Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stock and Rearing
2.2. Measurement of Lifespan in Drosophila melanogaster
2.3. Measurement of Fly Fecundity
2.4. Body Weight
2.5. Determination of the Heat Flow Using Isothermal Calorimetry
2.6. Statistical Analysis
3. Results
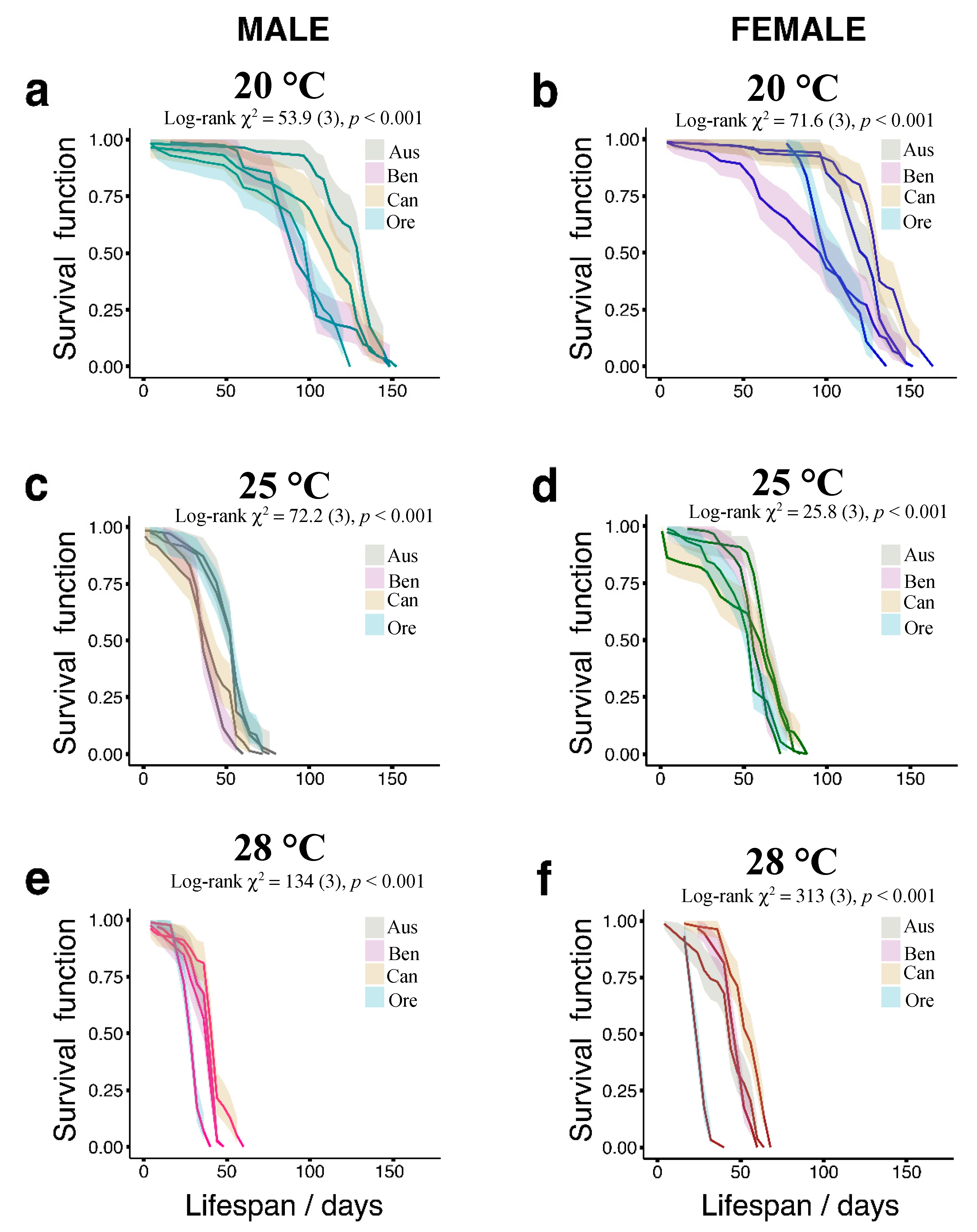
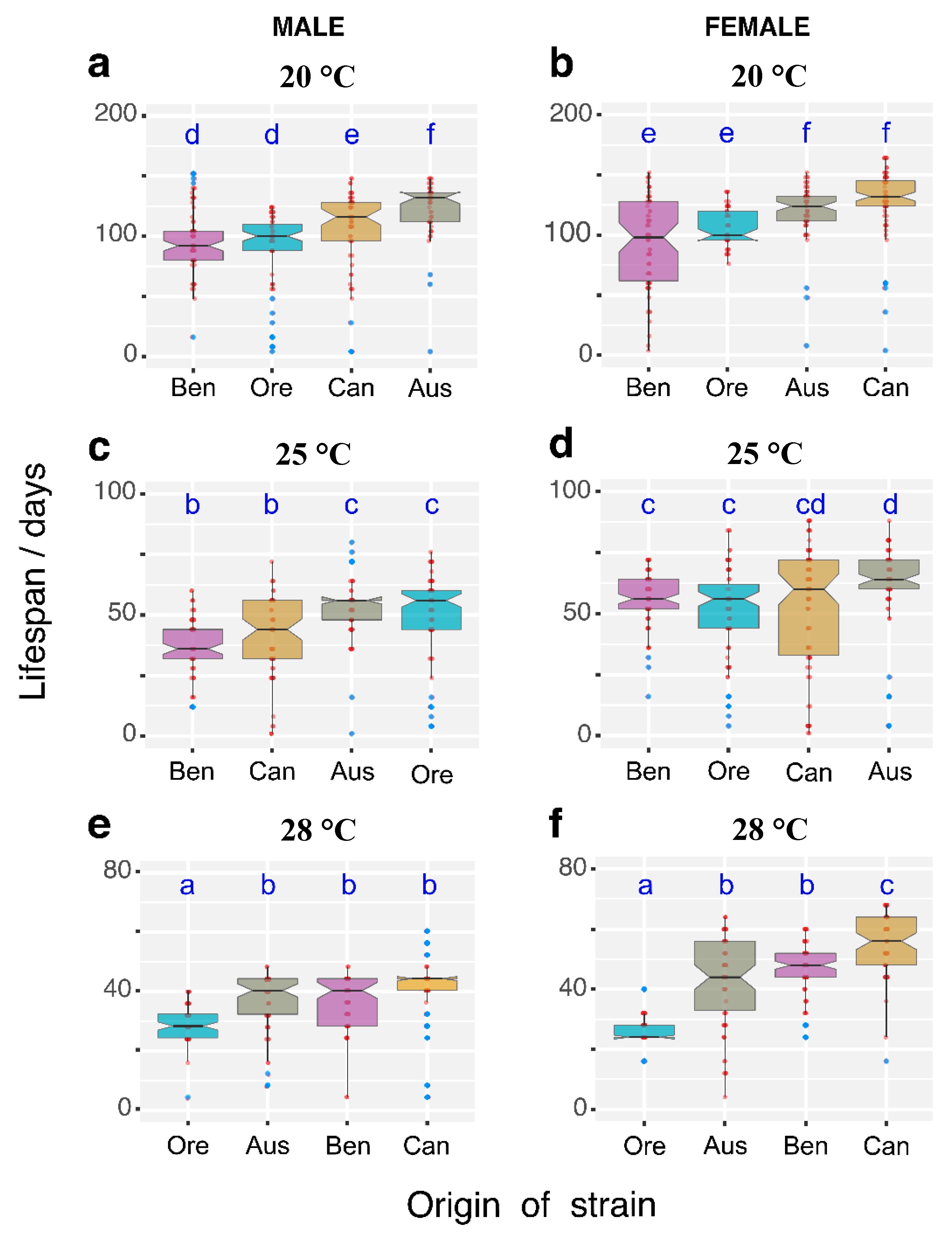
3.1. Temperature Effects on Longevity
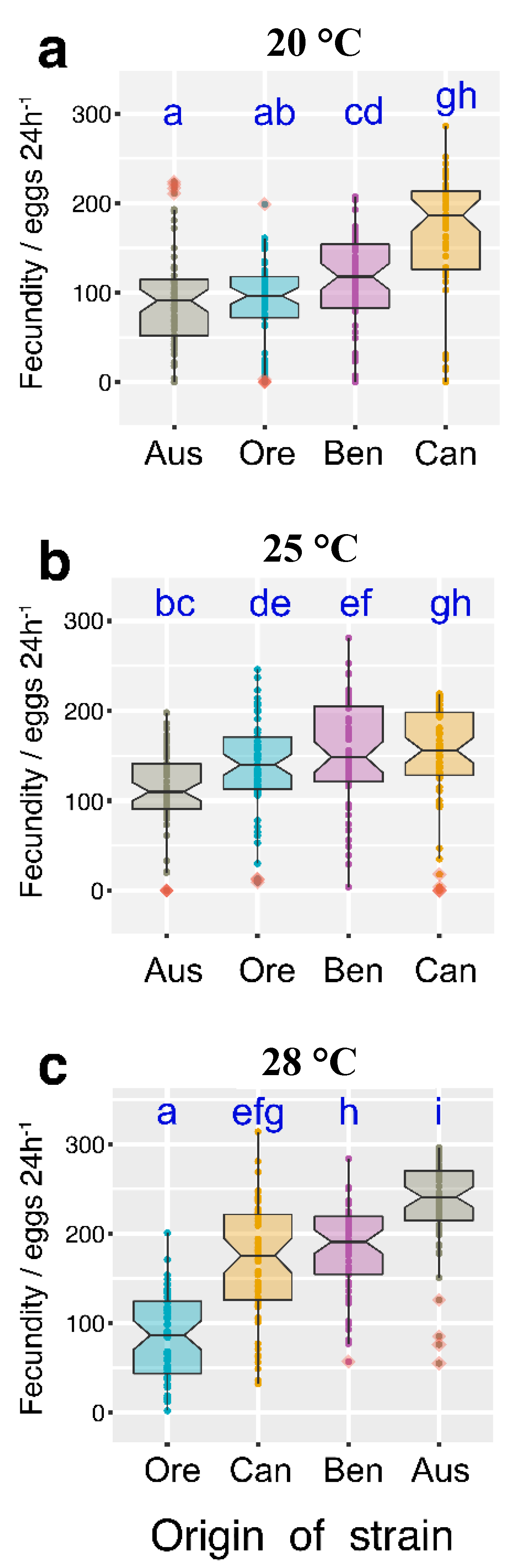
3.2. Temperature Effect on Fertility
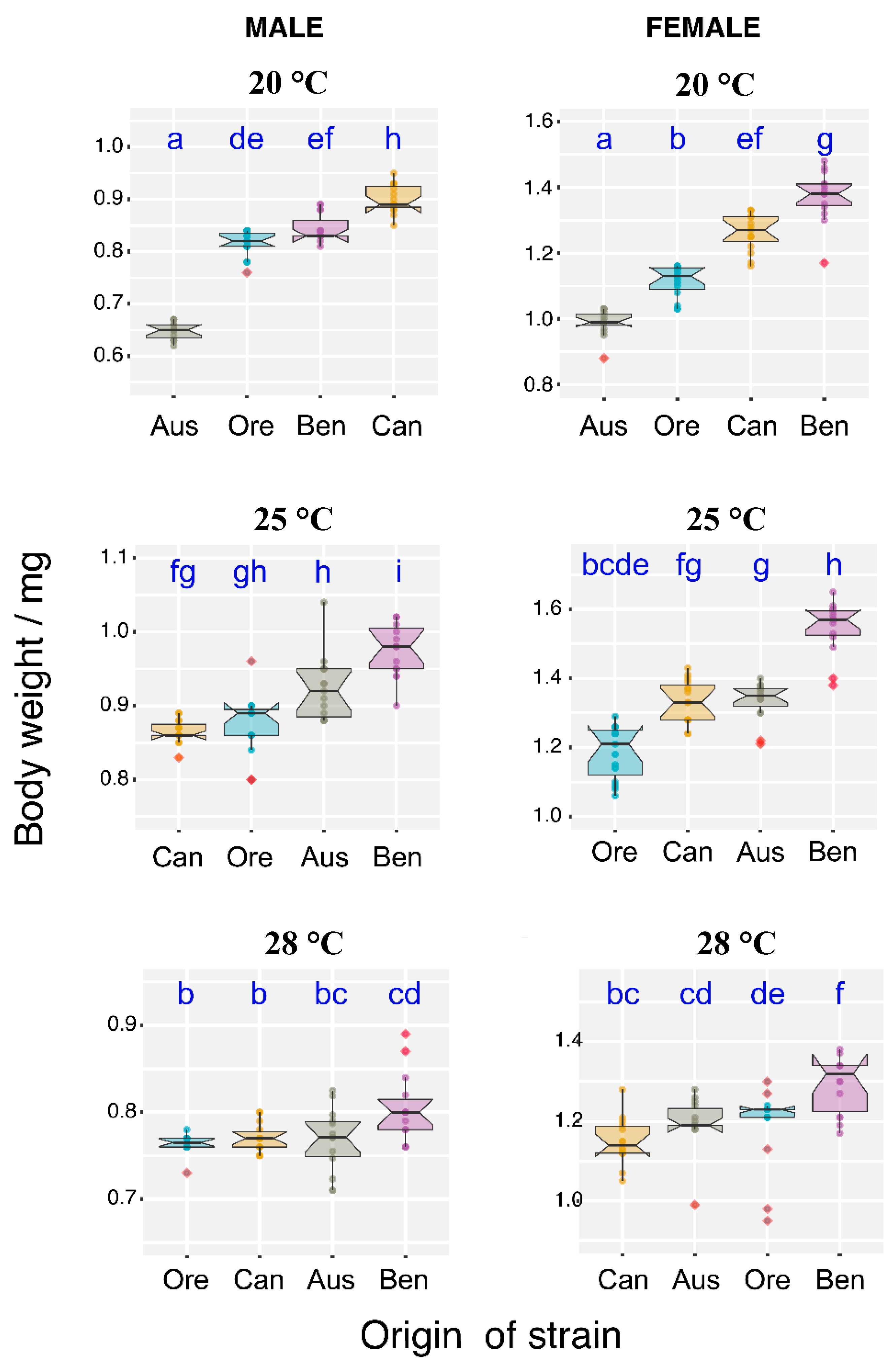
3.3. Temperature Effect on Body Weight
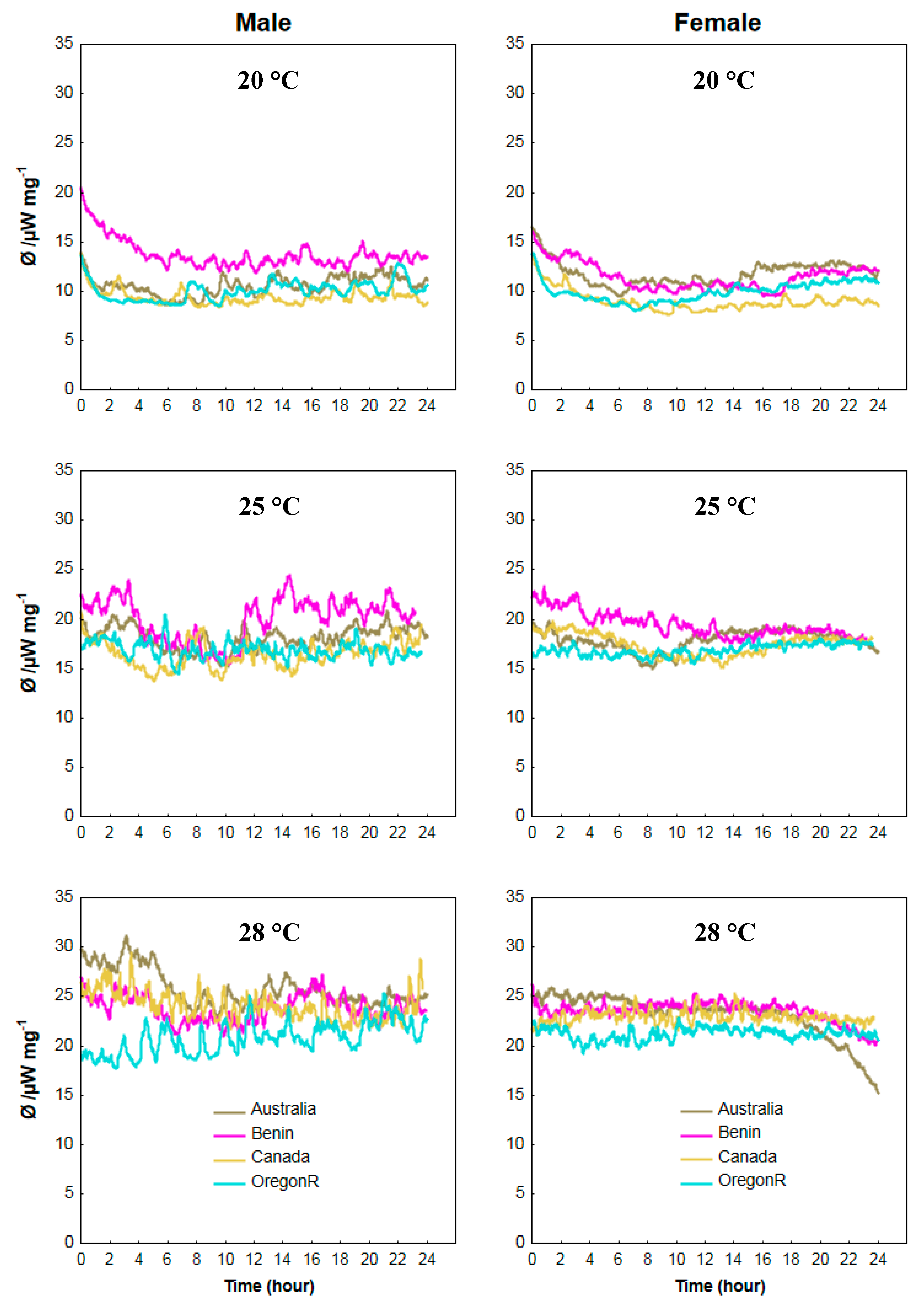
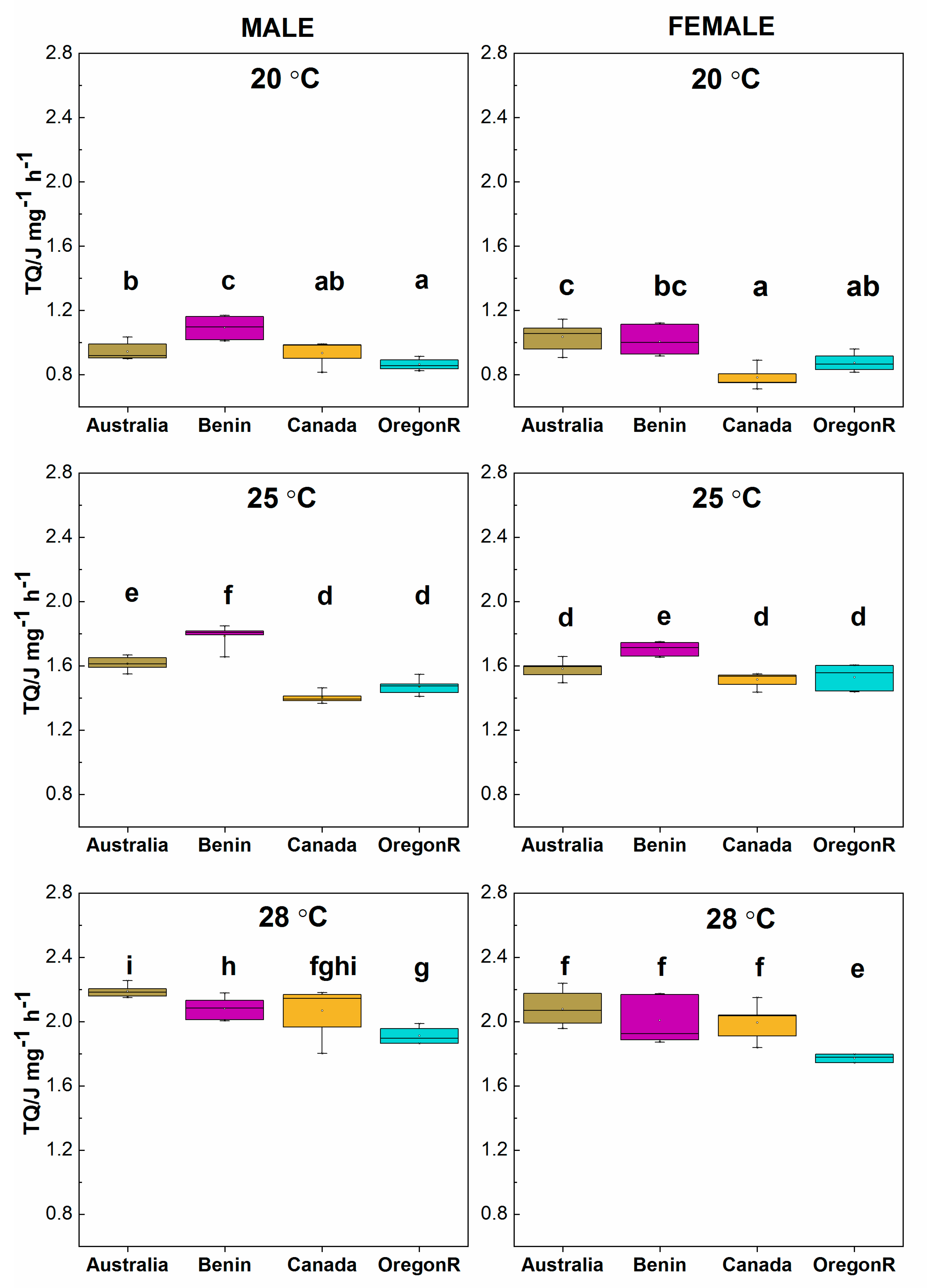
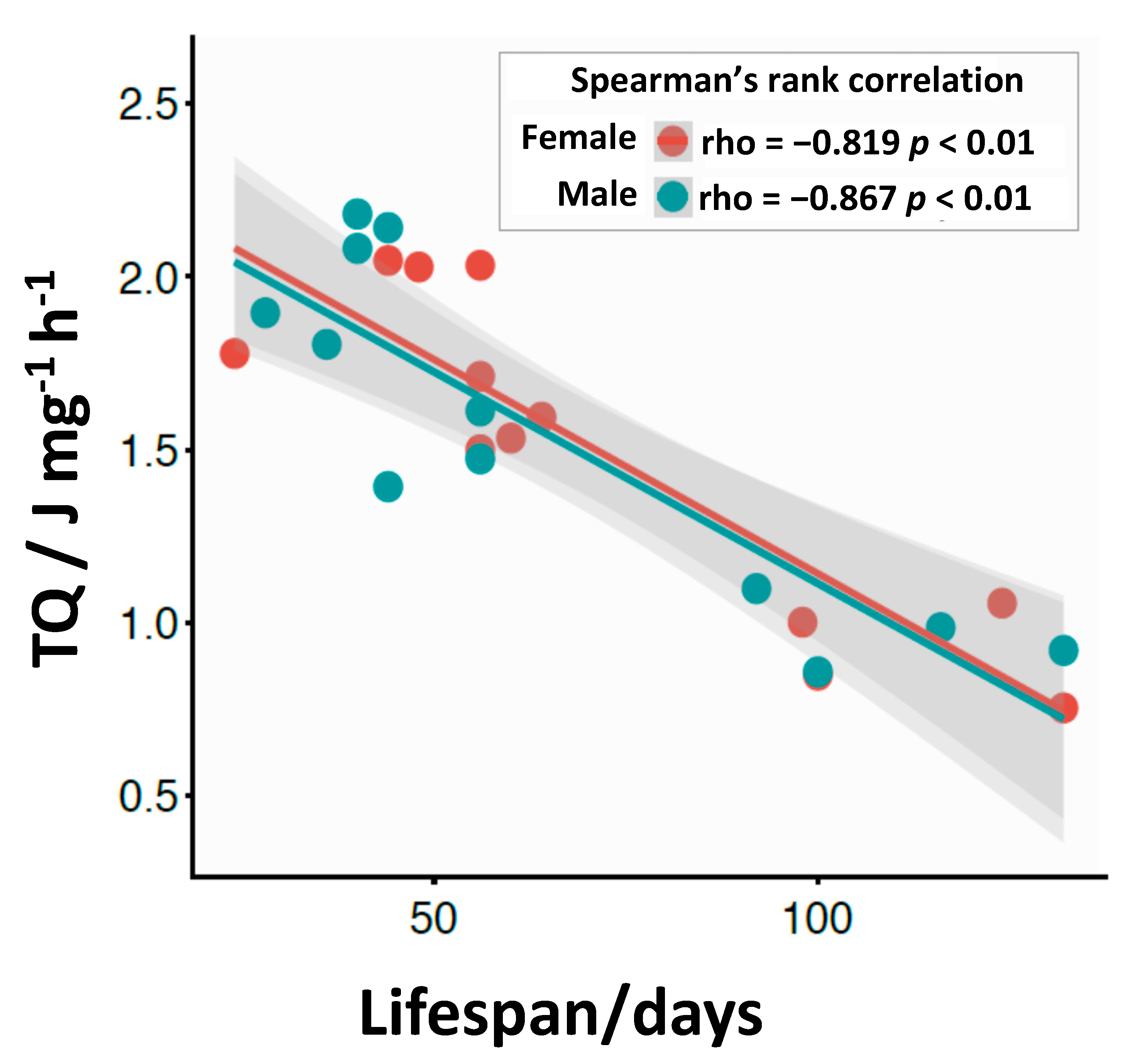
3.4. Calorimetric Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reznick, D.N.; Bryant, M.J.; Roff, D.; Ghalambor, C.K.; Ghalambor, D.E. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 2004, 431, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Callahan, D.; Topinkova, E. Is aging a preventable or curable disease? Drugs Aging 1998, 13, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Biological aging is no longer an unsolved problem. Biogerontol. Mech. Interv. 2007, 1100, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.J.; Madden, R.; Paul, G.; Taddei, F. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 2005, 3, 295–300. [Google Scholar] [CrossRef]
- Loeb, J.; Northrop, J.H. Is there a temperature coefficient for the duration of life? Proc. Natl. Acad. Sci. USA 1916, 2, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Landis, G.N.; Folk, D.; Wehr, N.B.; Hoe, N.; Waskar, M.; Abdueva, D.; Skvortsov, D.; Ford, D.; Luu, A.; et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007, 8, 1–27. [Google Scholar] [CrossRef]
- Landis, G.N.; Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef]
- Delabaere, L.; Ertl, H.A.; Massey, D.J.; Hofley, C.M.; Sohail, F.; Bienenstock, E.J.; Sebastian, H.; Chiolo, I.; LaRocque, J.R. Aging impairs double-strand break repair by homologous recombination in Drosophila germ cells. Aging Cell 2017, 16, 320–328. [Google Scholar] [CrossRef]
- Cannon, L.; Zambon, A.C.; Cammarato, A.; Zhang, Z.; Vogler, G.; Munoz, M.; Taylor, E.; Cartry, J.; Bernstein, S.I.; Melov, S.; et al. Expression patterns of cardiac aging in Drosophila. Aging Cell 2017, 16, 82–92. [Google Scholar] [CrossRef]
- Partridge, L.; Alic, N.; Bjedov, I.; Piper, M.D.W. Ageing in Drosophila: The role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 2011, 46, 376–381. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sen, S.; Chatterjee, R.; Roy, D.; James, J.; Thirumurugan, K. Context-and dose-dependent modulatory effects of naringenin on survival and development of Drosophila melanogaster. Biogerontology 2016, 17, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Helfand, S.L.; Rogina, B. Molecular genetics of aging in the fly: Is this the end of the beginning? Bioessays 2003, 25, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M. The Effects of Temperature and of Egg-Laying on the Longevity of Drosophila-Subobscura. J. Exp. Biol. 1958, 35, 832–842. [Google Scholar]
- Partridge, L.; Farquhar, M. Sexual-Activity Reduces Lifespan of Male Fruitflies. Nature 1981, 294, 580–582. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Partridge, L. Dietary restriction in Drosophila: Delayed aging or experimental artefact? PLoS Genet. 2007, 3, e57. [Google Scholar] [CrossRef]
- Seong, K.M.; Yu, M.; Lee, K.S.; Park, S.; Jin, Y.; Min, K.J. Curcumin Mitigates Accelerated Aging after Irradiation in Drosophila by Reducing Oxidative Stress. Biomed Res. Int. 2015, 2015, 425380. [Google Scholar] [CrossRef]
- Miquel, J.; Lundgren, P.R.; Bensch, K.G.; Atlan, H. Effects of Temperature on Life-Span, Vitality and Fine-Structure of Drosophila-Melanogaster. Mech. Ageing Dev. 1976, 5, 347–370. [Google Scholar] [CrossRef]
- Conti, B. Considerations on temperature, longevity and aging. Cell. Mol. Life Sci. 2008, 65, 1626–1630. [Google Scholar] [CrossRef]
- Rubner, M. Das Problem der Lebensdauer und Seine Beziehungen zu Wachstum und Ernarhrung; R. Oldenburg: Muenchen, Germany, 1908. [Google Scholar]
- Hofman, M.A. Energy-Metabolism, Brain Size and Longevity in Mammals. Q. Rev. Biol. 1983, 58, 495–512. [Google Scholar] [CrossRef]
- Molon, M.; Szajwaj, M.; Tchorzewski, M.; Skoczowski, A.; Niewiadomska, E.; Zadrag-Tecza, R. The rate of metabolism as a factor determining longevity of the Saccharomyces cerevisiae yeast. Age 2016, 38, 11. [Google Scholar] [CrossRef]
- Borowiak-Sobkowiak, B. Effect of Temperature on the Biological Parameters of Aphis Craccivora (Hemiptera Aphididae) on Robinia Pseudoacacia. Redia-G. Di Zool. 2017, 100, 65–71. [Google Scholar]
- Mehrparvar, M.; Hatami, B. Effect of temperature on some biological parameters of an Iranian population of the Rose Aphid, Macrosiphum rosae (Hemiptera: Aphididae). Eur. J. Entomol. 2007, 104, 631–634. [Google Scholar] [CrossRef]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic Defense Response of Apple Aphid Aphis pomi to Increased Temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef]
- Zhou, S.J.; Criddle, R.S.; Mitcham, E.J. Metabolic response of Platynota stultana pupae to controlled atmospheres and its relation to insect mortality response. J. Insect Physiol. 2000, 46, 1375–1385. [Google Scholar] [CrossRef]
- Zhou, S.J.; Criddle, R.S.; Mitcham, E.J. Metabolic response of Platynota stultana pupae during and after extended exposure to elevated CO2 and reduced O-2 atmospheres. J. Insect Physiol. 2001, 47, 401–409. [Google Scholar] [CrossRef]
- Joyal, J.J.; Hansen, L.D.; Coons, D.R.; Booth, G.M.; Smith, B.N.; Mill, D.D. Calorespirometric determination of the effects of temperature, humidity, low O-2 and high CO2 on the development of Musca domestica pupae. J. Therm. Anal. Calorim. 2005, 82, 703–709. [Google Scholar] [CrossRef]
- Neven, L.G.; Lehrman, N.J.; Hansen, L.D. Effects of temperature and modified atmospheres on diapausing 5th instar codling moth metabolism. J. Therm. Biol. 2014, 42, 9–14. [Google Scholar] [CrossRef]
- Schmolz, E.; Dewitz, R.; Schricker, B.; Lamprecht, I. Energy metabolism of European (Apis mellifera carnica) and Egyptian (A-m. lamarckii) honeybees. J. Therm. Anal. Calorim. 2001, 65, 131–140. [Google Scholar] [CrossRef]
- Molon, M.; Zadrag-Tecza, R. Effect of temperature on replicative aging of the budding yeast Saccharomyces cerevisiae. Biogerontology 2016, 17, 347–357. [Google Scholar] [CrossRef]
- Molon, M.; Zebrowski, J. Phylogenetic relationship and Fourier-transform infrared spectroscopy-derived lipid determinants of lifespan parameters in the Saccharomyces cerevisiae yeast. FEMS Yeast Res. 2017, 17, fox031. [Google Scholar]
- Trotta, V.; Calboli, F.C.F.; Ziosi, M.; Guerra, D.; Pezzoli, M.C.; David, J.R.; Cavicchi, S. Thermal plasticity in Drosophila Melanogaster: A comparison of geographic populations. BMC Evol. Biol. 2006, 6, 67. [Google Scholar] [CrossRef][Green Version]
- Williams, B.R.; van Heerwaarden, B.; Dowling, D.K.; Sgro, C.M. A multivariate test of evolutionary constraints for thermal tolerance in Drosophila melanogaster. J. Evol. Biol. 2012, 25, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Andrews, R. The Effect of Reproductive Activity on the Longevity of Male Drosophila-Melanogaster Is Not Caused by an Acceleration of Aging. J. Insect Physiol. 1985, 31, 393–395. [Google Scholar] [CrossRef]
- Priest, N.K.; Roach, D.A.; Galloway, L.F. Mating-induced recombination in fruit flies. Evolution 2007, 61, 160–167. [Google Scholar] [CrossRef]
- MacLellan, K.; Whitlock, M.C.; Rundle, H.D. Sexual selection against deleterious mutations via variable male search success. Biol. Lett. 2009, 5, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Renault, D. Metabolic effects of CO2 anaesthesia in Drosophila melanogaster. Biol. Lett. 2012, 8, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Linford, N.J.; Bilgir, C.; Ro, J.; Pletcher, S.D. Measurement of Lifespan in Drosophila melanogaster. Jove-J. Vis. Exp. 2013, e50068. [Google Scholar] [CrossRef]
- Clark, T.G.; Bradburn, M.J.; Love, S.B.; Altman, D.G. Survival analysis part I: Basic concepts and first analyses. Br. J. Cancer 2003, 89, 232–238. [Google Scholar] [CrossRef]
- Kapahi, P.; Zid, B.M.; Harper, T.; Koslover, D.; Sapin, V.; Benzer, S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004, 14, 885–890. [Google Scholar] [CrossRef]
- Peto, R.; Pike, M.C.; Armitage, P.; Breslow, N.E.; Cox, D.R.; Howard, S.V.; Mantel, N.; McPherson, K.; Peto, J.; Smith, P.G. Design and Analysis of Randomized Clinical-Trials Requiring Prolonged Observation of Each Patient. 2. Analysis and examples. Br. J. Cancer 1977, 35, 1–39. [Google Scholar] [CrossRef]
- Wilcox, R. Introduction to Robust Estimation and Hypothesis Testing, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 1–690. [Google Scholar]
- Krzywinski, M.; Altman, N. Visualizing samples with box plots. Nat. Methods 2014, 11, 119–120. [Google Scholar] [CrossRef]
- Prat, H. Analyse micro-calorimetrique des variations de la thermogenese chez divers insectes. Can. J. Zool. 1954, 32, 172–197. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span-From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Dowling, D.K.; Williams, B.R.; Garcia-Gonzalez, F. Maternal sexual interactions affect offspring survival and ageing. J. Evol. Biol. 2014, 27, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J. Variation in mitochondrial genotype has substantial lifespan effects which may be modulated by nuclear background. Aging Cell 2008, 7, 795–804. [Google Scholar] [CrossRef]
- Kellermann, V.; van Heerwaarden, B.; Sgro, C.M. How important is thermal history? Evidence for lasting effects of developmental temperature on upper thermal limits in Drosophila melanogaster. Proc. R. Soc. B-Biol. Sci. 2017, 284, 20170447. [Google Scholar] [CrossRef]
- Leiser, S.F.; Begun, A.; Kaeberlein, M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell 2011, 10, 318–326. [Google Scholar] [CrossRef]
- Van Voorhies, W.A.; Ward, S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc. Natl. Acad. Sci. USA 1999, 96, 11399–11403. [Google Scholar] [CrossRef]
- Zwaan, B.J.; Bijlsma, R.; Hoekstra, R.F. On the Developmental Theory of Aging. 2. The Effect of Developmental Temperature on Longevity in Relation to Adult Body Size in Drosophila-Melanogaster. Heredity 1992, 68, 123–130. [Google Scholar] [CrossRef]
- Sarup, P.; Sorensen, P.; Loeschcke, V. The long-term effects of a life-prolonging heat treatment on the Drosophila melanogaster transcriptome suggest that heat shock proteins extend lifespan. Exp. Gerontol. 2014, 50, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. Aging intervention, prevention, and therapy through hormesis. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2004, 59, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. Aging, anti-aging, and hormesis. Mech. Ageing Dev. 2004, 125, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. Principles and practice of hormetic treatment of ageing and age-related diseases. Hum. Exp. Toxicol. 2008, 27, 151–154. [Google Scholar] [CrossRef]
- Keil, G.; Cummings, E.; de Magalhaes, J.P. Being cool: How body temperature influences ageing and longevity. Biogerontology 2015, 16, 383–397. [Google Scholar] [CrossRef]
- Gao, G.Z.; Perkins, L.E.; Zalucki, M.P.; Lu, Z.Z.; Ma, J.H. Effect of temperature on the biology of Acyrthosiphon gossypii Mordvilko (Homoptera: Aphididae) on cotton. J. Pest Sci. 2013, 86, 167–172. [Google Scholar] [CrossRef]
- Pakyari, H.; Fathipour, Y.; Enkegaard, A. Effect of Temperature on Life Table Parameters of Predatory Thrips Scolothrips longicornis (Thysanoptera: Thripidae) Fed on Twospotted Spider Mites (Acari: Tetranychidae). J. Econ. Entomol. 2011, 104, 799–805. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Guo, J.Y.; Chen, H.S.; Wan, F.H. Effects of Temperature on Survival, Development, Longevity, and Fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a Potential Biological Control Agent Against Ambrosia artemisiifolia (Asterales: Asteraceae). Environ. Entomol. 2010, 39, 1021–1027. [Google Scholar] [CrossRef]
- Durak, R.; Borowiak-Sobkowiak, B. Influence of temperature on the biological parameters of the anholocyclic species Cinara tujafilina (Hemiptera: Aphidoidea). Cent. Eur. J. Biol. 2013, 8, 570–577. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chiu, Y.C. Ambient temperature influences aging in an annual fish (Nothobranchius rachovii). Aging Cell 2009, 8, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.K.; Walford, R.L. Mid-Life Temperature-Transfer Effects on Life-Span of Annual Fish. J. Gerontol. 1975, 30, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Munch, S.B.; Salinas, S. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc. Natl. Acad. Sci. USA 2009, 106, 13860–13864. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Sanchez-Alavez, M.; Winsky-Sommerer, R.; Morale, M.C.; Lucero, J.; Brownell, S.; Fabre, V.; Huitron-Resendiz, S.; Henriksen, S.; Zorrilla, E.P.; et al. Transgenic mice with a reduced core body temperature have an increased life span. Science 2006, 314, 825–828. [Google Scholar] [CrossRef]
- Gribble, K.E.; Moran, B.M.; Jones, S.; Corey, E.L.; Welch, D.B.M. Congeneric variability in lifespan extension and onset of senescence suggest active regulation of aging in response to low temperature. Exp. Gerontol. 2018, 114, 99–106. [Google Scholar] [CrossRef]
- Le Bourg, E. Life-time protection against severe heat stress by exposing young Drosophila melanogaster flies to a mild cold stress. Biogerontology 2016, 17, 409–415. [Google Scholar] [CrossRef]
- Pearl, R. The Rate of Living: Being an Account of Some Experimental Studies on the Biology of Life Duration; University of London Press LTD.: London, UK, 1928; pp. 147–185. [Google Scholar]
- Lints, F.A.; Lints, C.V. Influence of Preimaginal Environment on Fecundity and Aging in Drosophila-Melanogaster Hybrids. 3. Developmental Speed and Life-Span. Exp. Gerontol. 1971, 6, 427–445. [Google Scholar] [CrossRef]
- Mayer, P.J.; Baker, G.T. Genetic-aspects of drosophila as a model system of eukaryotic aging. Int. Rev. Cytol. -A Surv. Cell Biol. 1985, 95, 61–102. [Google Scholar]
- Partridge, L.; Fowler, K.; Trevitt, S.; Sharp, W. An examination of the effects of males on the survival and egg-production rates of female drosophila-melanogaster. J. Insect Physiol. 1986, 32, 925–929. [Google Scholar] [CrossRef]
- Partridge, L.; Green, A.; Fowler, K. Effects of egg-production and of exposure to males on female survival in drosophila-melanogaster. J. Insect Physiol. 1987, 33, 745–749. [Google Scholar] [CrossRef]
- Hunter, A.S. Effects of temperature on drosophila. 1. Respiration of d melanogaster grown at different temperatures. Comp. Biochem. Physiol. 1964, 11, 411–417. [Google Scholar] [CrossRef]
- Sohal, R.S.; Allen, R.G.; Farmer, K.J.; Procter, J. Effect of physical-activity on superoxide-dismutase, catalase, inorganic peroxides and glutathione in the adult male housefly, musca-domestica. Mech. Ageing Dev. 1984, 26, 75–81. [Google Scholar] [CrossRef]
- Mitrovski, P.; Hoffmann, A.A. Postponed reproduction as an adaptation to winter conditions in Drosophila melanogaster: Evidence for clinal variation under semi-natural conditions. Proc. R. Soc. B-Biol. Sci. 2001, 268, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Rosewell, J.; Shorrocks, B. The implication of survival rates in natural-populations of drosophila-capture recapture experiments on domestic species. Biol. J. Linn. Soc. 1987, 32, 373–384. [Google Scholar] [CrossRef]
- Vaught, R.C.; Voigt, S.; Dobler, R.; Clancy, D.J.; Reinhardt, K.; Dowling, D.K. Interactions between cytoplasmic and nuclear genomes confer sex-specific effects on lifespan in Drosophila melanogaster. J. Evol. Biol. 2020, 33, 694–713. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mołoń, M.; Dampc, J.; Kula-Maximenko, M.; Zebrowski, J.; Mołoń, A.; Dobler, R.; Durak, R.; Skoczowski, A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects 2020, 11, 470. https://doi.org/10.3390/insects11080470
Mołoń M, Dampc J, Kula-Maximenko M, Zebrowski J, Mołoń A, Dobler R, Durak R, Skoczowski A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects. 2020; 11(8):470. https://doi.org/10.3390/insects11080470
Chicago/Turabian StyleMołoń, Mateusz, Jan Dampc, Monika Kula-Maximenko, Jacek Zebrowski, Agnieszka Mołoń, Ralph Dobler, Roma Durak, and Andrzej Skoczowski. 2020. "Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity" Insects 11, no. 8: 470. https://doi.org/10.3390/insects11080470
APA StyleMołoń, M., Dampc, J., Kula-Maximenko, M., Zebrowski, J., Mołoń, A., Dobler, R., Durak, R., & Skoczowski, A. (2020). Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects, 11(8), 470. https://doi.org/10.3390/insects11080470





