Suppression of Transferrin Expression Enhances the Susceptibility of Plutella xylostella to Isaria cicadae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Microorganisms
2.2. cDNA Cloning
2.3. Sequence Analysis of PxTrf
2.4. Reverse Transcriptase Quantitative PCR
2.5. Identification of PxTrf Protein in P. xylostella
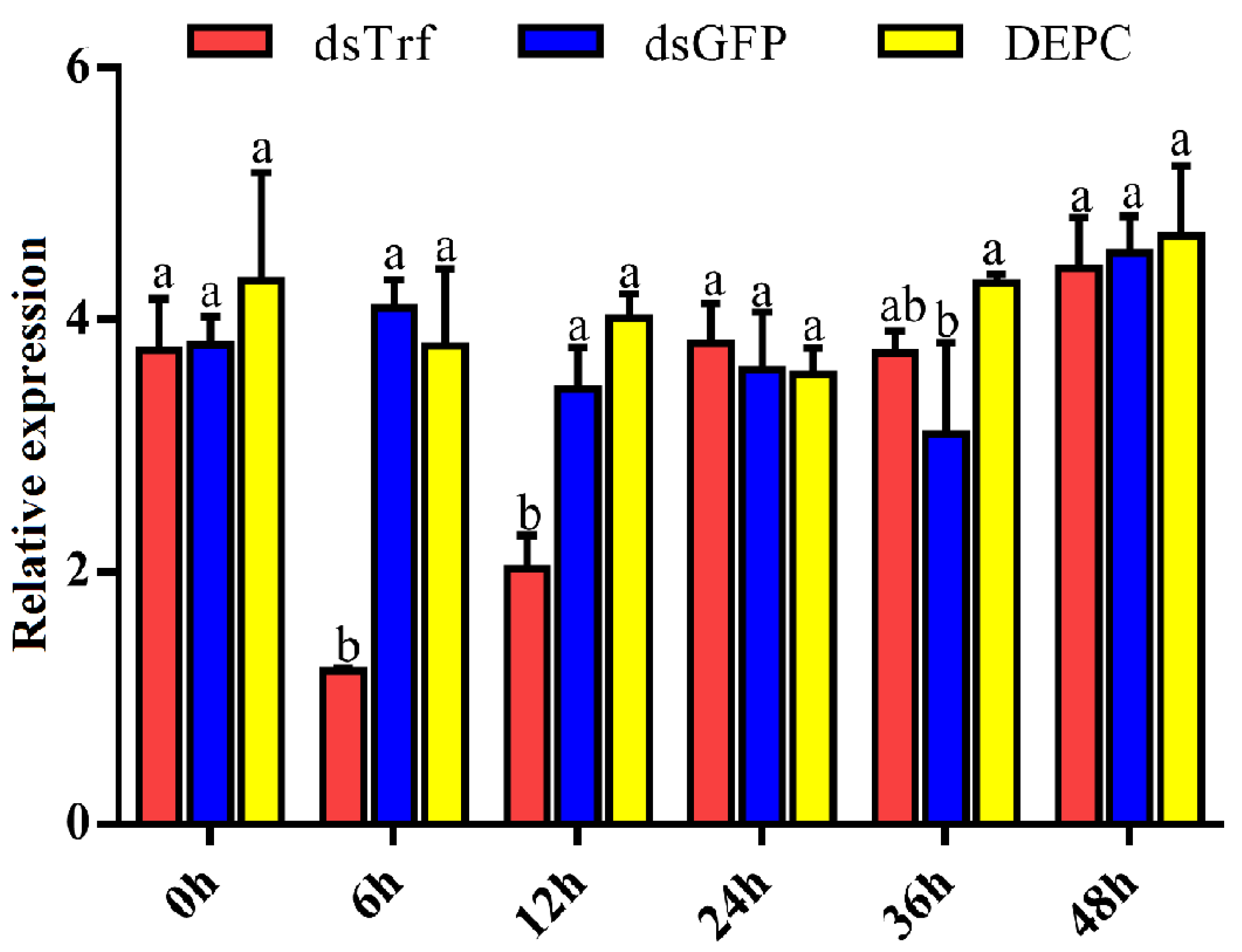
2.6. RNA Interference
2.7. Hemocyte Nodulation Assay
2.8. Larvae Pupation Analysis
2.9. I. cicadae Bioassay
2.10. Statistical Analysis
3. Results
3.1. cDNA Cloning and Sequence Analysis of PxTrf
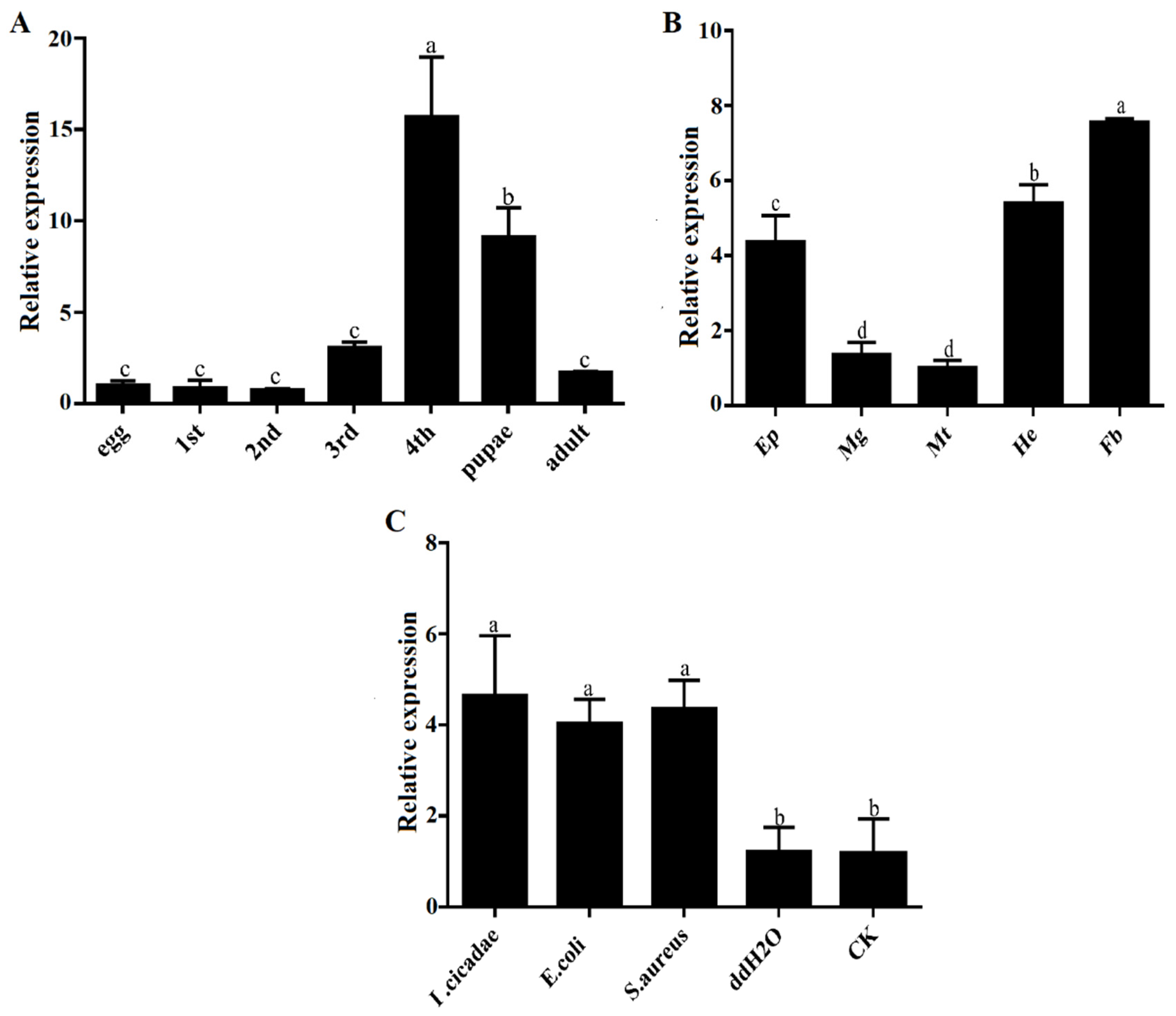
3.2. Expression Profiles of PxTrf
3.3. Identification of PxTrf Protein in P. xylostella
3.4. Analysis of the Efficacy of PxTrf RNAi
3.5. Bioassay of PxTrf Silencing P. xylostella to I. cicadae Infection
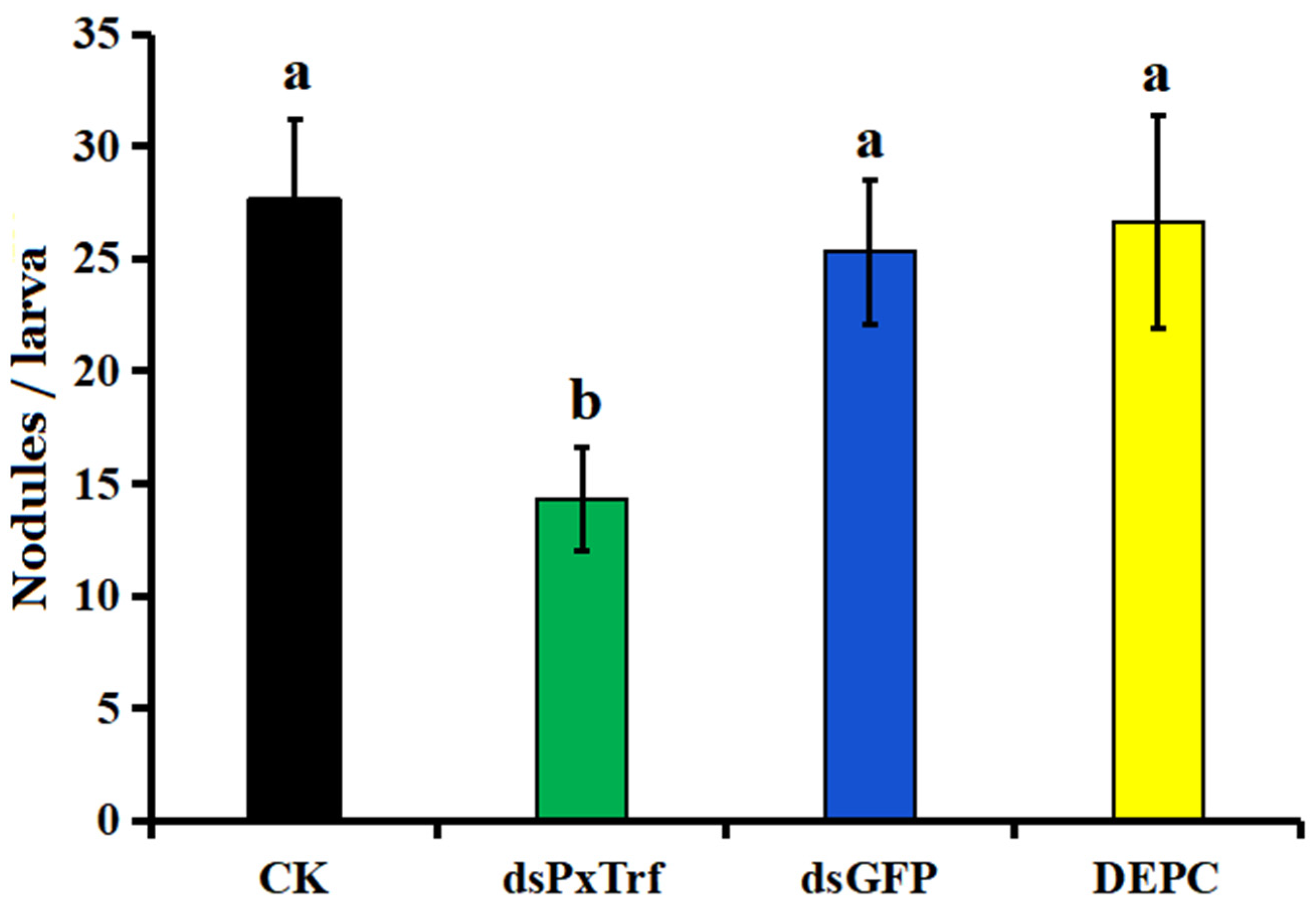
3.6. Hemocyte Nodulation Assay
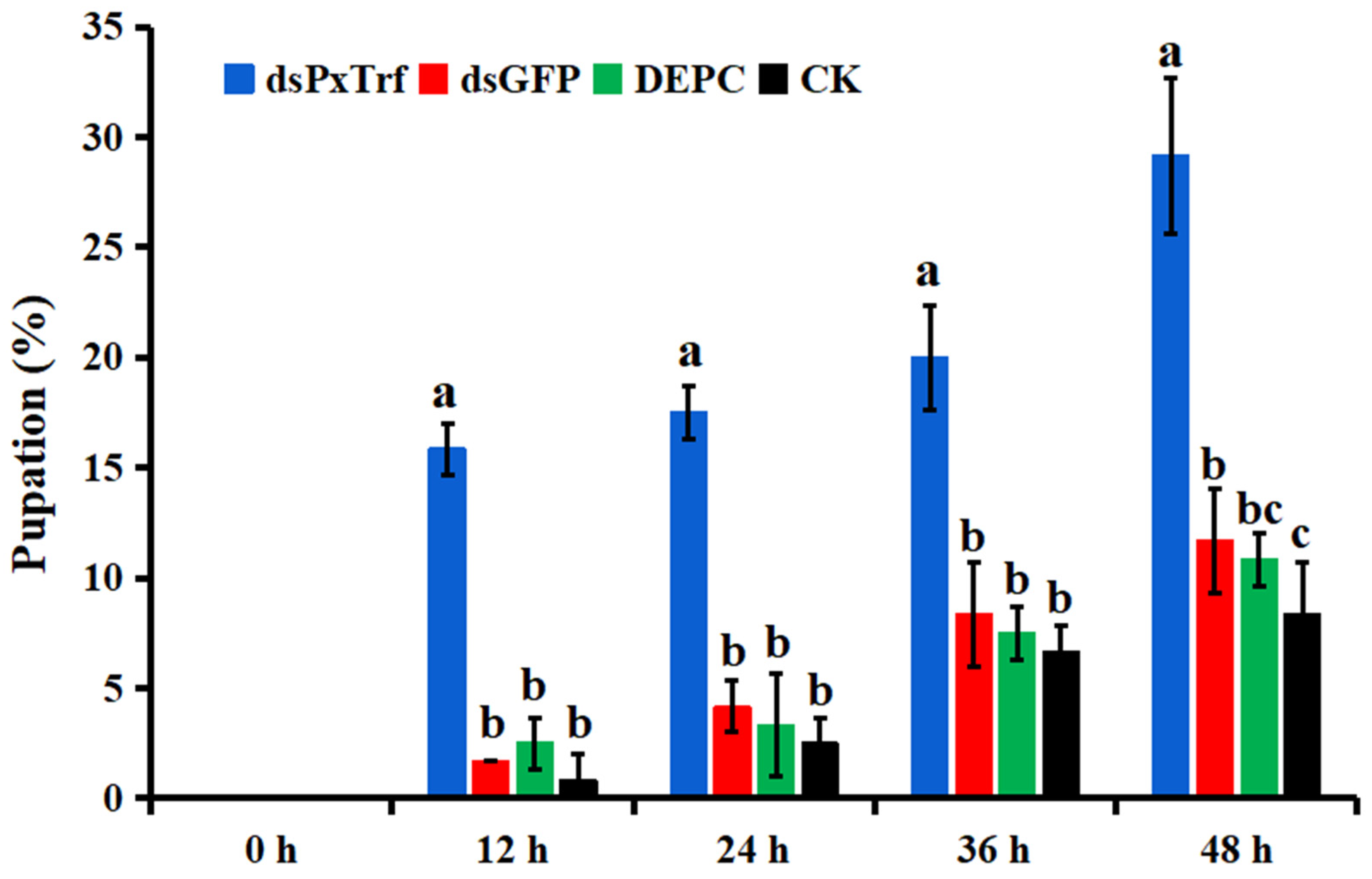
3.7. Larvae Pupation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Feng, X.; Liu, S.S.; You, M.; Furlong, M.J. Biology, ecology, and management of the diamondback moth in China. Annu. Rev. Entomol. 2016, 61, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.L.S.; Sobreiro, A.I.; Couto, I.F.S.; Silva, R.M.; Pereira, F.F.; Heredia-Vieira, S.C.; Cardoso, C.A.L.; Mauad, M.; Scalon, S.P.Q.; Verza, S.S.; Mussury, R.M. Chemical compounds and bioactivity of aqueous extracts of Alibertia spp. in the control of Plutella xylostella L. (Lepidoptera: Plutellidae). Insects 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.; Li, X.; Liu, Y.; Gao, X.; Liang, P. Global identification of microRNAs associated with chlorantraniliprole resistance in diamondback moth Plutella xylostella (L.). Sci Rep. 2017, 7, e40713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.; Xu, M.; Shi, H.; Gao, X.; Liang, P. Genome-wide identification of lncRNAs associated with chlorantraniliprole resistance in diamondback moth Plutella xylostella (L.). BMC Genom. 2017, 18, e380. [Google Scholar] [CrossRef]
- Li, X.; Shi, H.; Gao, X.; Liang, P. Characterization of UDP-glucuronosyltransferase genes and their possible roles in multi-insecticide resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 695–704. [Google Scholar] [CrossRef]
- Hajek, A.E.; Delalibera, I. Fungal pathogens as classical biological control agents against arthropods. Biocontrol 2010, 55, 147–158. [Google Scholar] [CrossRef]
- Levitin, A.; Whiteway, M. Drosophila innate immunity and response to fungal infections. Cell. Microbiol. 2008, 10, 1021–1026. [Google Scholar] [CrossRef]
- Fang, M.; Chai, Y.; Chen, G.; Wang, H.; Huang, B. N6-(2-hydroxyethyl)-adenosine exhibits insecticidal activity against Plutella xylostella via adenosine receptors. PLoS ONE 2016, 11, e0162859. [Google Scholar] [CrossRef] [Green Version]
- Geiser, D.L.; Winzerling, J.J. Insect transferrins: Multifunctional proteins. Biochim. et Biophys. Acta 2012, 1820, 437–451. [Google Scholar] [CrossRef]
- Luck, A.N.; Mason, A.B. Transferrin-mediated cellular iron delivery. Curr. Top. Membr. 2012, 69, 3–35. [Google Scholar]
- Guo, S.; Frazer, D.M.; Anderson, G.J. Iron homeostasis: Transport, metabolism, and regulation. Curr. Opin. Clin. Nutr. Metab. Care. 2016, 19, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A. Molecular evolution of the transferrin family and associated receptors. Biochim. Biophys. Acta 2012, 1820, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Friedman, R. Evolutionary diversification of the vertebrate transferrin multi-gene family. Immunogenetics 2014, 66, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.T.; Ho, J.Z.; Ho, B.; Ding, J.L. Iron-withholding strategy in innate immunity. Immunobiology 2006, 211, 295–314. [Google Scholar] [CrossRef]
- Hirai, M.; Watanabe, D.; Chinzei, Y. A juvenile hormone-repressible transferrinlike protein from the bean bug, Riptortus clavatus: cDNA sequence analysis and protein identification during diapause and vitellogenesis. Arch. Insect Biochem. Physiol. 2000, 44, 17–26. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Choo, Y.M.; Kim, I.; Je, Y.H.; Woo, S.D.; Lee, S.M.; Park, H.C.; Sohn, H.D.; Jin, B.R. Insect transferrin functions as an antioxidant protein in a beetle larva. Comp. Biochem. Physiol. B 2008, 150, 161–169. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, Q.; Lu, Y.; Zhao, Q.; Gao, X. A transferrin gene associated with development and 2-tridecanone tolerance in Helicoverpa armigera. Insect Mol. Biol. 2015, 24, 155–166. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, E.; Spellman, P.T.; Rubin, G.M.; Lemaitre, B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl. Acad. Sci. USA 2001, 98, 12590–12595. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.J.; Crozier, Y.C.; Crozier, R.H. Isolation and characterization of a termite transferrin gene up-regulated on infection. Insect Mol. Biol. 2003, 12, 1–7. [Google Scholar] [CrossRef]
- Guz, N.; Attardo, G.M.; Wu, Y.; Aksoy, S. Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans). J. Insect Physiol. 2007, 53, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, E.Y.; Lee, J.K.; Kwon, O.Y.; Hwang, J.S.; Kim, I.; Kang, S.W.; Lee, W.J.; Ding, J.L.; You, K.H.; Goo, T.W. Bombyx mori transferrin: Genomic structure, expression and antimicrobial activity of recombinant protein. Developmental Comp. Immunol. 2009, 33, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Brummett, L.M.; Kanost, M.R.; Gorman, M.J. The immune properties of Manduca sexta transferrin. Insect Biochem. Mol. Biol. 2017, 81, 1–9. [Google Scholar]
- Lehane, M.J.; Gibson, W.; Lehane, S.M. Differential expression of fat body genes in Glossina morsitans morsitans following infection with Trypanosoma brucei brucei. Int. J. Parasitol. 2008, 38, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Dang, X.; Zheng, X.; Wang, Y.; Wang, L.; Ye, L.; Jiang, J. Antimicrobial peptides from the edible insect Musca domestica and their preservation effect on chilled pork. J. Food Process. Preserv. 2019, e14369. [Google Scholar] [CrossRef]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, Y. A viral histone H4 suppresses expression of a transferrin that plays a role in the immune response of the diamondback moth, Plutella xylostella. Insect Mol. Biol. 2010, 19, 567–574. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Cheng, Y.; Zak, O.; Aisen, P.S.; Harrison, C.; Walz, T. Structure of the human transferrin receptor-transferrin complex. Cell 2004, 116, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Lambert, L.A.; Perri, H.; Halbrooks, P.J.; Mason, A.B. Evolution of the transferrin family: Conservation of residues associated with iron and anion binding. Comp. Biochem. Physiol. B 2005, 142, 129–141. [Google Scholar] [CrossRef]
- Jamroz, R.C.; Gasdaska, J.R.; Bradfield, J.Y.; Law, J.H. Transferrin in a cockroach: Molecular cloning characterization, and suppression by juvenile hormone. Proc. Natl. Acad. Sci. USA 1993, 90, 1320–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.Y.; Lee, K.S.; Choo, Y.M.; Kim, I.; Hwang, J.S.; Sohn, H.D.; Jin, B.R. Molecular cloning and characterization of a transferrin cDNA from the white-spotted flower chafer, Protaetia brevitarsis. DNA Seq. 2008, 19, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.; Dunkov, B.C.; Dimov, S.; Ralchev, K.; Law, J.H. Drosophila melanogaster ferritin: cDNA encoding a light chain homologue, temporal and tissue specific expression of both subunit types. Insect Biochem. Mol. Biol. 2002, 32, 295–302. [Google Scholar] [CrossRef]
- Harizanova, N.; Georgieva, T.; Dunkov, B.C.; Yoshiga, T.; Law, J.H. Aedes aegypti transferrin. Gene structure, expression pattern, and regulation. Insect Mol. Biol. 2005, 14, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kim, B.Y.; Lee, K.S.; Yoon, H.J.; Cui, Z.; Lu, W.; Jia, J.M.; Kim, D.H.; Sohn, H.D.; Jin, B.R. Molecular characterization of iron binding proteins, transferrin and ferritin heavy chain subunit, from the bumblebee Bombus ignitus. Comp. Biochem. Physiol. B 2009, 152, 20–27. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef]
- Ezzati-Tabrizi, R.; Farrokhi, N.; Talaei-Hassanloui, R.; Alavi, S.M.; Hosseininaveh, V. Insect inducible antimicrobial peptides and their applications. Curr. Protein Pept. Sci. 2013, 14, 698–710. [Google Scholar] [CrossRef]
- Dunkov, B.; Georgieva, T. Insect iron binding proteins: Insights from the genomes. Insect Biochem. Mol. Biol. 2006, 36, 300–309. [Google Scholar] [CrossRef]
- Huebers, H.A.; Huebers, E.; Finch, C.A.; Webb, B.A.; Truman, J.W.; Riddiford, L.M.; Martin, A.W.; Massover, W.H. Iron binding proteins and their roles in the tobacco hornworm, Manduca sexta (L.). J. Comp. Physiol. B 1988, 158, 291–300. [Google Scholar] [CrossRef]
- Bartfeld, N.S.; Law, J.H. Isolation and molecular cloning of transferrin from the tobacco hornworm, Manduca sexta. J. Biol. Chem. 1990, 265, 21684–21691. [Google Scholar] [PubMed]
- Kurama, T.; Kurata, S.; Natori, S. Molecular characterization of an insect Transferrin and its selective incorporation into eggs during oogenesis. Eur. J. Biochem. 1995, 228, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Levy, F.; Bulet, P.; Ehret-Sabatier, L. Proteomic analysis of the systemic immune response of Drosophila. Mol. Cell. Proteom. 2004, 3, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaible, U.E.; Kaufmann, S.H.E. Iron and microbial infection. Nat. Rev. Microbiol. 2004, 2, 946–953. [Google Scholar] [CrossRef]
- Barber, M.F.; Elde, N.C. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 2014, 346, 1362–1366. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, K.W.; Spellberg, B. Transferrin-mediated iron sequestration as a novel therapy for bacterial and fungal infections. Curr. Opin. Microbiol. 2015, 27, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Garner, B.; Brown, E.; Taplin, M.; Garcia, A.; Williams-Mapp, B. Transferrin impacts Bacillus thuringiensis biofilm levels. Biomed. Res. Int. 2016, 2016, e3628268. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Pantapalangkoor, P.; Tan, B.; Bruhn, K.W.; Ho, T.; Nielsen, T.; Skaar, E.P.; Zhang, Y.; Bai, R.; Wang, A.; Doherty, T.M.; Spellberg, B. Transferrin iron starvation therapy for lethal bacterial and fungal infections. J. Infect. Dis. 2014, 210, 254–264. [Google Scholar] [CrossRef]
- Ned, R.M.; Swat, W.; Andrews, N.C. Transferrin receptor 1 is differentially required in lymphocyte development. Blood 2003, 102, 3711–3718. [Google Scholar] [CrossRef]
- Macedo, M.F.; de Sousa, M.; Ned, R.M.; Mascarenhas, C.; Andrews, N.C.; Correia-Neves, M. Transferrin is required for early T-cell differentiation. Immunology 2004, 112, 543–549. [Google Scholar] [CrossRef]
- Lin, J.R.; Hu, J. SeqNLS: Nuclear localization signal prediction based on frequent pattern mining and linear motif scoring. PLoS ONE 2013, 8, e76864. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W.; Riddiford, L.M. The evolution of insect metamorphosis: A developmental and endocrine view. Philos. Trans. R. Soc. B 2019, 374, e20190070. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Primers | Sequence (5′–3′) | Function |
|---|---|---|
| PxTrf-CF | ATCAGTGACCATGATAGTGAAAATAGCCAT | Cloning |
| PxTrf-CR | GTATGTATGTTTATGCAACACCACAAGATG | |
| PxTrf-QF | TAGCGTCAGCATCCAACAAG | qRT-PCR |
| PxTrf-QR | TCCAAGCTTTTTCAGGCACT | |
| Actin-F | TGGCACCACACCTTCTAC | qRT-PCR |
| Actin-R | CATGATCTGGGTCATCTTCT | |
| dsTrf-F | TAATACGACTCACTATAGGGTGTGCGGAAGTTCTTTGGG | RNAi |
| dsTrf-R | TAATACGACTCACTATAGGGCTACGTTATCACCTGTAGGGTT | |
| dsGFP-F | TAATACGACTCACTATAGGGAGGGCGAGGGCGATGCCACC | RNAi |
| dsGFP-R | TAATACGACTCACTATAGGGTGTACTCCAGCTTGTGCCCC |
| Sample | Protein | Score | Matches | Sequences | emPAI |
|---|---|---|---|---|---|
| Naïve | Transferrin | 167 | 9 (6) | 8 (5) | 0.23 |
| IC | Transferrin | 290 | 22 (13) | 15 (10) | 0.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Hao, Z.; Wang, L.; Li, S.; Guo, Y.; Dang, X. Suppression of Transferrin Expression Enhances the Susceptibility of Plutella xylostella to Isaria cicadae. Insects 2020, 11, 281. https://doi.org/10.3390/insects11050281
Xu H, Hao Z, Wang L, Li S, Guo Y, Dang X. Suppression of Transferrin Expression Enhances the Susceptibility of Plutella xylostella to Isaria cicadae. Insects. 2020; 11(5):281. https://doi.org/10.3390/insects11050281
Chicago/Turabian StyleXu, Huihui, Zhongping Hao, Lifang Wang, Shuangjiao Li, Yuruo Guo, and Xiangli Dang. 2020. "Suppression of Transferrin Expression Enhances the Susceptibility of Plutella xylostella to Isaria cicadae" Insects 11, no. 5: 281. https://doi.org/10.3390/insects11050281
APA StyleXu, H., Hao, Z., Wang, L., Li, S., Guo, Y., & Dang, X. (2020). Suppression of Transferrin Expression Enhances the Susceptibility of Plutella xylostella to Isaria cicadae. Insects, 11(5), 281. https://doi.org/10.3390/insects11050281





