Abstract
Chitin is one the main components of the insect cuticle, and chitin synthase (CHS) is an important enzyme required for chitin formation. CHS has been characterized in various insect species, but the structure and biochemical properties in Spodoptera litura have not been determined. In this study, we identified two CHS genes, SlCHS1 and SlCHS2, which encode proteins with 1565 and 1520 amino acid residues, respectively. Transcriptional analysis suggested that SlCHS1 has a high expression level in the integument whereas SlCHS2 showed the highest expression level in the midgut. During S. litura growth and development, SlCHS1 and SlCHS2 were both predominantly expressed in the fourth-instar larval stage. In addition, the expression of SlCHS1 and SlCHS2 could be induced by 20-hydroxyecdysone (20E). Silencing of SlCHS1 by RNA interference significantly inhibited the pupation and molting of S. litura larvae (RNAi), while knockdown of SlCHS2 had no significant effects on the S. litura phenotype. These results may provide a new molecular target for control of S. litura.
1. Introduction
Chitin is a linear amino polysaccharide polymer made up of β-1,4-N-acetyl-d-glucosamine (GlcNAc) and is the second most abundant biological polymer after cellulose in nature. Chitin has been detected in a wide variety of organisms, ranging from the simplest algae to fungi, nematodes and arthropods [1,2]. In insects, chitin is essential for the structural integrity of the cuticle, and the peritrophic matrix (PM) lining the midgut epithelium, which protects the intestinal epithelium from mechanical disruption and invasion by various pathogens [3,4]. The balance of chitin content is essential for the growth and development (molting) of insects [5]. However, humans and higher animals appear to be completely devoid of chitin. Therefore, insecticides using chitin metabolism as targets can effectively control pests, but have no effect on humans. Chitin biosynthesis starts with the disaccharide trehalose, culminating in the polymerization of the N-acetyl glucosamine subunits by chitin synthase to produce chitin microfibrils [6]. In addition, this process requires various enzymes including hexokinase (HK), glucose-6-phosphate isomerase (G6PI), glutamine-fructose-6-phosphate aminotransferase (GFAT), glucosamine-6-phosphate N-acetyltransferase (GNPNA), phosphoglucosamine mutase (PAGM) and UDP-N-acetylglucosamine pyrophosphorylase (UAP). The last step is the chitin biosynthetic pathway catalyzed by chitin synthase (CHS), which can catalyze the transfer of sugar moieties from activated sugar donors to specific acceptors [7].
Chitin synthase (CHS, EC 2.4.1.16) is an integral membrane glycosyltransferase that is essential for chitin chain polymerization and deposition in insect chitinous structures [8]. CHS is a transmembrane protein that plays an important role in chitin synthesis [9]. CHS cDNA sequences have been characterized in several insect species, including Drosophila melanogaster [10], Manduca sexta [11], Tribolium castaneum [1], Locusta migratoria [12], Spodopetra frugiperda [13], and Diaphorina citri [14]. In insects, CHS are divided into two groups, namely, CHS1 and CHS2, based on domain composition, sequence homology, tissue localization and physiological role [15]. CHS1 is exclusively expressed in the epidermis underlying the cuticular exoskeleton and related ectodermal cells such as tracheal cells, while CHS2 is highly expressed in the midgut, and its coding enzyme is responsible for the synthesis of PM-associated chitin [16].
In recent years, RNA interference (RNAi) has been widely used to research the functions of CHS in different species. Chen et al. revealed that the cuticle of Spodoptera exigua larvae was disordered and that the epithelial walls did not expand uniformly after silencing CHSA [17]. In Leptinotarsa decemlineata, knockdown of CHSAa, CHSAb and CHSB in second- and fourth-instar larvae lowered chitin contents in whole body and integument samples and thinned tracheal taenidia [18]. However, the functions of CHS have not been reported in Spodoptera litura. RNAi refers to highly conserved cellular mechanisms in which Argonaute (Ago) family proteins bind small RNAs to trigger the degradation of longer RNAs through the sequence complementarity [19]. Delivery of dsRNA in insects can induce cascades of the RNAi pathway by which the enzyme Dicer2 cleaves dsRNA into fragments of siRNAs that bind to the RNA-induced silencing complex (RISC), which recognizes the target mRNA, binds to it and triggers degradation of the homologue endogenous mRNA [20,21]. Since the first discovery of this process in Caenorhabditis elegans by Fire et al., gene knockdown through RNAi induced by double stranded RNA (dsRNA) has been widely applied for the management of insect pests [22].
Spodoptera litura (Lepidoptera: Noctuidae) is an important herbivorous pest responsible for widespread economic damage to numerous field vegetables and ornamental plants in tropical and subtropical regions [23]. At present, control of S. litura is primarily achieved through the application of various chemical insecticides. However, S. litura has evolved high resistance to every class of pesticides used against it [24]. Shad et al. revealed that S. litura shows a high level of resistance to spinosad, indoxacarb, and methoxyfenozide [25]. In addition, many field populations of S. litura have developed resistance to multiple insecticides in South Asia, including chlorpyrifos, β-cypermethrin and methomyl [26]. Therefore, it is highly important to identify environmentally friendly methods to control S. litura. Chitin is found in insect exoskeletons, but is not found in vertebrates [27]. Therefore, we considered using chitin metabolism-related genes as targets to control S. litura.
In the current study, two cDNAs encoding chitin synthase (SlCHS1 and SlCHS2) were identified from the genome database of S. litura, and their spatial and temporal expression profiles were analyzed. In addition, the expression levels of SlCHS1 and SlCHS2 can be induced by 20E. Furthermore, silencing of SlCHS1 influences S. litura larvae pupation and molting. However, silencing of SlCHS2 has no significant influence for molting of S. litura. Herein we explore whether these genes can provide useful information on CHSs as targets for the identification of novel insecticides?
2. Materials and Methods
2.1. Spodoptera Litura Rearing and Tissue Preparation
S. litura larvae were collected from the orange orchard at the National Navel Orange Engineering Research Center (NORC), Gannan Normal University, Ganzhou, China. Larvae were reared in culture dishes on an artificial diet at 27 °C and 70%–75% relative humidity, with a photoperiod of 12 h light and 12 h dark, until they became adult moths. The main components of the artificial diet include corn starch, soybean flour, agar, yeast powder, sorbic acid and cholesterol. All male and female adults were placed in a plastic case, and moderate hydromel was added to keep the plural adults alive. The produced eggs were reared based on the above conditions. S. litura were collected at different developmental stages, including larvae, pupae and adults. Moreover, the first day of sixth-instar larvae were dissected to obtain various tissues, including the integument, head, Malpighian tubule, fat body and midgut. The midgut was cleaned using precooled DEPC-water to remove the remaining food debris, and stored at −80 °C.
20E treatment was performed according to a previous report with some modifications [28]. In brief, a total of 2 μg of 20E was dissolved in 4 μL of dimethyl sulfoxide (DMSO) to prepare the working solution and then injected into larvae on the first day their sixth instar. DMSO was injected into other first day, sixth-instar larvae, as a control. The midgut and integument samples were collected after 1, 12, 36 and 48 h, and stored at −80 °C. Each treatment was repeated with three biological replicates.
2.2. RNA Isolation and cDNA Synthesis
To analyze the spatiotemporal expression patterns of SlCHS1 and SlCHS2, S. litura total RNA was extracted from different tissues of sixth-instar larvae (integument, head, Malpighian tubule, fat body and midgut) and at different developmental stages (second-instar, third-instar, fourth-instar, fifth-instar, and sixth-instar larvae, and pupae and adults) using the animal tissue total RNA kit (Simgen, Hangzhou, Zhejiang, China). RNA concentration and purity were assayed using a NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, New York, NY, USA) at absorbance ratios of A260/230 and A260/280. The integrity of total RNA was confirmed using standard agarose gel electrophoresis with ethidium bromide (EB) staining. Total RNA was reverse-transcribed in a 20 μL reaction system using a Fast 1st strand cDNA Synthesis kit (with gDNase) (Simgen) according to the manufacturer’s instructions. In brief, 2.0 μL of 5 × gRNA buffer and 1 μg of total RNA were mixed, and then RNase-free water was added to reach 10 μL, which was then incubated at 42 °C for 3 min. Afterward, 4 μL of 5 × RT buffer and 2 μL of RT enzyme mix were added and incubated at 95 °C for 3 min. The cDNA was stored at −20 °C for later use.
2.3. Sequencing Analysis of SlCHS1 and SlCHS2
To identify chitin synthase genes in S. litura, a TBLASTIN search of the S. litura genome database (https://www.ncbi.nlm.nih.gov/genome/14271?genome_assembly_id = 350050) was performed using the amino acid sequences of D. melanogaster CHS1 (NM_079509.3), D. melanogaster CHS2 (NM_079485.4), M. sexta CHS1 (AY062175.2) and M. sexta CHS2 (AY821560.1) as queries. This resulted in the identification of two cDNA sequence that we have named as SlCHS1 and SlCHS2. To confirm the correctness of the two candidate CHS sequences, reverse transcription PCR (RT-PCR) was carried out to amplify the full-length ORF sequence using gene-specific primers (Table 1). The deduced amino acid sequences of SlCHS1 and SlCHS2 were analyzed by using DASTAR software. The open reading frame (ORF) was predicted according to the ORF finder tool (https://www.ncbi.nlm.nih.gov/orffinfer/). The molecular weight (MW) and isogenic point (pI) of SlCHS1 and SlCHS2 were calculated using ExPASy (http://web.expasy.org/compute_pi). The signal peptides of SlCHS1 and SlCHS2 were predicted using SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP). The functional domain was predicted by using SMART software (http://smart.embl-heidelberg.de/). The membrane-spanning domain was predicted by TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The phylogenetic tree was constructed with MEGA 7.0 software using the neighbor-joining method with 1000-fold bootstrap resampling. Protein sequences from different insect species were obtained from GenBank (http://www.ncbi.nlm.nih.gov/) and used in the phylogenetic analysis (Table S1).

Table 1.
Primers used in this study.
2.4. RT-qPCR Analysis of SlCHS1 and SlCHS2 Expression Levels
RT-qPCR was performed to analyze the relative expression levels of SlCHS1 and SlCHS2. The primers were designed using Primer Premier 5.0 software (Table 1). The 20-µL reaction mixture for RT-qPCR contained 10 µL of SYBR II, 8 µL of ddH2O, 0.5 µL of forward primer, 0.5 µL of reverse primer, and 1.0 µL of cDNA template. The thermal cycling profile consisted of an initial denaturation at 95 °C for 60 s and 40 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 10 s. The reactions were performed with a LightCycle® 96 PCR Detection System (Roche, Basel, Switzerland). Relative expression levels were calculated by using the 2−∆∆Ct method. There were three biological replicates and three technique replicates for each sample. The reference gene chosen for analysis of SlCHS1 and SlCHS2 in different tissues and different developmental stages was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All data were analyzed using a one-way analysis of variance (ANOVA) and Tukey’s test.
2.5. dsRNA Synthesis and RNAi Analysis
dsRNA targeting SlCHS1 and SlCHS2 was synthesized using the T7 RioMAX Express RNAi System (Promega, San Luis Obispo, CA, USA) based on the manufacturer’s instructions. The forward and reverse primers were designed to amplify 439 bp and 421 bp for SlCHS1 and SlCHS2, respectively (Table 1). GFP dsRNA was used as a control. The final concentration of dsRNA for injection was adjusted to 300 ng/µL and 500 ng/µL using DEPC water as the working solution. To ensure the RNAi efficiency, pre-pupal S. litura larvae were injected with 10 µL of SlCHS1 and SlCHS2 dsRNA using a microinjector (Sangon Biotech, Shanghai, China), and the midgut and integument were collected at 24 h and stored at -80 °C. After 24 h, all live insects were collected to isolate total RNA and synthesize cDNA. The effect of dsSlCHS1 and dsSlCHS2 on gene expression was evaluated by RT-qPCR. A total of three biological replicates were used for each experiment. All data were analyzed using ANOVA and Tukey’s test.
3. Results
3.1. cDNA and Deduced Amino Acid Sequences of SlCHS1 and SlCHS2
In total, two chitin synthase genes, SlCHS1 and SlCHS2 were identified from S. litura. The cDNA sequence of SlCHS1 (GenBank accession number: XM_022964624.1) contains an ORF of 4698 bp encoding a protein of 1565 amino acid residues with a predicted MW of 178.1 kDa and a pI of 6.56. SlCHS2 (GenBank accession number: XM_022821184.1) contains an ORF of 4563 bp encoding a protein of 1520 amino acid residues with a predicted MW of 174.1 kDa and a pI of 5.83. In terms of protein structure, SlCHS1 contains three domains, including an N-terminal domain (residues 1-561) with nine transmembrane helices, a putative catalytic domain (residues 562-900) and a C-terminal domain (residues 901-1565) with an additional seven transmembrane helices (Figure S1). SlCHS2 also has three domains, including an N-terminal domain (residues 1-552) with nine transmembrane helices, a putative catalytic domain (residues 553-891) and a C-terminal domain (residues 892-1520) with an additional seven transmembrane helices (Figure S2). In addition, both SlCHS1 and SlCHS2 contain signature sequences (EDR and QRRRW). By using NetNGlyc 1.0 software analysis, SlCHS1 contains six glycosylation sites, and SlCHS2 contains five glycosylation sites, suggesting that these proteins are glycosylated. The deduced amino acid sequences of these two chitin synthases exhibited seven highly conserved motifs (Figure 1). Based on the amino acid sequences of CHS from different insect species, a phylogenetic tree was generated using MEGA 5.0 to investigate the evolutionary relationship of SlCHS1 and SlCHS2 among the selected insect species. Insect CHSs can be divided into two classes, CHS1 and CHS2. The results showed that both SlCHS1 and SlCHS2 maintained high identity to S. exigua CHS1 and CHS2, respectively (Figure 2).
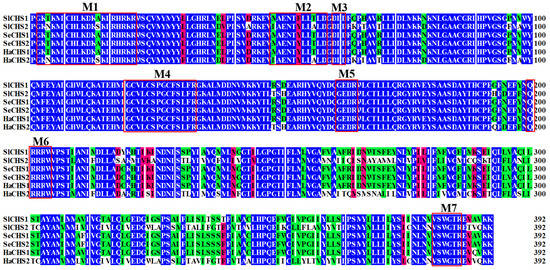
Figure 1.
Multiple sequence alignment of the conserved catalytic domain of the chitin synthases (CHSs) from three insect species. CHSs are from Spodoptera litura (Sl), Spodoptera exigua (Se) and Helicoverpa armigera (Ha). Seven characteristic motifs (M 1–7) for insect chitin synthases are indicated with red boxes.
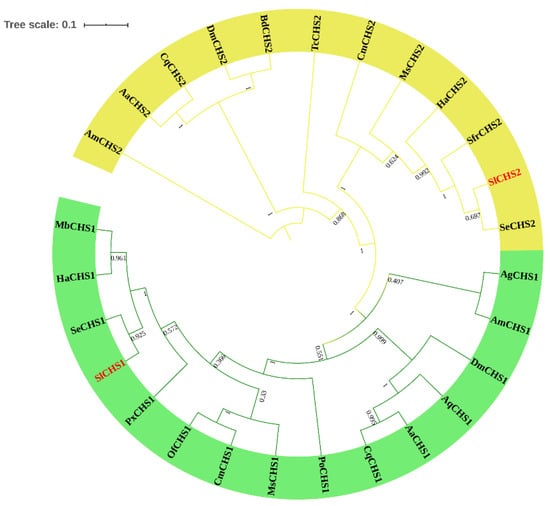
Figure 2.
Phylogenetic relationship analysis of chitin synthases (CHSs) in different insect species. A phylogenetic tree was constructed using MEGA 5.0 software using the neighbor-joining method with a bootstrap value of 1000. CHSs are from Spodoptera litura (Sl), Spodoptera exigua (Se), Spodoptera frugiperda (Sfr), Helicoverpa armigera (Ha), Manduca sexta (Ms), Cnaphalocrocis medinalis (Cm), Tribolium castaneum (Tc), Apis mellifera (Am), Aedes aegypti (Aa), Culex quinquefasciatus (Cq), Drosophila melanogaster (Dm), Bactrocera dorsalis (Bd), Aphis glycines (Ag), Anopheles quadrimaculatus (Aq), Ostrinia furnacalis (Of), Plutella xylostella (Px), Mamestra brassicae (Mb) and Phthorimaea operculella (Po).
3.2. Spatiotemporal Expression Patterns of SlCHS1 and SlCHS2
The expression profiles of SlCHS1 and SlCHS2 in different developmental stages and different tissues were investigated by RT-qPCR. For tissue expression analysis, the first day of sixth-instar larvae were dissected to obtain different tissues, including the integument, head, Malpighian tubules, fat body and midgut, and the results suggested that both SlCHS1 and SlCHS2 were expressed in all S. litura tissues. Notably, SlCHS1 was predominantly expressed in the integument followed by the head. Low expression of SlCHS1 was detected in the Malpighian tubules. The expression level of SlCHS1 in the integument was 380.2 times that in the Malpighian tubules, and its expression in the head was 191.7 times that of the Malpighian tubules. However, SlCHS2 had the highest expression level in the midgut, whereas it showed low expression in the integument, head and fat body. The expression level of SlCHS2 in the midgut was 4687.5 times that in the integument (Figure 3). For developmental stage expression analysis, the expression levels of SlCHS1 and SlCHS2 increased from the second-instar larvae to the fourth-instar larval stage and then decreased from the fourth-instar larvae to the sixth-instar larval stage. The SlCHS1 expression level showed a significant fluctuation at the pupal stage, while the SlCHS2 expression level had no significant change from the sixth-instar larval stage to the adult stage (Figure 3).

Figure 3.
Expression patterns of SlCHS1 and SlCHS2 in different tissues of sixth-instar larvae and at different developmental stages of the larvae of S. litura. Relative mRNA levels of SlCHS1 and SlCHS2 as examined using RT-qPCR. Data were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and are represented as the means ± standard errors of the means from three independent experiments. Relative expression levels were calculated using the 2−∆∆Ct method. Statistical analysis was performed using SPSS software. All data were analyzed using ANOVA and Tukey’s test. The significant differences are indicated by a different letter, for example, a, b, and c (p < 0.05).
3.3. Analysis of the Expression Levels of SlCHS1 and SlCHS2 after 20E Treatment
20E is the key factor that controls the metamorphosis of insects. To analyze whether 20E regulates the transcriptional expression of SlCHS1 and SlCHS2, 20E was injected into sixth-instar larvae. The results revealed that 20E can induce the expression levels of SlCHS1 and SlCHS2 (Figure 4). The expression level of SlCHS1 showed no significant change between 20E treatment group and the control at 1 h. However, the SlCHS1 expression level was significantly upregulated at 12 h after 20E treatment compared with the control group and then sharply decreased from 12 h to 36 h. The expression level of SlCHS2 had no significant change from 1 h to 12 h after 20E treatment. However, compared with the control group, the SlCHS2 expression level was significantly upregulated from 36 h to 48 h. These results indicated that the expression levels of SlCHS1 and SlCHS2 were affected by 20E.
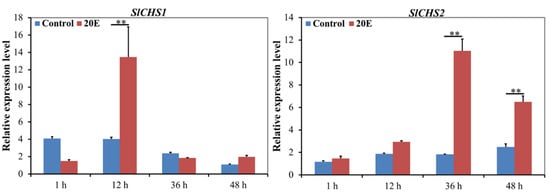
Figure 4.
Expression levels of SlCHS1 and SlCHS2 after 20E treatment in S. litura. Data were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and are represented as the means ± standard errors of the means from three independent experiments. Relative expression levels were calculated using the 2−∆∆Ct method. Statistical analysis was performed using SPSS software. All data were analyzed using ANOVA and Tukey’s test. Significant differences are indicated by ** (p < 0.01).
3.4. RNAi-Based Silencing of SlCHS1 and SlCHS2 and Phenotype Analysis
To investigate the effect of SlCHS1 and SlCHS2 on S. litura molting, RNAi was performed by injection of dsRNA. The silencing efficiency was scrutinized in different concentrations of dsRNA by RT-qPCR. The results showed that neither SlCHS1 nor SlCHS2 could be silenced effectively at 24 h after injection of 300 ng/μL dsRNA. However, the expression levels of SlCHS1 and SlCHS2 were significantly (p < 0.01) reduced after injection of 500 ng/μL dsRNA at 24 h compared with the dsGFP control treatment (Figure 5A,B). After injection of dsSlCHS1, the S. litura larvae could not develop into pupae normally, and the adults were unable to molt completely. However, the larvae treated with dsSlCHS2 were not affected and pupated normally (Figure 5C). These results suggest that SlCHS1 might play an important role during S. litura larval molting, while SlCHS2 has no significant effect on S. litura molting.
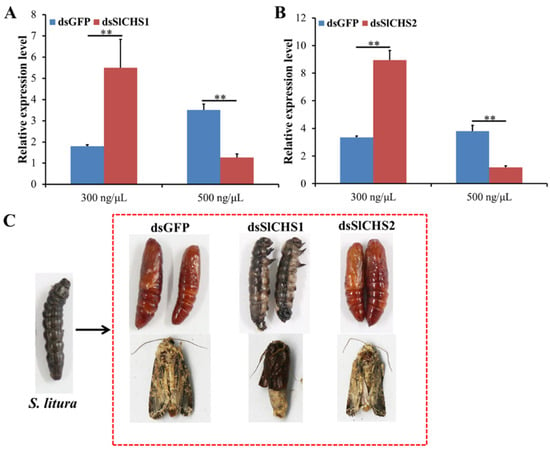
Figure 5.
Effect on S. litura after RNA interference of SlCHS1 and SlCHS2. (A) Relative expression levels of SlCHS1 when S. litura was treated with dsSlCHS1 and dsGFP at different concentrations. (B) Relative expression levels of SlCHS2 when S. litura was treated with dsSlCHS2 and double-stranded dsGFP at different concentrations. The mean expression level is represented for three biological replicates. Relative expression levels were calculated using the 2−∆∆Ct method. Statistical analysis was performed using SPSS software. All data were analyzed using ANOVA and Tukey’s test. Significant differences are indicated by ** (p < 0.01). (C) Representative phenotypes of S. litura at 24 h after dsGFP, dsSlCHS1 and dsSlCHS2 treatment.
4. Discussion
Spodoptera litura is one of the major agricultural pests worldwide and can seriously damage some important economic crops, including soybean, cotton, tobacco and cruciferous vegetables [29]. To date, the control of S. litura has been mainly dependent on chemical insecticides [30]. However, the long-term use of these insecticides not only pollutes the environment and affects human health, but also leads to the development of insect resistance against certain pesticides, including organophosphate and some biogenic insecticides [31,32]. Therefore, it is critical to find environmentally friendly insecticides for the control of S. litura. In recent years, some key genes from S. litura were identified as potential targets for the control of S. litura. Ji et al. revealed that silencing of S. litura nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome P450 reductases (SlCPRs) increased larval mortality by 34.6% (LC15 dose) and 53.5% (LC50 dose) by RNAi [33]. Wang et al. also found that RNAi-mediated silencing of S. litura cytochrome P450 monooxygenases 321B1 (SlCYP321B1) further increased mortality by 25.6% and 38.9% when fifth-instar larvae were exposed to chlorpyrifos and β-cypermethrin [26]. However, these target genes also exhibited some defects because of insect insecticide resistance. In contrast, chitin forms the insect exoskeleton, plays important roles in physiological systems, and provides physical, chemical and biological protection. In the present study, we identified two chitin synthase genes from the S. litura genome database. The structural domain analysis suggested that both SlCHS1 and SlCHS2 contain 16 transmembrane helices (Figures S1 and S2). Depending on the number of predicted transmembrane helices, the N-terminus faces either the extracellular space or the cytoplasm. However, the C-terminal region is predicted to face the extracellular space and may be involved in protein– protein interactions or oligomerization [34]. Additionally, we also found that both SlCHS1 and SlCHS2 contain two typical chitin synthase signature motifs, including EDR and QRRRW, which are essential for the catalytic mechanism [35]. Moreover, SlCHS1 and SlCHS2 contain six and five glycosylation sites, respectively, suggesting that these proteins are glycosylated. Protein glycosylation is the covalent attachment of an oligosaccharide chain to a protein backbone and is considered to be a very common protein modification [36]. These results indicated that S. litura CHS plays an important role in chitin synthesis.
To further investigate the functions of SlCHS1 and SlCHS2, we analyzed their expression patterns in different tissues and at different developmental stages, as well as 20E treatment. The results showed that SlCHS1 had high expression in the integument and head, while SlCHS2 had high expression in the midgut (Figure 3). In insects, chitin functions as a scaffold material, supporting the cuticle of the epidermis [37]. In S. exigua, northern blot analysis also revealed that SeCHSA is transcribed preferentially in the cuticle and tracheae [38]. Qu et al. revealed that the Ostrinia furnacalis chitin synthase A (OfCHSA) transcript was preferentially expressed in the epidermis [39]. In addition, the localization of SlCHS1 was confirmed in the integument and midgut. Therefore, we speculated that SlCHS1 may play a critical role in the process of cuticle formation. The insect midgut epithelium is commonly lined by an invertebrate-unique structure, the peritrophic matrix (PM), which facilitates the digestion of food and the protection of the gut epithelium [40]. We considered that SlCHS2 may be involved in chitin formation in PM. In T. castaneum, the CHSB gene is the major or sole contributor to PM chitin synthesis [41]. At different developmental stages, both SlCHS1 and SlCHS2 showed high expression in the fourth-instar larval stage (Figure 3). In Ectropis oblique, CHSA expression was the strongest in third- and fourth-instar larvae [42]. However, the SlCHS1 expression level showed a significant fluctuation at the pupal stage. During the growth and development of O. furnacalis, OfCHSA was mainly expressed during larval–larval molting and larval–pupal transformation [39]. Therefore, we speculated that OfCHSA also plays an important role in larval–pupal transformation. 20E plays critical roles in insect development and binds to the nuclear receptor heterodimer ecdysone receptor (EcR)/ultraspiracle (USP) [43]. In insects, the process of chitin biosynthesis is strictly coordinated with the cycle of molts. Thus, chitin biosynthesis may be regulated by 20E [15,44]. In this study, we found that both SlCHS1 and SlCHS2 expression levels can be induced after 20E treatment (Figure 4). In a previous report, analysis of mRNA levels showed that BmCHSA-2b was responsive to 20E [45]. In Bactrocera dorsalis, 20E induced the expression of BdCHS1 and its variants [46]. Based on these results, we speculated that 20E can bind EcR and USP to form the ligand–receptor complex 20E-EcR/USP, and the receptor complex directly activates SlCHS1 and SlCHS2 expression thereafter.
RNAi has already proven its usefulness in functional genomics research in insects, but it also has considerable potential for the control of pest insects [47]. For the effective silencing of target genes, it is critical to transmit dsRNA into the body of insects to disrupt the expression of target genes [48]. Mechanistic studies have shown that double-stranded ribonucleases (dsRNases), endosomal entrapment, deficient function of the core machinery, and inadequate immune stimulation, contribute to limited RNAi efficiency [49]. Compared with the Coleoptera, Lepidoptera insects appear to have a low RNAi efficiency because dsRNAs were degraded more easily by RNase [50]. To ensure the RNAi efficiency, prepupa S. litura larvae were used for RNAi analysis. The results showed that the transformation of larval–pupae and pupae–adults was disrupted after silencing of SlCHS1, while S. litura larvae showed no obvious change after silencing of SlCHS2 (Figure 5). In T. castaneum, TcCHS1-specific RNAi disrupted all three types of molt (larval–larval, larval–pupal and pupal–adult), while TcCHS2-specific RNAi had no effect on metamorphosis [41]. In S. exigua, the cuticle was disordered, and the epithelial walls of larval tracheae did not expand uniformly after injection of dsSeCHSA [21]. These results demonstrated that SlCHS1 was mainly involved in the formation of epidermal structure and that SlCHS2 was associated with PM formation.
5. Conclusions
Two cDNA sequences of SlCHS1 and SlCHS2 were identified based on genome database of S. litura. RT-qPCR analysis suggested that SlCHS1 and SlCHS2 were highly expressed in the integument and midgut, respectively. Developmental stage expression analysis showed that both SlCHS1 and SlCHS2 were predominantly expressed in the fourth-instar larval stage. In addition, the expression of SlCHS1 and SlCHS2 could be induced by 20E. Furthermore, silencing of SlCHS1 significantly inhibited the pupation and molting of S. litura larvae by RNAi. These results suggest that SlCHS1 and SlCHS2 were involved in the formation of chitin in integument and peritrophic matrix (PM), respectively. In our following study, we will design some inhibitors directly targeted at S. litura chitin synthases and combined with RNAi to control S. litura.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/4/253/s1, Table S1: Sequences and relevant information used for phylogenetic analysis of the chitin synthase genes. Figure S1: Nucleotide and deduced amino acid sequences of SlCHS1 cDNA from S. litura. Figure S2: Nucleotide and deduced amino acid sequences of SlCHS2 cDNA from S. litura.
Author Contributions
Conceptualization, H.-Z.Y. and Z.-J.L.; methodology, N.-Y.L. and Y.-X.X.; software, H.-Z.Y.; investigation, Q.Z. and Y.W.; writing—original draft preparation, H.-Z.Y.; writing—review and editing, H.-Z.Y. and Z.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (31960116), Ministry of Science and Technology of China (2018YFD0201504), the Educational Commission of Jiangxi Province of China (GJJ180747), and Science and Technology Department of Jiangxi Province (20171BCB23074), and the earmarked fund for Jiangxi Agriculture Research System (JXARS-07).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arakane, Y.; Hogenkamp, D.G.; Zhu, Y.C.; Kramer, K.J.; Specht, C.A.; Beeman, R.W.; Kanost, M.R.; Muthukrishnan, S. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem. Mol. Biol. 2004, 34, 291–304. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. Macromolecules 2017, 97, 331–338. [Google Scholar] [CrossRef]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Stannard, J. Antioxidant defense of the midgut epithelium by the peritrophic envelope in caterpillars. J. Insect Physiol. 2004, 50, 783–790. [Google Scholar] [CrossRef]
- Arakane, Y.; Specht, C.A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Chitin synthase are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 959–962. [Google Scholar] [CrossRef]
- Tang, B.; Chen, X.F.; Liu, Y.; Tian, H.G.; Liu, J.; Hu, J.; Xu, W.H.; Zhang, W.Q. Characterization and expression patterns of a membrane-bound trehalase from Spodoptera exigua. BMC Mol. Biol. 2008, 9, 51. [Google Scholar] [CrossRef]
- Shang, F.; Xiong, Y.; Xia, W.K.; Wei, D.D.; Wei, D.; Wang, J.J. Identification, characterization and functional analysis of a chitin synthase gene in the brown citrus aphid, Toxoptera citricida (Hemiptera, Aphididae). Insect Mol. Biol. 2016, 25, 422–430. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Tellam, R.L.; Vuocolo, T.; Johnson, S.E.; Jarmey, J.; Pearson, R.D. Insect chitin synthase. Eur. J. Biochem. 2001, 267, 6025–6043. [Google Scholar] [CrossRef]
- Gagou, M.E.; Kapsetaki, M.; Turberg, A.; Kafetzopoulos, D. Stage-specific expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila metamorphosis. Insect Biochem. Mol. Biol. 2002, 32, 141–146. [Google Scholar] [CrossRef]
- Hogenkamp, D.G.; Arakane, Y.; Zimoch, L.; Merzendorfer, H.; Kramer, K.J.; Beeman, R.W.; Kanost, M.R.; Specht, C.A.; Muthukrishnan, S. Characterization of a gut-specific transcript and differential tissue expression of alternatively spliced mRNAs during development. Insect Biochem. Mol. Biol. 2005, 35, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Liu, X.J.; Zhang, J.Q.; Li, D.Q.; Sun, Y.; Guo, Y.P.; Ma, E.B.; Zhu, K.Y. Silencing of two alternative splicing-derived mRNA variants of chitin synthase 1 gene by RNAi is lethal to the oriental migratory locust, Locusta migratoria manilensis (Meyen). Insect Biochem. Mol. Biol. 2010, 40, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, R.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Terra, W.R.; Ferreira, C. Sequences of cDNAs and expression of genes encoding chitin synthase and chitinase in the midgut of Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2005, 35, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Huang, Y.L.; Yu, H.Z.; Li, N.Y.; Xie, Y.X.; Zhang, Q.; Zeng, X.D.; Hu, H.; Huang, A.J.; Yi, L.; et al. Silencing of chitin synthase gene is lethal to the Asian citrus psyllid, Diaphorina citri. Int. J. Mol. Sci. 2019, 20, 3734. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Ashfaq, M.; Sonoda, S.; Tsumuki, H. Developmental and tissue-specific expression of CHS1 from Plutella xylostella and its response to chlorfluazuron. Pestic. Biochem. Phys. 2007, 89, 20–30. [Google Scholar] [CrossRef]
- Chen, X.F.; Tian, H.G.; Zou, L.Z.; Tang, B.; Hu, J.; Zhang, W.Q. Distribution of Spodoptera exigua larval development by silencing chitin synthase A with RNA silence. Bull. Entomol. Res. 2008, 98, 613–619. [Google Scholar] [CrossRef]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, J.H.; Liu, Y.; Belles, X.; Lee, H.J. Oral delivery of dsRNA to German cockroach protects dsRNA from degradation and induces RNAi response. Pest. Manag. Sci. 2017, 73, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 19, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yin, Y.P.; Li, Y.; Mahmud, M.S.; Wang, Z.K. Identification and analysis of genes differentially expressed in the Spodoptera litura fat body in response to the biocontrol fungus, Nomuraea rileyi. Comp. Biochem. Phys. B 2012, 163, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Phys. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Shad, S.A.; Sayyed, A.H.; Fazal, S.; Saleem, M.A.; Zaka, S.M.; Ali, M. Field evolved resistance to carbamates organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). J. Pest. Sci. 2012, 85, 153–162. [Google Scholar] [CrossRef]
- Wang, R.L.; Zhu-Salzman, K.; Baerson, S.R.; Xin, X.W.; Li, J.; Su, Y.J.; Zeng, R.S. Identification of a novel cytochrome P450 CYP321B1 gene from tobacco cutworm (Spodoptera litura) and RNA interference to evaluate its role in commonly used insecticides. Insect Sci. 2017, 24, 235–247. [Google Scholar] [CrossRef]
- Guan, S.P.; Mok, Y.K.; Koo, K.N.; Chu, K.L.; Wong, W.S. Chitinases: Biomarkers for human diseases. Protein Pept. Lett. 2009, 16, 490–498. [Google Scholar] [CrossRef]
- Chen, W.J.; Huang, L.X.; Hu, D.; Liu, L.Y.; Gu, J.; Huang, J.H.; Feng, Q.L. Cloning, expression and chitin-binding activity of two peritrophin-like protein genes in the common cutworm, Spodoptera litura. Insect Sci. 2014, 21, 449–458. [Google Scholar] [CrossRef]
- Ahmad, M.; Mehmood, R. Monitoring of resistance to new chemistry insecticides in Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. J. Econ. Entomol. 2015, 108, 1279–1288. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.G.; Yao, X.G.; Gong, C.W.; Shen, L.T. Effects of bistrifluron resistance on the biological traits of Spodoptera litura (Fab.) (Noctuidae: Lepidoptera). Ecotoxicology 2019, 28, 323–332. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Brevault, T.; Carriere, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, B.; Shoaib, F.; Muhammad, Z.A.; Adeel, R. Resistance and detoxification enzyme activities to bifenthrin in Oxycarenus hyalinipennis (Hemiptera: Lygaeidae). Crop. Prot. 2018, 111, 17–22. [Google Scholar]
- Ji, H.Y.; Staehelin, C.; Jiang, Y.P.; Liu, S.W.; Ma, Z.H.; Su, Y.J.; Zhang, J.E.; Wang, R.L. Tobacco cutworm (Spodoptera litura) larvae silenced in the NADPH-cytochrome P450 reductase gene show increased susceptibility to phoxim. Int. J. Mol. Sci. 2019, 20, 3839. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, P.; Stetefeld, J.; Strelkov, S.V. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001, 11, 82–88. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Specht, C.A.; Dittmer, N.T.; Muthukrishnan, S.; Kanost, M.R.; Kramer, K.J. Sequence of a cDNA and expression of the gene encoding a putative epidermal chitin synthase of Manduca sexta. Insect Biochem. Mol. Biol. 2002, 32, 1497–1506. [Google Scholar] [CrossRef]
- Vandenborre, G.; Smagghe, G.; Ghesquiere, B.; Menschaert, G.; Rao, R.N.; Gevaert, K.; Van Damme, E.J. Diversity in protein glycosylation among insect species. PLoS ONE 2011, 6, e16682. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function, and regulation of chitin synthase and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Chen, X.F.; Yang, X.; Kumar, N.S.; Tang, B.; Sun, X.J.; Qiu, X.M.; Hu, J.; Zhang, W.Q. The class A chitin synthase gene of Spodoptera exigua: Molecular cloning and expression patterns. Insect Biochem. Mol. Biol. 2007, 37, 409–417. [Google Scholar] [CrossRef]
- Qu, M.B.; Yang, Q. A novel alternative splicing site of class A chitin synthase from the insect Ostrinia furnacalis-Gene organization, expression pattern and physiological significance. Insect Biochem. Mol. Biol. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Kumar, N.S.; Tang, B.; Chen, X.F.; Tian, H.G.; Zhang, W.Q. Molecular cloning, expression pattern and comparative analysis of chitin synthase gene B in Spodoptera exigua. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 447–453. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef]
- Liang, Y.R.; Lin, C.; Wang, R.R.; Ye, J.H.; Lu, J.L. Cloning and expression pattern of chitin synthase (CHS) gene in epidermis of Ectropis oblique Prout. Afr. J. Biotechnol. 2010, 9, 5279–5308. [Google Scholar]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The Juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, Z.K.; Liang, Y.K.; Pang, R.; Zhang, W.Q. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2013, 43, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.F.; Zhang, J.; Lyu, H.; Liu, J.; Ding, Y.; Feng, Q.L.; Song, Q.S.; Zheng, S.C. BmCHSA-2b, a Lepidoptera specific alternative splicing variant of epidermal chitin synthase, is required for pupal wing development in Bombyx mori. Insect Biochem. Mol. Biol. 2017, 87, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Xu, K.K.; Cong, L.; Wang, J.J. Identification, mRNA expression, and functional analysis of chitin synthase 1 gene and its two alternative splicing variants in oriental fruit fly, Bactrocera dorsalis. Int. J. Biol. Sci. 2013, 9, 331–342. [Google Scholar] [CrossRef]
- Copper, A.M.W.; Silver, K.; Zhang, J.Z.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest. Manag. Sci. 2018, 75, 1. [Google Scholar] [CrossRef]
- Tao, X.Y.; Xue, X.Y.; Huang, Y.P.; Chen, X.Y.; Mao, Y.B. Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Eol. 2012, 21, 4371–4385. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.Z.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing of RNA interference in insects. Pest. Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Guan, R.B.; Li, H.C.; Miao, X.X. Prediction of effective RNA interference targets and pathway-related genes in lepidopteran insects by RNA sequencing analysis. Insect Sci. 2018, 25, 356–367. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).