Migratory Take-Off Behaviour of the Mongolian Grasshopper Oedaleus asiaticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Field Site
2.2. Take-Off Behaviour Observations
2.3. Regression Models with Meteorological Data
2.4. Statistical Analysis
3. Results
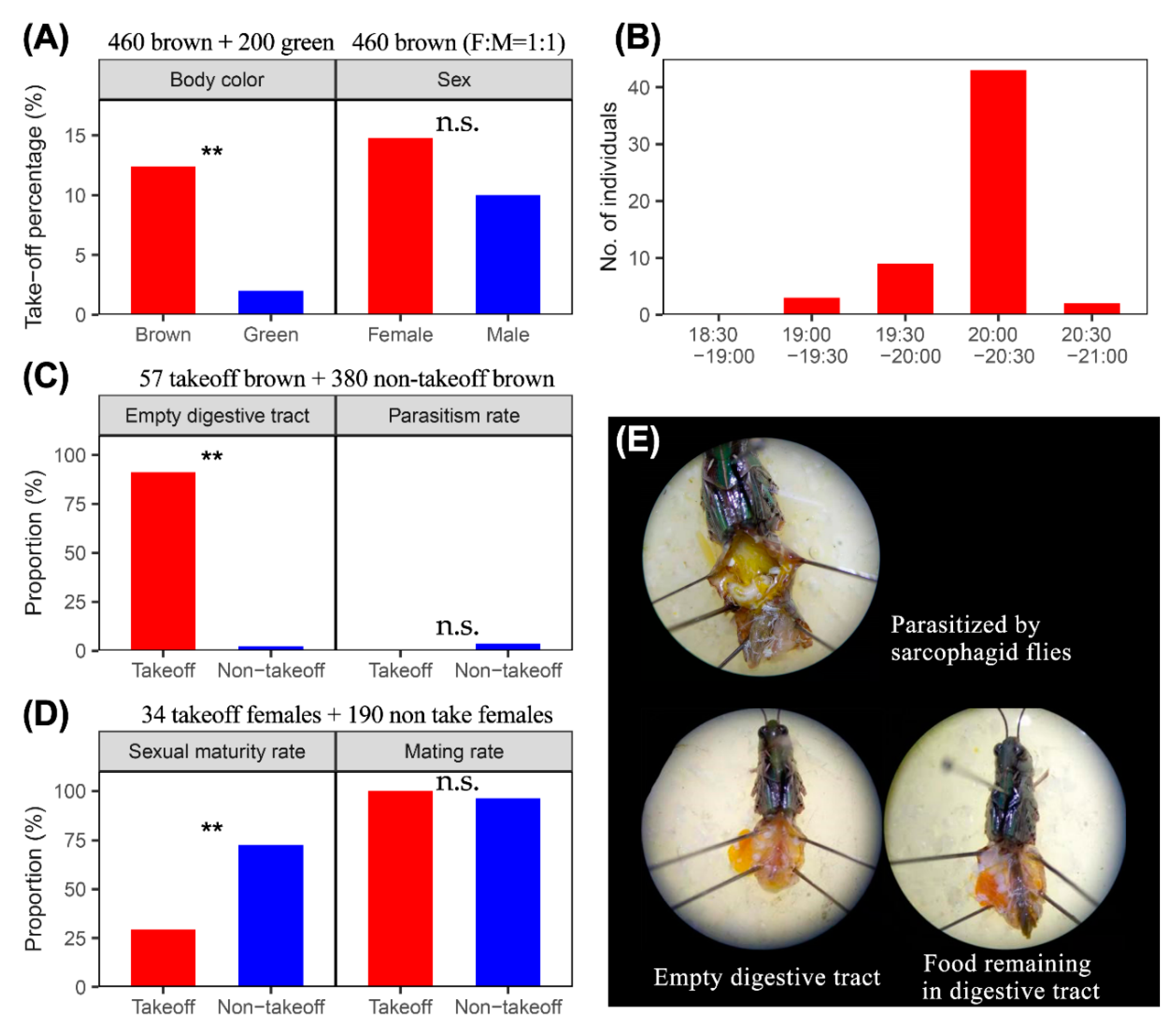
3.1. Observation of the Take-Off of O. asiaticus
3.2. Take-Off Related to the Physiological State and Parasitization Rate of O. asiaticus
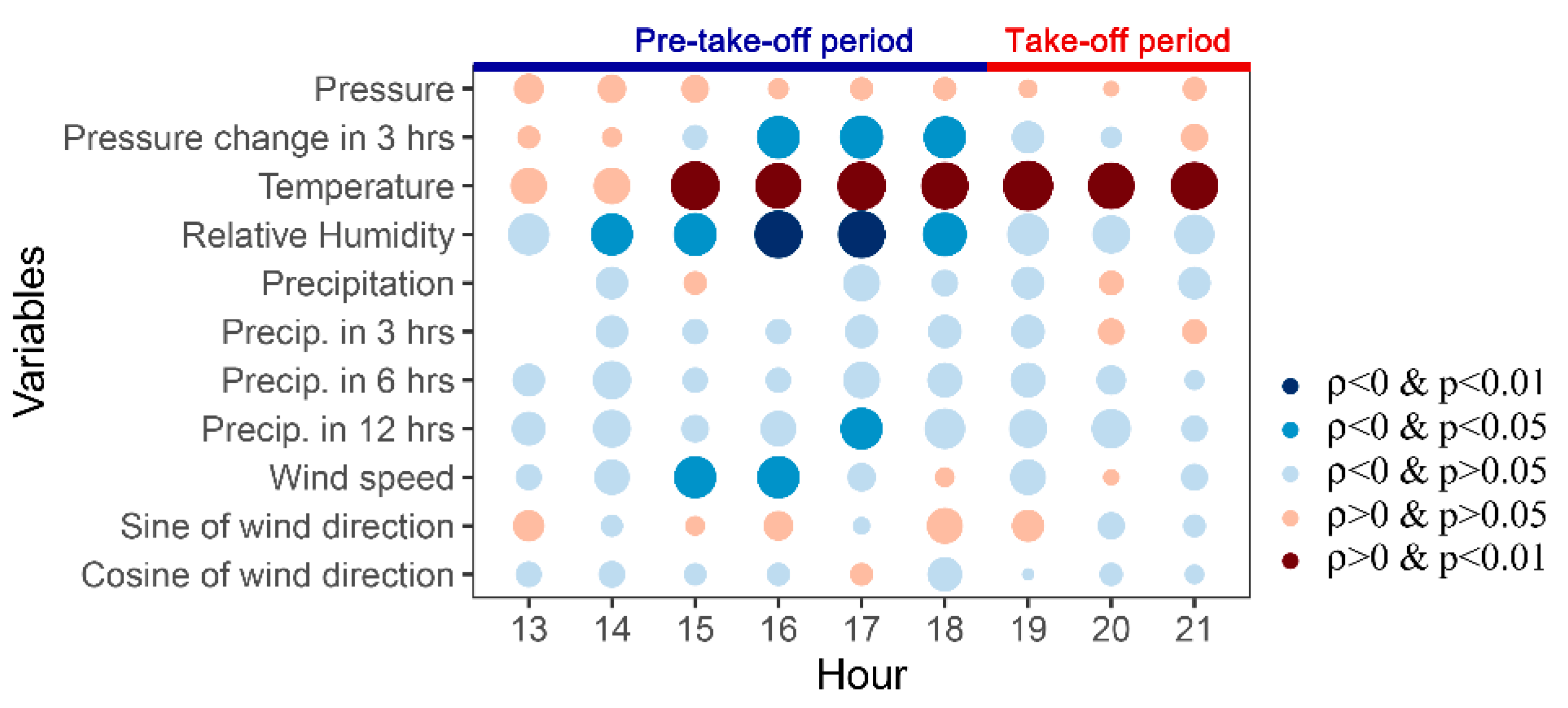
3.3. Take-Off Behaviour of O. asiaticus and Weather Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and grasshopper management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Latchininsky, A.V.; Sword, G.; Sergeev, M.; Cigliano, M.M.; Lecoq, M. Locusts and grasshoppers: behavior, ecology, and biogeography. Psyche 2011. [Google Scholar] [CrossRef]
- Le Gall, M.; Overson, R.; Cease, A. A global review on locusts (Orthoptera: Acrididae) and their interactions with livestock grazing practices. Front. Ecol. Evol. 2019, 7, 263. [Google Scholar] [CrossRef]
- Centre for Overseas Pest Research. The Locust and Grasshopper Agricultural Manual; Centre for Overseas Pest Research: London, UK, 1982; p. 690. [Google Scholar]
- Farrow, R.A. Flight and migration in Acridoids. In The Biology of Grasshoppers; Chapman, R.F., Joern, A., Eds.; John Wiley: London, UK, 1990; pp. 227–314. [Google Scholar]
- Van Huis, A.; Cressman, K.; Magor, J.I. Preventing desert locust plagues: optimizing management interventions. Entomol. Exp. Appl. 2007, 122, 191–214. [Google Scholar] [CrossRef]
- Lecoq, M. Forecasting systems for migrant pests. III. Locusts and grasshoppers in West Africa and Madagascar. In Insect Migration: Tracking Resources through Space and Time; Drake, V.A., Gatehouse, A.G., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 377–395. [Google Scholar]
- Wang, X.; Fang, X.; Yang, P.; Jiang, X.; Jiang, F.; Zhao, D.; Li, B.; Cui, F.; Wei, J.; Ma, C.; et al. The locust genome provides insight into swarm formation and long-distance flight. Nature Commun. 2014, 5, 2957. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.M. A taxonomic revision of the genus Oedaleus Fieber (Orthoptera: Acrididae). Bull. Br. Mus. Nat. Hist. 1981, 42, 83–183. [Google Scholar]
- Cease, A.J.; Elser, J.J.; Ford, C.F.; Hao, S.G.; Kang, L.; Harrison, J.F. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 2012, 335, 467–469. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Zhang, Z.; Pan, X.; Ma, A. Studies on the composition of Acridoidea fauna and its regional distribution in Nei Mongol (Inner Mongolia) autonomous region. Entomotaxonomia 1990, 12, 171–191. [Google Scholar]
- Zhang, L.; Yan, Y.H.; Wang, G.Q. A preliminary survey on the epizootics of infection of Nosema locustae grasshoppers in rangeland. Acta Agrestia Sin. 1995, 3, 223–229. [Google Scholar]
- Zhou, X.R.; Chen, Y.; Guo, Y.H.; Pang, B.P. Population dynamics of Oedaleus asiaticus on desert grasslands in Inner Mongolia. Chinese J. Appl. Entomol. 2012, 49, 1598–1603. [Google Scholar]
- Pan, J.M. Rangeland grasshoppers and their control strategies in Inner Mongolia. Grassl. China 2002, 24, 66–69. [Google Scholar]
- Liu, L.; Guo, A.H. Analysis of meteorological and ecological conditions of grasshopper Infestation in Inner Mongolia in 2004. Acta Meteorol. Sin. 2004, 11, 55–57. [Google Scholar]
- Gao, S.-J.; Wei, Y.S.; Temuer Liu, A.; Xu, L.B.; Wang, N. The flight ability of Oedaleus asiaticus and its relationship to population density. Pratac. Sci. 2012, 29, 1915–1919. [Google Scholar]
- Xiang, J.I.A.N.G.; Maimaitiming, Z.L. Nocturnal migration of grasshopper (Acrididae: Oedaleus asiaticus). Acta Agrestia Sin. 2003, 11, 75–77. [Google Scholar]
- Cease, A.J.; Hao, S.; Kang, L.; Elser, J.J.; Harrison, J.F. Are color or high rearing density related to migratory polyphenism in the band-winged grasshopper, Oedaleus asiaticus? J. Insect Physiol. 2010, 56, 926–936. [Google Scholar] [CrossRef]
- Cease, A.J.; Harrison, J.F.; Hao, S.; Niren, D.C.; Zhang, G.; Kang, L.; Elser, J.J. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus). R. Soc. Open Sci. 2017, 4. [Google Scholar] [CrossRef]
- Drake, V.A.; Reynolds, D.R. Radar Entomology: Observing Insect Flight and Migration; CABI: Wallingford, UK, 2012. [Google Scholar]
- Schaefer, G.W. Radar observations of insect flight. In Insect Flight; Rainey, R.C., Ed.; Blackwell Scientific: Oxford, UK, 1976; pp. 157–197. [Google Scholar]
- Riley, J.R.; Reynolds, D.R. Radar-based studies of the migratory flight of grasshoppers in the middle Niger area of Mali. Proc. R. Soc. B 1979, 204, 67–82. [Google Scholar]
- Han, H.B.; Wang, N.; Xu, L.B.; Gao, S.J.; Liu, A.P. Deqinghala. Grading criteria for the ovarian development of Oedaleus decorus asiaticus and differences between two types of ovarian development. Plant Prot. (Beijing, China) 2018, 44, 135–138. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Rogerson, P.A. Statistical Methods for Geography; Sage: London, UK, 2001. [Google Scholar]
- Dingle, H.; Drake, V.A. What is migration. Bioscience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Chapman, J.W.; Drake, V.A. Insect migration. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press, Salt Lake City: Salt Lake City, UT, USA, 2019; Volume 3, pp. 573–580. [Google Scholar]
- Riley, J.R.; Reynolds, D.R. A long-range migration of grasshoppers observed in the Sahelian zone of Mali by two radars. J. Animal Ecol. 1983, 52, 167–183. [Google Scholar] [CrossRef]
- Simpson, S.J.; Sword, G.A. Locusts. Curr. Biol. 2008, 18, R364–R366. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, X.Y.; Zhou, Y.; Liu, B.W.; Zheng, W.; Li, R.Q.; Wang, J.; Yu, J. The analysis of large-scale gene expression correlated to the phase changes of the migratory locust. PNAS 2004, 101, 17611–17615. [Google Scholar] [CrossRef]
- Han, H.B.; Zhou, X.R.; Pang, B.P.; Zhang, M.Z.; Li, H.P. Microsatellite marker analysis of the genetic diversity of Oedaleus asiaticus (Orthoptera: Acrididae) populations in Inner Mongolia, northern China. Acta Entomol. Sin. 2013, 56, 79–87. [Google Scholar]
- Lambert, M.R.K. Observations on the laboratory take-off behaviour of the desert locust, Schistocerca gregaria (Forskål) (Orthoptera: Acrididae). Bull. Entomol. Res. 1981, 71, 481. [Google Scholar] [CrossRef]
- Lambert, M.R.K. Some factors affecting flight in field populations of the Australian plague locust, Chortoicetes terminifera (Walker), in New South Wales. Anim. Behav. 1972, 20, 205–217. [Google Scholar] [CrossRef]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen: London, UK, 1969. [Google Scholar]
- Roff, D.A.; Fairbairn, D.J. The evolution and genetics of migration in insects. Bioscience 2007, 57, 155–164. [Google Scholar] [CrossRef]
- Rankin, M.A.; Burchsted, J.C.A. The cost of migration in insects. Annu. Rev. Entomol. 1992, 37, 533–559. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Sappington, T.W. Relationship of flight and reproduction in beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae), a migrant lacking the oogenesis-flight syndrome. J. Insect Physiol. 2010, 56, 1631–1637. [Google Scholar] [CrossRef]
- Zhao, X.C.; Feng, H.Q.; Wu, B.; Wu, X.F.; Liu, Z.F.; Wu, K.M.; McNeil, J.N. Does the onset of sexual maturation terminate the expression of migratory behaviour in moths? a study of the oriental armyworm, Mythimna separata. J. Insect Physiol. 2009, 55, 1039–1043. [Google Scholar]
- Zheng, D.B.; Hu, G.; Yang, F.; Du, X.D.; Yang, H.B.; Zhang, G.; Qi, G.J.; Liang, Z.L.; Zhang, X.X.; Cheng, X.N.; et al. Ovarian development status and population characteristics of Sogatella furcifera (Horváth) and Nilaparvata lugens (Stål): implication for pest forecasting. J. Appl. Entomol. 2009, 138, 67–77. [Google Scholar] [CrossRef]
- Hu, G.; Lim, K.S.; Horvitz, N.; Clark, S.J.K.; Reynolds, D.R.; Sapir, N.; Chapman, J.W. Mass seasonal bioflows of high-flying insect migrants. Science 2016, 354, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.K.; Isard, S.A. Atmospheric scales of biotic dispersal. Agr. Forest Meteorol. 1999, 97, 263–274. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). Weather and Desert Locusts (WMO-No. 1175); Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
| Independent Variable | Estimate | Standard Error | z-Value | p-Value |
|---|---|---|---|---|
| Intercept | −3.85 | 2.07 | −1.862 | 0.0626 |
| Total precipitation in last 12 h at 17:00 h | −2.49 | 1.55 | −1.608 | 0.1077 |
| Temperature at 15:00 h | 0.203 | 0.06 | 3.138 | 0.0017 |
| Wind speed at 15:00 h | −0.369 | 0.69 | −5.278 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-P.; Tu, X.-B.; Lin, P.-J.; Li, S.; Xu, C.-M.; Wang, X.-Q.; Reynolds, D.R.; Chapman, J.; Zhang, Z.-H.; Hu, G. Migratory Take-Off Behaviour of the Mongolian Grasshopper Oedaleus asiaticus. Insects 2020, 11, 416. https://doi.org/10.3390/insects11070416
Wang Y-P, Tu X-B, Lin P-J, Li S, Xu C-M, Wang X-Q, Reynolds DR, Chapman J, Zhang Z-H, Hu G. Migratory Take-Off Behaviour of the Mongolian Grasshopper Oedaleus asiaticus. Insects. 2020; 11(7):416. https://doi.org/10.3390/insects11070416
Chicago/Turabian StyleWang, Yun-Ping, Xiong-Bing Tu, Pei-Jiong Lin, Shuang Li, Chao-Min Xu, Xin-Qiao Wang, Don R. Reynolds, Jason Chapman, Ze-Hua Zhang, and Gao Hu. 2020. "Migratory Take-Off Behaviour of the Mongolian Grasshopper Oedaleus asiaticus" Insects 11, no. 7: 416. https://doi.org/10.3390/insects11070416
APA StyleWang, Y.-P., Tu, X.-B., Lin, P.-J., Li, S., Xu, C.-M., Wang, X.-Q., Reynolds, D. R., Chapman, J., Zhang, Z.-H., & Hu, G. (2020). Migratory Take-Off Behaviour of the Mongolian Grasshopper Oedaleus asiaticus. Insects, 11(7), 416. https://doi.org/10.3390/insects11070416








