The Gram-Positive Bacterium Leuconostoc pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of Drosophila Species
2.2. Maintenance of Aphids
2.3. Cultivation of L. pseudomesenteroides and Preparation of Extracts
2.4. Feeding of Drosophila Species (Oral Infection Route)
2.5. Injection of Drosophila Species (Septic Infection Route)
2.6. Feeding of Aphids (Oral Infection Route)
2.7. Injection of Aphids (Septic Infection Route)
2.8. Data Analysis
3. Results
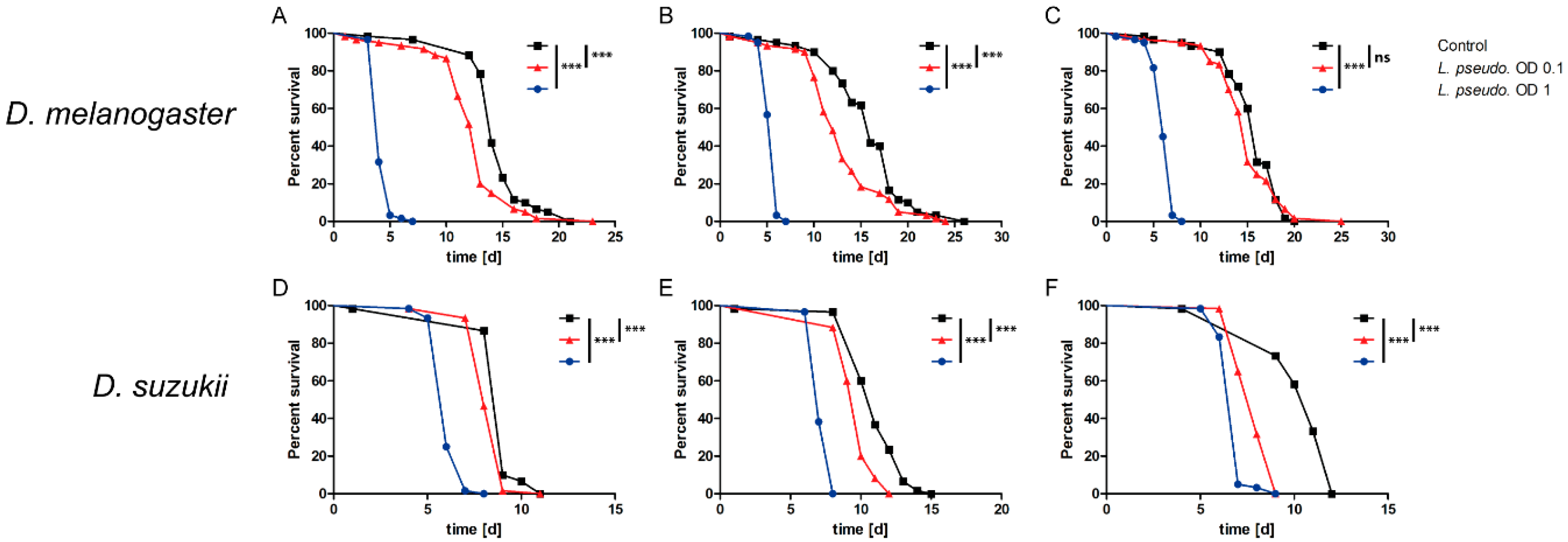
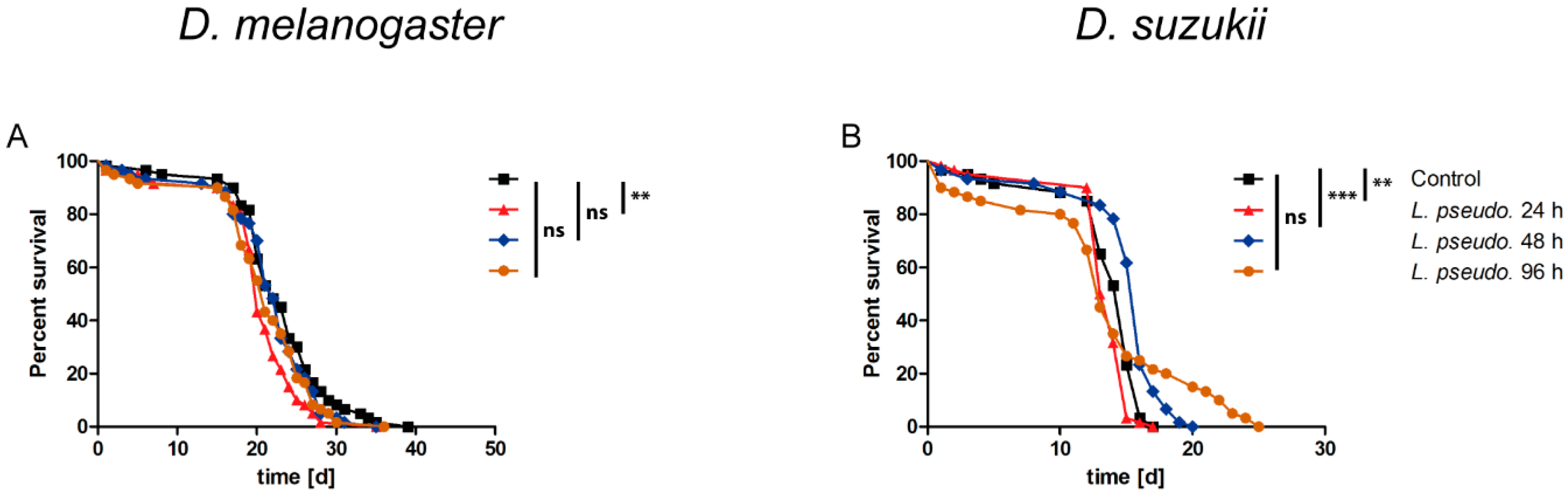
3.1. Oral Infection of Drosophilids with L. pseudomesenteroides
3.2. Septic Infection of Drosophilids with L. pseudomesenteroides
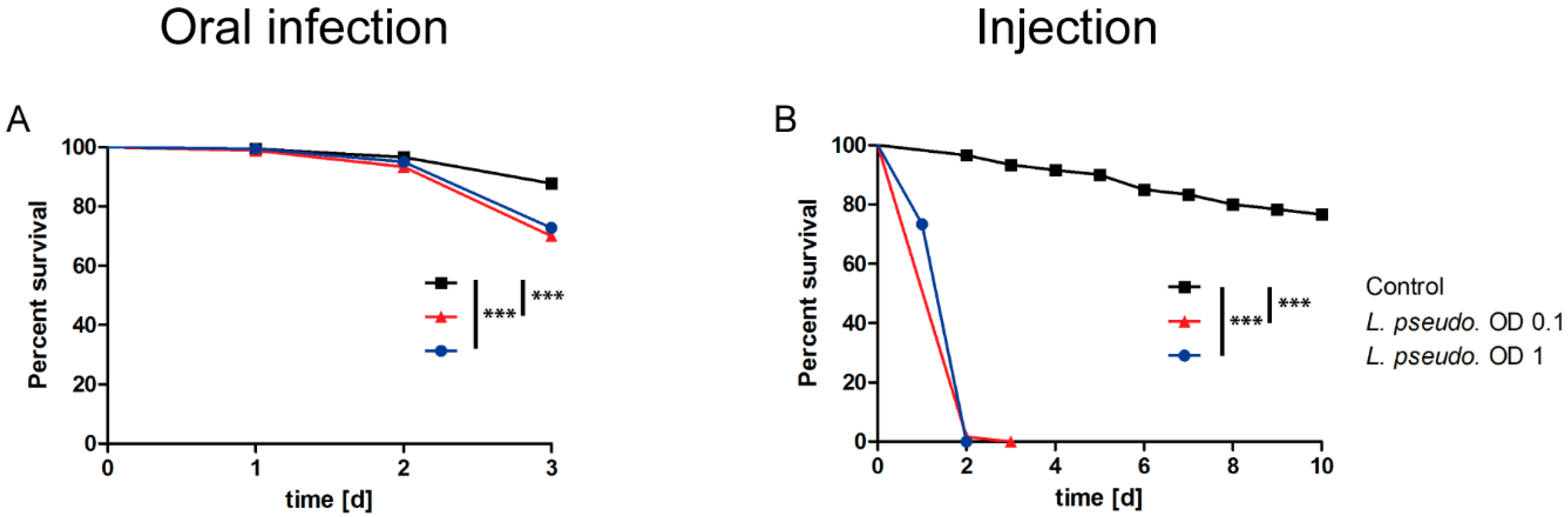
3.3. Oral Infection of A. pisum with L. pseudomesenteroides
3.4. Septic Infection of A. pisum with L. pseudomesenteroides
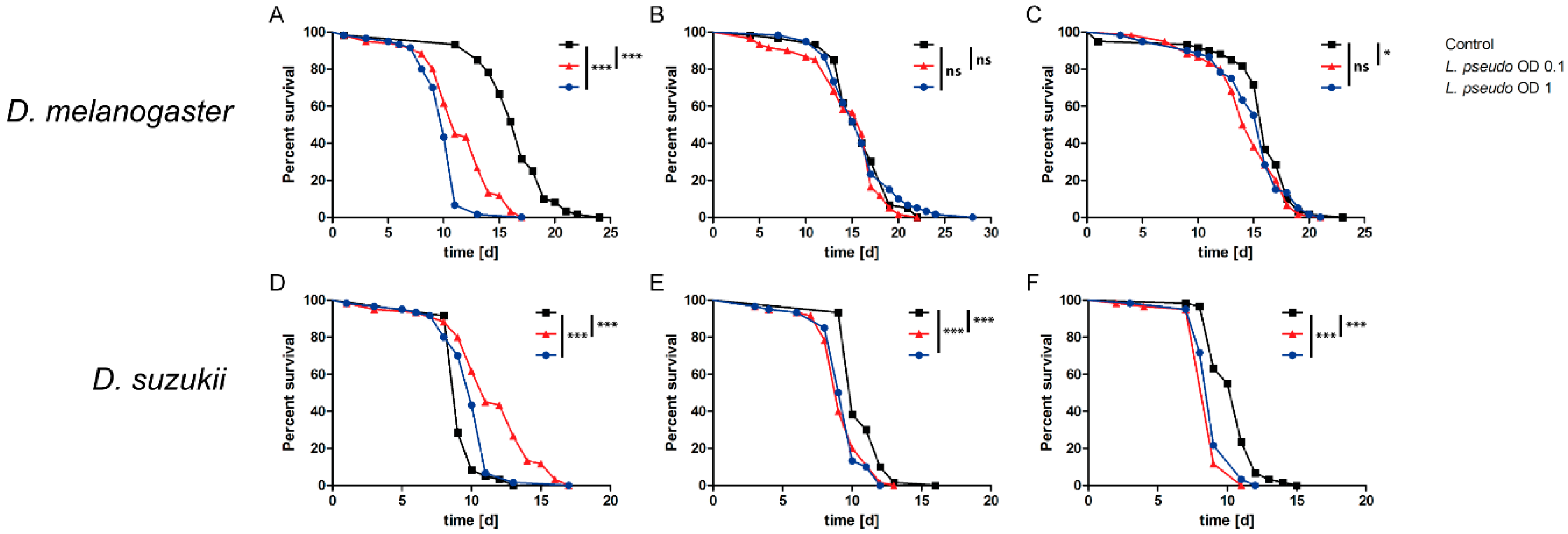
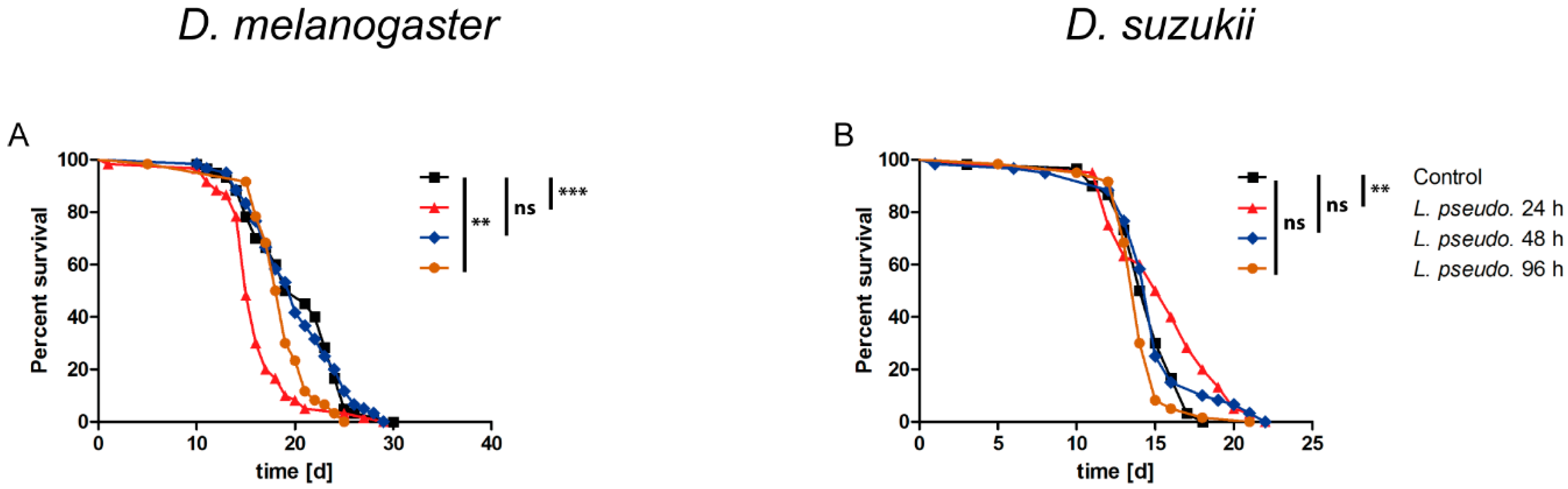
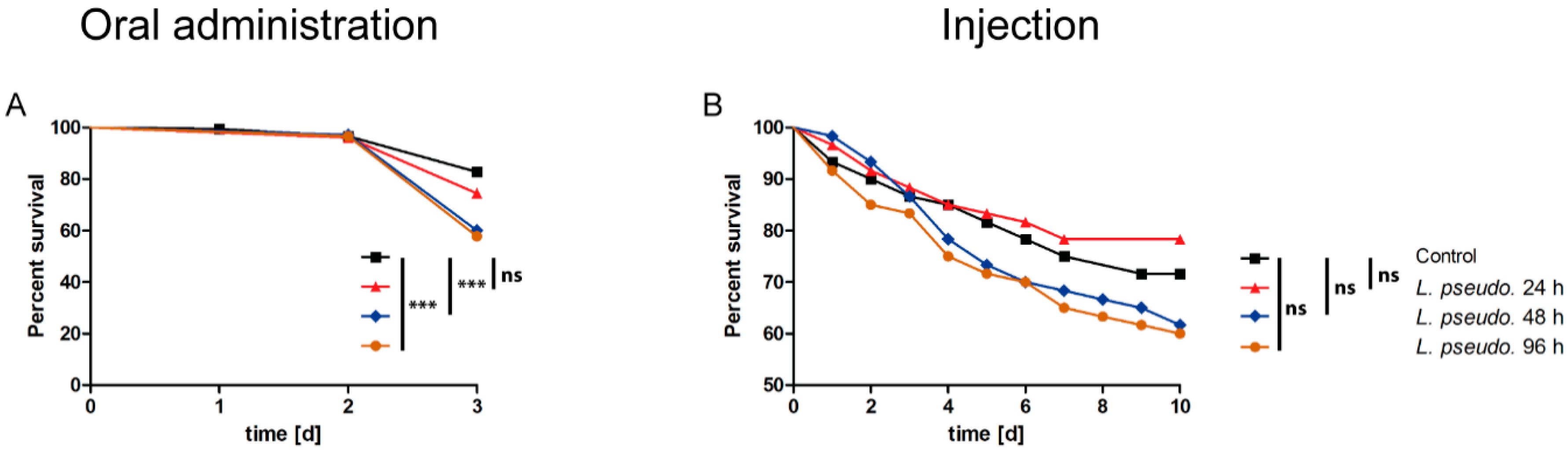
3.5. Oral Administration of L. pseudomesenteroides Extracts to Drosophilids
3.6. Septic Administration of L. pseudomesenteroides Extracts to Drosophilids
3.7. Oral Administration of L. pseudomesenteroides Extracts to A. pisum
3.8. Septic Administration of L. pseudomesenteroides Extracts to A. pisum
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- van Emden, H.F.; Harrington, R. (Eds.) Aphids as Crop. Pests, 2nd ed.; CABI: Wallingford, UK; Boston, MA, USA, 2017. [Google Scholar]
- Will, T.; Vilcinskas, A. Aphid-proof plants: Biotechnology-based approaches for aphid control. Adv. Biochem. Eng. Biotechnol. 2013, 136, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. (Ed.) Biology and Ecology of Aphids; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of synthetic and botanical pesticides on beneficial insects. Agric. Sci. 2016, 7, 364–372. [Google Scholar] [CrossRef]
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag. Sci. 2017, 73, 672–677. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Bizzarri, M.F.; Bishop, A.H. The ecology of Bacillus thuringiensis on the Phylloplane: Colonization from soil, plasmid transfer, and interaction with larvae of Pieris brassicae. Microb. Ecol. 2008, 56, 133–139. [Google Scholar] [CrossRef]
- Porcar, M.; Gómez, F.; Gruppe, A.; Gómez-Pajuelo, A.; Segura, I.; Schröder, R. Hymenopteran specificity of Bacillus thuringiensis strain PS86Q3. Biol. Control 2008, 45, 427–432. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Boyetchko, S.M.; Längle, T. Social and economic drivers shaping the future of biological control: A Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol. Control 2010, 52, 221–229. [Google Scholar] [CrossRef]
- Kil, Y.; Seo, M.; Kang, D.; Oh, S.; Cho, H.; Youn, Y.; Yasunaga-Aoki, C.; Yu, Y. Effects of enterobacteria (Burkholderia sp.) on development of Riptortus pedestris. J. Fac. Agric. Kyushu Univ. 2014, 59, 77–84. [Google Scholar]
- Cordova-Kreylos, A.L.; Fernandez, L.E.; Koivunen, M.; Yang, A.; Flor-Weiler, L.; Marrone, P.G. Isolation and characterization of Burkholderia rinojensis sp. nov., a non-Burkholderia cepacia complex soil bacterium with insecticidal and miticidal activities. Appl. Environ. Microbiol. 2013, 79, 7669–7678. [Google Scholar] [CrossRef]
- He, H.; Ratnayake, A.S.; Janso, J.E.; He, M.; Yang, H.Y.; Loganzo, F.; Shor, B.; O’Donnell, C.J.; Koehn, F.E. Cytotoxic Spliceostatins from Burkholderia sp. and Their Semisynthetic Analogues. J. Nat. Prod. 2014, 77, 1864–1870. [Google Scholar] [CrossRef]
- Martin, P.A.W.; Hirose, E.; Aldrich, J.R. Toxicity of Chromobacterium subtsugae to Southern Green Stink Bug (Heteroptera: Pentatomidae) and Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2007, 100, 680–684. [Google Scholar] [CrossRef]
- Koivunen, M.; Chanbusarakum, L.; Fernández, L.; Asolkar, R.; Tan, E.; Wallner, D.; Marrone, P. Development of a new microbial insecticide based on Chromobacterium subtsugae. IOBC/WPRS Bull. 2009, 45, 183–186. [Google Scholar]
- Martin, P.A.W.; Shropshire, A.D.S.; Gundersen-Rindal, D.; Blackburn, M. Chromobacterium subtsugae sp. nov. for Control of Insect Pests. U.S. Patent 7,244,607, 8 February 2007. [Google Scholar]
- Mertz, F.P.; YAO, R.C. Saccharopolyspora spinosa sp. nov. Isolated from Soil Collected in a Sugar Mill Rum Still. Int. J. Syst. Bacteriol. 1990, 40, 34–39. [Google Scholar] [CrossRef]
- Kirst, H.A.; Michel, K.H.; Martin, J.W.; Creemer, L.C.; Chio, E.H.; Yao, R.C.; Nakatsukasa, W.M.; Boeck, L.D.; Occolowitz, J.L.; Paschal, J.W.; et al. A83543A-D, unique fermentation-derived tetracyclic macrolides. Tetrahedron Lett. 1991, 32, 4839–4842. [Google Scholar] [CrossRef]
- Kirst, H.A. The spinosyn family of insecticides: Realizing the potential of natural products research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crouse, G.D.; Durst, G. Natural products as insecticides: The biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag. Sci. 2001, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, L. Insect pathogenic bacteria in integrated pest management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Schetelig, M.F.; Lee, K.-Z.; Otto, S.; Talmann, L.; Stökl, J.; Degenkolb, T.; Vilcinskas, A.; Halitschke, R. Environmentally sustainable pest control options for Drosophila suzukii. J. Appl. Entomol. 2018, 142, 3–17. [Google Scholar] [CrossRef]
- Lee, K.-Z.; Vilcinskas, A. Analysis of virus susceptibility in the invasive insect pest Drosophila suzukii. J. Invertebr. Pathol. 2017, 148, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Gegner, T.; Carrau, T.; Vilcinskas, A.; Lee, K.-Z. The infection of Harmonia axyridis by a parasitic nematode is mediated by entomopathogenic bacteria and triggers sex-specific host immune responses. Sci. Rep. 2018, 8, 15938. [Google Scholar] [CrossRef] [PubMed]
- Heep, J.; Skaljac, M.; Grotmann, J.; Kessel, T.; Seip, M.; Schmidtberg, H.; Vilcinskas, A. Identification and functional characterization of a novel insecticidal decapeptide from the myrmicine ant Manica rubida. Toxins 2019, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T. Studies on Drosophila suzukii Mats. CAB Direct 1939, 49. Available online: https://www.cabi.org/isc/abstract/19410501073 (accessed on 25 July 2020).
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; de Toni, D.C.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. Biol. Sci. 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piškur, J.; Witzgall, P.; et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Zhu, J.; Park, K.-C.; Baker, T.C. Identification of odors from overripe mango that attract vinegar flies, Drosophila melanogaster. J. Chem. Ecol. 2003, 29, 899–909. [Google Scholar] [CrossRef]
- Barata, A.; Santos, S.C.; Malfeito-Ferreira, M.; Loureiro, V. New insights into the ecological interaction between grape berry microorganisms and Drosophila flies during the development of sour rot. Microb. Ecol. 2012, 64, 416–430. [Google Scholar] [CrossRef]
- Rombaut, A.; Guilhot, R.; Xuéreb, A.; Benoit, L.; Chapuis, M.P.; Gibert, P.; Fellous, S. Invasive Drosophila suzukii facilitates Drosophila melanogaster infestation and sour rot outbreaks in the vineyards. R. Soc. Open Sci. 2017, 4, 170117. [Google Scholar] [CrossRef]
- Roberts, D.B. Drosophila melanogaster: The model organism. Entomol. Exp. Appl. 2006, 121, 93–103. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Lemaitre, B. Gut homeostasis in a microbial world: Insights from Drosophila melanogaster. Nat. Rev. Microbiol. 2013, 11, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Bost, A.; Franzenburg, S.; Adair, K.L.; Martinson, V.G.; Loeb, G.; Douglas, A.E. How gut transcriptional function of Drosophila melanogaster varies with the presence and composition of the gut microbiota. Mol. Ecol. 2018, 27, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.L.; Wilson, M.; Bost, A.; Douglas, A.E. Microbial community assembly in wild populations of the fruit fly Drosophila melanogaster. ISME J. 2018, 12, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Brisson, J.A.; Stern, D.L. The pea aphid, Acyrthosiphon pisum: An emerging genomic model system for ecological, developmental and evolutionary studies. Bioessays 2006, 28, 747–755. [Google Scholar] [CrossRef]
- Porcar, M.; Grenier, A.-M.; Federici, B.; Rahbé, Y. Effects of Bacillus thuringiensis delta-endotoxins on the pea aphid (Acyrthosiphon pisum). Appl. Environ. Microbiol. 2009, 75, 4897–4900. [Google Scholar] [CrossRef]
- Haviland, D.R.; Beers, E.H. Chemical control programs for Drosophila suzukii that comply with international limitations on pesticide residues for exported sweet cherries. J. Integr. Pest Manag. 2012, 3, F1–F6. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Rosensteel, D.O.; Hardin, J.A.; Sial, A.A.; Burrack, H.J. Season-long programs for control of Drosophila suzukii in southeastern U.S. blueberries. Crop. Protect. 2016, 81, 76–84. [Google Scholar] [CrossRef]
- Markow, T.A. The natural history of model organisms: The secret lives of Drosophila flies. eLife 2015, 4, e06793. [Google Scholar] [CrossRef]
- Gress, B.E.; Zalom, F.G. Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manag. Sci. 2019, 75, 1270–1276. [Google Scholar] [CrossRef]
- Smirle, M.J.; Zurowski, C.L.; Ayyanath, M.-M.; Scott, I.M.; MacKenzie, K.E. Laboratory studies of insecticide efficacy and resistance in Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) populations from British Columbia, Canada. Pest Manag. Sci. 2017, 73, 130–137. [Google Scholar] [CrossRef]
- Hiebert, N.; Carrau, T.; Bartling, M.; Vilcinskas, A.; Lee, K.-Z. Identification of entomopathogenic bacteria associated with the invasive pest Drosophila suzukii in infested areas of Germany. J. Invertebr. Pathol. 2020, 173, 107389. [Google Scholar] [CrossRef] [PubMed]
- Johanningsmeier, S.; McFeeters, R.F.; Fleming, H.P.; Thompson, R.L. Effects of Leuconostoc mesenteroides starter culture on fermentation of cabbage with reduced salt concentrations. J. Food Sci. 2007, 72, M166–M172. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.-Y.; Park, W.-S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Guo, J.; Axel, C.; Arendt, E.K.; Kildea, S.; Coffey, A. Control of Zymoseptoria tritici cause of septoria tritici blotch of wheat using antifungal Lactobacillus strains. J. Appl. Microbiol. 2016, 121, 485–494. [Google Scholar] [CrossRef]
- Visser, R.; Holzapfel, W.H.; Bezuidenhout, J.J.; Kotzé, J.M. Antagonism of lactic acid bacteria against phytopathogenic bacteria. Appl. Environ. Microbiol. 1986, 52, 552–555. [Google Scholar] [CrossRef]
- Laitila, A.; Alakomi, H.-L.; Raaska, L.; Mattila-Sandholm, T.; Haikara, A. Antifungal activities of two Lactobacillus plantarum strains against Fusarium moulds in vitro and in malting of barley. J. Appl. Microbiol. 2002, 93, 566–576. [Google Scholar] [CrossRef]
- Lazzeri, A.M.; Mangia, N.P.; Mura, M.E.; Floris, I.; Satta, A.; Ruiu, L. Potential of novel food-borne Lactobacillus isolates against the honeybee pathogen Paenibacillus larvae. Biocontrol Sci. Technol. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Torres, M.J.; Rocha, V.F.; Audisio, M.C. Laboratory evaluation of Lactobacillus johnsonii CRL1647 metabolites for biological control of Musca domestica. Entomol. Exp. Appl. 2016, 159, 347–353. [Google Scholar] [CrossRef]
- Akey, D.H.; Beck, S.D. Continuous rearing of the pea aphid, Acyrthosiphon pisum, on a holidic diet. Ann. Entomol. Soc. Am. 1971, 64, 353–356. [Google Scholar] [CrossRef]
- Schnepf, E.; Crickmore, N.; van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef]
- Höfte, H.; Whiteley, H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kuldeep Gupta, M.K.; Sanjeev Kumar, L.G. A fine-tuned management between physiology and immunity maintains the gut microbiota in insects. Biochem. Physiol. 2015, 4, 182. [Google Scholar] [CrossRef]
- Lin, Q.-C.; Zhai, Y.-F.; Zhang, A.-S.; Men, X.-Y.; Zhang, X.-Y.; Zalom, F.G.; Zhou, C.-G.; Yu, Y. Comparative developmental times and laboratory life tables for Drosophlia suzukii and Drosophila melanogaster (Diptera: Drosophilidae). Fla. Entomol. 2014, 97, 1434–1442. [Google Scholar] [CrossRef]
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Rendon, D.; Buser, J.; Tait, G.; Lee, J.C.; Walton, V.M. Survival and fecundity parameters of two Drosophila suzukii (Diptera: Drosophilidae) morphs on variable diet under suboptimal temperatures. J. Insect Sci. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Kacsoh, B.Z.; Schlenke, T.A. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS ONE 2012, 7, e34721. [Google Scholar] [CrossRef] [PubMed]
- Chaplinska, M.; Gerritsma, S.; Dini-Andreote, F.; Falcao Salles, J.; Wertheim, B. Bacterial communities differ among Drosophila melanogaster populations and affect host resistance against parasitoids. PLoS ONE 2016, 11, e0167726. [Google Scholar] [CrossRef]
- Wong, A.C.N.; Vanhove, A.S.; Watnick, P.I. The interplay between intestinal bacteria and host metabolism in health and disease: Lessons from Drosophila melanogaster. Dis. Model. Mech. 2016, 9, 271–281. [Google Scholar] [CrossRef]
- Martino, M.E.; Ma, D.; Leulier, F. Microbial influence on Drosophila biology. Curr. Opin. Microbiol. 2017, 38, 165–170. [Google Scholar] [CrossRef]
- Bing, X.; Gerlach, J.; Loeb, G.; Buchon, N. Nutrient-dependent impact of microbes on Drosophila suzukii development. mBio 2018, 9, e02199-17. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.N.A.; Ng, P.; Douglas, A.E. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 2011, 13, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Fleury, F.; Ris, N.; Allemand, R.; Fouillet, P.; Carton, Y.; Boulétreau, M. Ecological and genetic interactions in Drosophila-parasitoids communities: A case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. In Drosophila Melanogaster, Drosophila Simulans: So Similar, So Different; Capy, P., Gibert, P., Boussy, I., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 181–194. [Google Scholar]
- Valtierra-de-Luis, D.; Villanueva, M.; Caballero, J.; Matas, I.M.; Williams, T.; Caballero, P. Quantification of dose-mortality responses in adult Diptera: Validation using Ceratitis capitata and Drosophila suzukii responses to spinosad. PLoS ONE 2019, 14, e0210545. [Google Scholar] [CrossRef] [PubMed]
- Nehme, N.T.; Liégeois, S.; Kele, B.; Giammarinaro, P.; Pradel, E.; Hoffmann, J.A.; Ewbank, J.J.; Ferrandon, D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007, 3, e173. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- van Vu, H.; Hong, S.I.; Kim, K. Selection of entomopathogenic fungi for aphid control. J. Biosci. Bioeng. 2007, 104, 498–505. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F.; Abdel-Salam, A.H. Natural incidence of Cladosporium spp. as a bio-control agent against whiteflies and aphids in Egypt. J. Appl. Entomol. 2003, 127, 228–235. [Google Scholar] [CrossRef]
- Harada, H.; Ishikawa, H. Probiotic effect of Lactobacillus sp. DS-12 in flounder (Paralichthys olivaceus). J. Gen. Appl. Microbiol. 1997, 43, 363–367. [Google Scholar] [CrossRef]
- Haynes, S.; Darby, A.C.; Daniell, T.J.; Webster, G.; van Veen, F.J.F.; Godfray, H.C.J.; Prosser, J.I.; Douglas, A.E. Diversity of bacteria associated with natural aphid populations. Appl. Environ. Microbiol. 2003, 69, 7216–7223. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef]
- Laughton, A.M.; Garcia, J.R.; Altincicek, B.; Strand, M.R.; Gerardo, N.M. Characterisation of immune responses in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 2011, 57, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Leuconostoc pseudomesenteroides (ID 3416)—Genome—NCBI. Available online: https://www.ncbi.nlm.nih.gov/genome/3416?genome_assembly_id=173100 (accessed on 27 March 2020).
- Sawa, N.; Okamura, K.; Zendo, T.; Himeno, K.; Nakayama, J.; Sonomoto, K. Identification and characterization of novel multiple bacteriocins produced by Leuconostoc pseudomesenteroides QU 15. J. Appl. Microbiol. 2010, 109, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Balay, D.R.; Dangeti, R.V.; Kaur, K.; McMullen, L.M. Purification of leucocin A for use on wieners to inhibit Listeria monocytogenes in the presence of spoilage organisms. Int. J. Food Microbiol. 2017, 255, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M. Antimicrobial properties of diacetyl. Appl. Environ. Microbiol. 1982, 44, 525–532. [Google Scholar] [CrossRef]
- Rothacher, L.; Ferrer-Suay, M.; Vorburger, C. Bacterial endosymbionts protect aphids in the field and alter parasitoid community composition. Ecology 2016, 97, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Skaljac, M.; Vogel, H.; Wielsch, N.; Mihajlovic, S.; Vilcinskas, A. Transmission of a protease-secreting bacterial symbiont among pea aphids via host plants. Front. Physiol. 2019, 10, 438. [Google Scholar] [CrossRef]
- Helander, I.M.; von Wright, A.; Mattila-Sandholm, T.-M. Potential of lactic acid bacteria and novel antimicrobials against Gram-negative bacteria. Trends Food Sci. Technol. 1997, 8, 146–150. [Google Scholar] [CrossRef]
- Jay, J.M.; Golden, D.A.; Loessner, M.J. Modern Food Microbiology, 7th ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.M.; van Gey Pittius, N.C.; Champion, P.A.D.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.J.; Bitter, W. Type VII secretion—Mycobacteria show the way. Nat. Rev. Microbiol. 2007, 5, 883–891. [Google Scholar] [CrossRef]
- Filloux, A. The type VI secretion system: A tubular story. EMBO J. 2009, 28, 309–310. [Google Scholar] [CrossRef]
- Kolter, R.; Siegele, D.A.; Tormo, A. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 1993, 47, 855–874. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiebert, N.; Kessel, T.; Skaljac, M.; Spohn, M.; Vilcinskas, A.; Lee, K.-Z. The Gram-Positive Bacterium Leuconostoc pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests. Insects 2020, 11, 471. https://doi.org/10.3390/insects11080471
Hiebert N, Kessel T, Skaljac M, Spohn M, Vilcinskas A, Lee K-Z. The Gram-Positive Bacterium Leuconostoc pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests. Insects. 2020; 11(8):471. https://doi.org/10.3390/insects11080471
Chicago/Turabian StyleHiebert, Nils, Tobias Kessel, Marisa Skaljac, Marius Spohn, Andreas Vilcinskas, and Kwang-Zin Lee. 2020. "The Gram-Positive Bacterium Leuconostoc pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests" Insects 11, no. 8: 471. https://doi.org/10.3390/insects11080471
APA StyleHiebert, N., Kessel, T., Skaljac, M., Spohn, M., Vilcinskas, A., & Lee, K.-Z. (2020). The Gram-Positive Bacterium Leuconostoc pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests. Insects, 11(8), 471. https://doi.org/10.3390/insects11080471




