hallman, G.J.; Hellmich, R.L. Ionizing radiation as a phytosanitary treatment against European corn borer (Lepidoptera: Crambidae) in ambient, low oxygen, and cold conditions. J. Econ. Entomol. 2009, 102, 64–68. [Google Scholar] [CrossRef]
- NAPPO (North American Plant Protection Organization), RSPM 34. Development of Phytosanitary Treatment Protocols for Regulated Arthropod Pests of Fresh Fruits or Vegetables; NAPPO: Ottawa, ON, Canada, 2011. [Google Scholar]
- Follett, P.A.; Neven, L.G. Phytosanitary irradiation: Does modified atmosphere packaging or controlled atmosphere storage creating a low oxygen environment threaten treatment efficacy? Radiat. Phys. Chem. 2020, 173. [Google Scholar] [CrossRef]
- Robertson, J.R.; Ryssekk, R.M.; Preisler, H.K.; Savin, N.E. Bioassays with Arthropods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 26–32. [Google Scholar]
- USDA Treatment Manual. Available online: https://www.aphis.usda.gov/import_export/plants/manuals/ports/downloads/treatment.pdf (accessed on 1 May 2020).
- Bustos, M.E.; Enkerlin, W.; Reyes, J.; Tolrdo, J. Irradiation of Mangoes as a postharvest quarantine treatment for fruit flies. J. Econ. Entomol. 2004, 97, 286–292. [Google Scholar] [CrossRef]
- Hallman, G.J.; Thomas, D.B. Ionizing radiation as a phytosanitary treatment against fruit flies (Diptera: Tephritidae): Efficacy in naturally versus artificially infested fruit. J. Econ. Entomol. 2010, 103, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.F.; Sun, X.L. Comparing lethal dose ratios using probit regression with arbitrary slopes. BMC Pharmacol. Toxicol. 2018, 19, 61. [Google Scholar] [CrossRef] [PubMed]
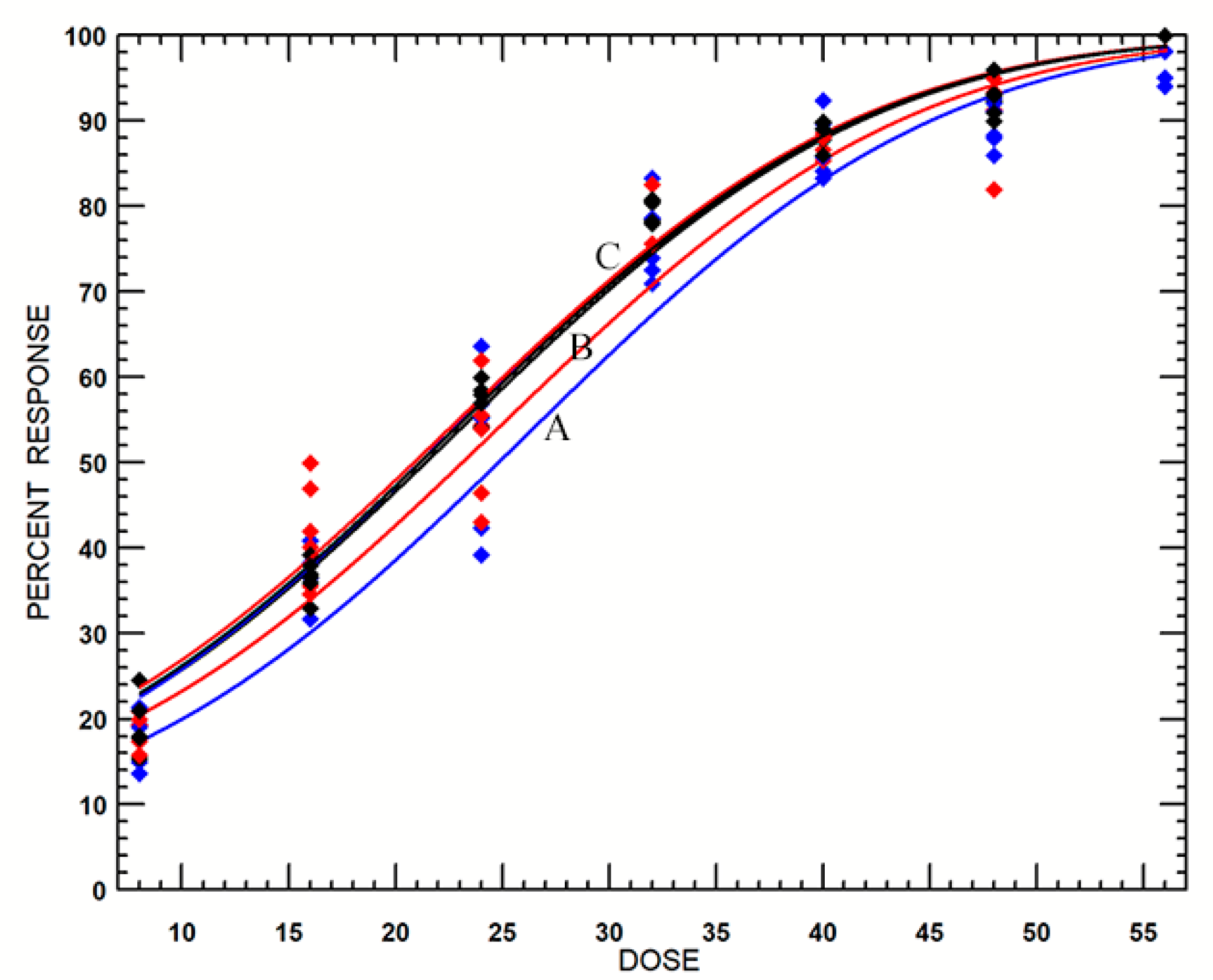
| O2 (%) | % Mortality at the Specified Radiation Dose (Mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Gy | 8 Gy | 16 Gy | 24 Gy | 32 Gy | 40 Gy | 48 Gy | 56 Gy | 64 Gy | |
| 0 | 6.8 ± 2.1a * | 10.4 ± 2.6a | 32.3 ± 5.1a | 41.3 ± 8.6a | 70.5 ± 1.6b | 83.2 ± 1.1a | 86.5 ± 1.4a | 95.5 ± 2.3b | 100.0 ± 0a |
| 2 | 8.0 ± 1.8a | 12.2 ± 1.9a | 36.9 ± 8.0a | 43.5 ± 6.3a | 78.5 ± 1.8a | 85.5 ± 1.4a | 87.6 ± 7.2a | 100.0 ± 0a | 100.0 ± 0a |
| 4 | 7.7 ± 3.2a | 13.2 ± 4.9a | 31.0 ± 3.4a | 52.3 ± 2.4a | 78.1 ± 1.3a | 86.9 ± 2.1a | 91.1 ± 1.8a | 100.0 ± 0a | 100.0 ± 0a |
| 6 | 7.0 ± 0.8a | 11.9 ± 4.3a | 32.1 ± 1.2a | 55.5 ± 4.8a | 78.5 ± 3.1a | 88.5 ± 3.6a | 91.4 ± 0.8a | 100.0 ± 0a | 100.0 ± 0a |
| 8 | 7.9 ± 0.4a | 10.9 ± 2.1a | 36.0 ± 6.6a | 53.8 ± 4.5a | 76.9 ± 3.8ab | 87.2 ± 0.3a | 93.5 ± 2.9a | 100.0 ± 0a | 100.0 ± 0a |
| 21 | 7.9 ± 1.6a | 12.0 ± 1.9a | 31.5 ± 1.1a | 54.8 ± 1.7a | 77.2 ± 1.7a | 87.3 ± 2.2a | 92.8 ± 2.7a | 100.0 ± 0a | 100.0 ± 0a |
| O2 (%) | Slope ± SE | Intercept ± SE | R2 | Estimated Dose for 100% Mortality (Gy) |
|---|---|---|---|---|
| 0 | 1.454 ± 0.047 | −5.640 ± 1.912 | 0.9772 | 65.8 |
| 2 | 1.618 ± 0.091 | −7.568 ± 3.313 | 0.9517 | 60.3 |
| 4 | 1.658 ± 0.059 | −6.668 ± 2.094 | 0.9769 | 58.3 |
| 6 | 1.665 ± 0.061 | −6.194 ± 2.198 | 0.9748 | 57.8 |
| 8 | 1.679 ± 0.048 | −6.505 ± 1.728 | 0.9845 | 57.5 |
| 21 | 1.683 ± 0.047 | −6.970 ± 1.673 | 0.9855 | 57.6 |
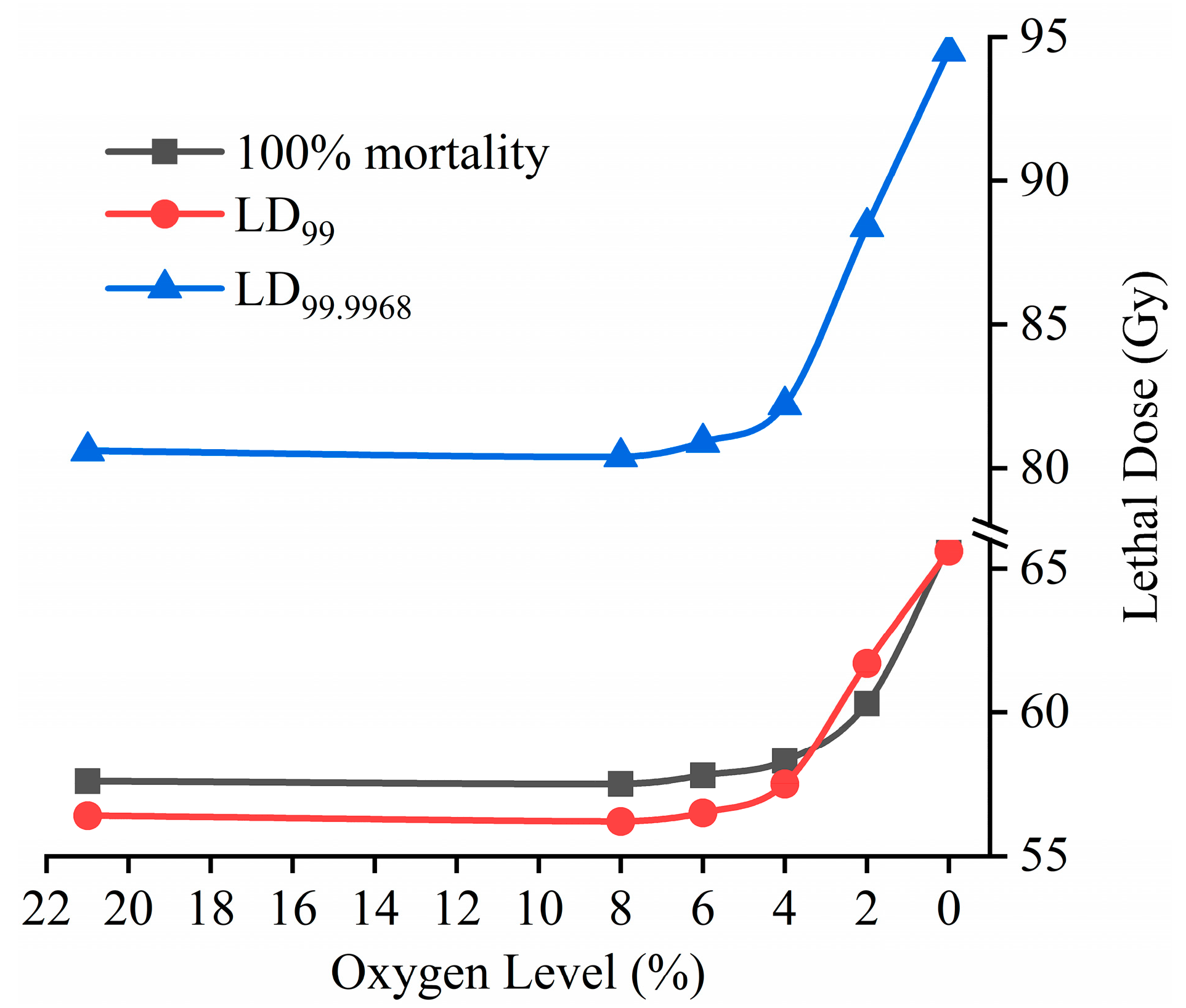
| O2 (%) | No. Treated | Slope ± SE | Intercept ± SE | Estimated Lethal Dose (95% CLs) (Gy) * | Hetero- Geneity | |
|---|---|---|---|---|---|---|
| LD99 | LD99.9968 | |||||
| 0 | 2155 | 0.058 ± 0.003 | −1.486 ± 0.09 | 65.6 (61.7–70.5)a | 94.5 (87.7–103.0)a | 1.61 |
| 2 | 2217 | 0.063 ± 0.003 | −1.540 ± 0.099 | 61.7 (56.6–68.6)a | 88.4 (79.7–100.5)ab | 3.13 |
| 4 | 2289 | 0.068 ± 0.003 | −1.557 ± 0.096 | 57.5 (54.4–61.2)b | 82.2 (76.9–88.8)b | 1.44 |
| 6 | 2335 | 0.069 ± 0.003 | −1.553 ± 0.092 | 56.5 (53.3–60.5)b | 80.9 (75.3–87.9)b | 1.76 |
| 8 | 2142 | 0.069 ± 0.003 | −1.579 ± 0.100 | 56.2 (53.3–59.8)b | 80.4 (75.2–86.7)b | 1.32 |
| 21 | 2103 | 0.069 ± 0.003 | −1.558 ± 0.100 | 56.4 (53.9–59.3)b | 80.6 (76.3–85.6)b | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, G.; Zhao, J.; Ma, F.; Liu, B.; Zhong, Y.; Song, Z.; Zhao, Q.; Chen, N.; Ma, C. Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres. Insects 2020, 11, 526. https://doi.org/10.3390/insects11080526
Zhan G, Zhao J, Ma F, Liu B, Zhong Y, Song Z, Zhao Q, Chen N, Ma C. Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres. Insects. 2020; 11(8):526. https://doi.org/10.3390/insects11080526
Chicago/Turabian StyleZhan, Guoping, Jupeng Zhao, Fuhuan Ma, Bo Liu, Yong Zhong, Zijiao Song, Qingying Zhao, Naizhong Chen, and Chen Ma. 2020. "Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres" Insects 11, no. 8: 526. https://doi.org/10.3390/insects11080526
APA StyleZhan, G., Zhao, J., Ma, F., Liu, B., Zhong, Y., Song, Z., Zhao, Q., Chen, N., & Ma, C. (2020). Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres. Insects, 11(8), 526. https://doi.org/10.3390/insects11080526






