Organic Mulch Increases Insect Herbivory by the Flea Beetle Species, Disonycha glabrata, on Amaranthus spp.
Abstract
1. Introduction
2. Materials and Methods
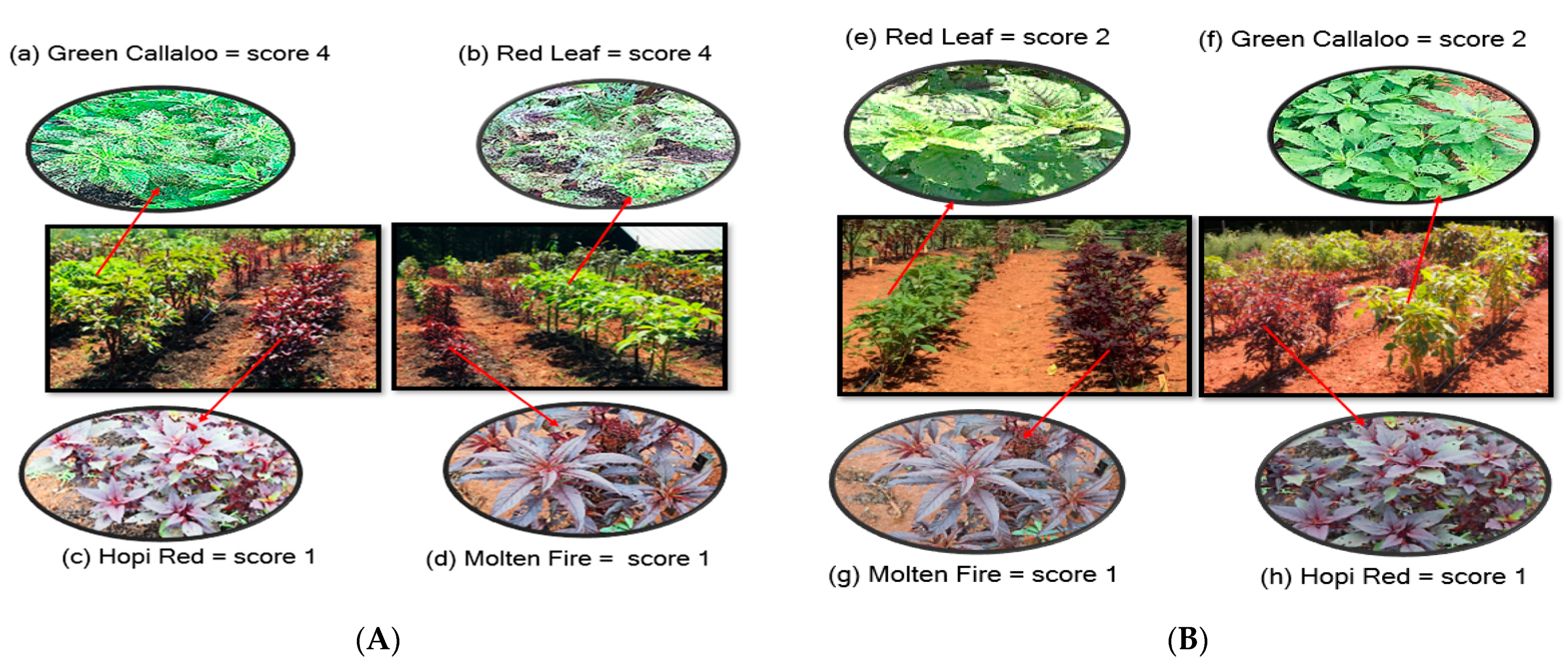
2.1. Field Screening for Resistance
2.2. Insect Sampling
2.3. Leaf Damage Measurement
2.4. Determination of Soil Moisture Content in Plots with and without Leaf Compost Mulch
2.5. Determination of Total Leaf Protein and Polyphenol Concentration among the Amaranthus spp.
2.5.1. Sample Preparation
2.5.2. Determination of Total Leaf Protein Concentration in the Amaranthus Varieties
2.5.3. Determination of Total Leaf Polyphenol Concentration in Amaranthus Varieties
2.6. Amaranth Leaf and Grain Yield
2.7. Data Analysis
3. Results
3.1. Diversity and Abundance of Insects Associated with Amaranth Varieties
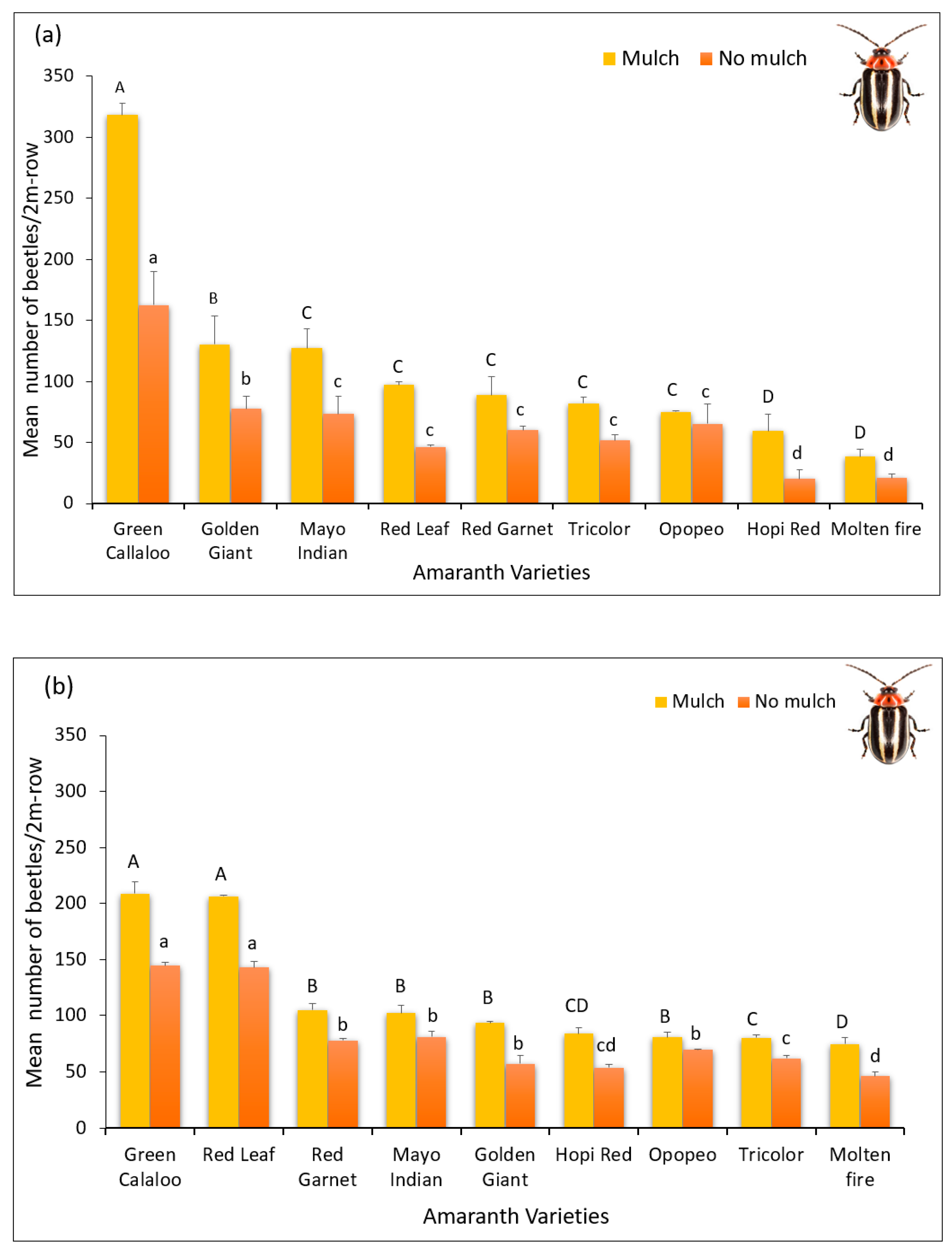
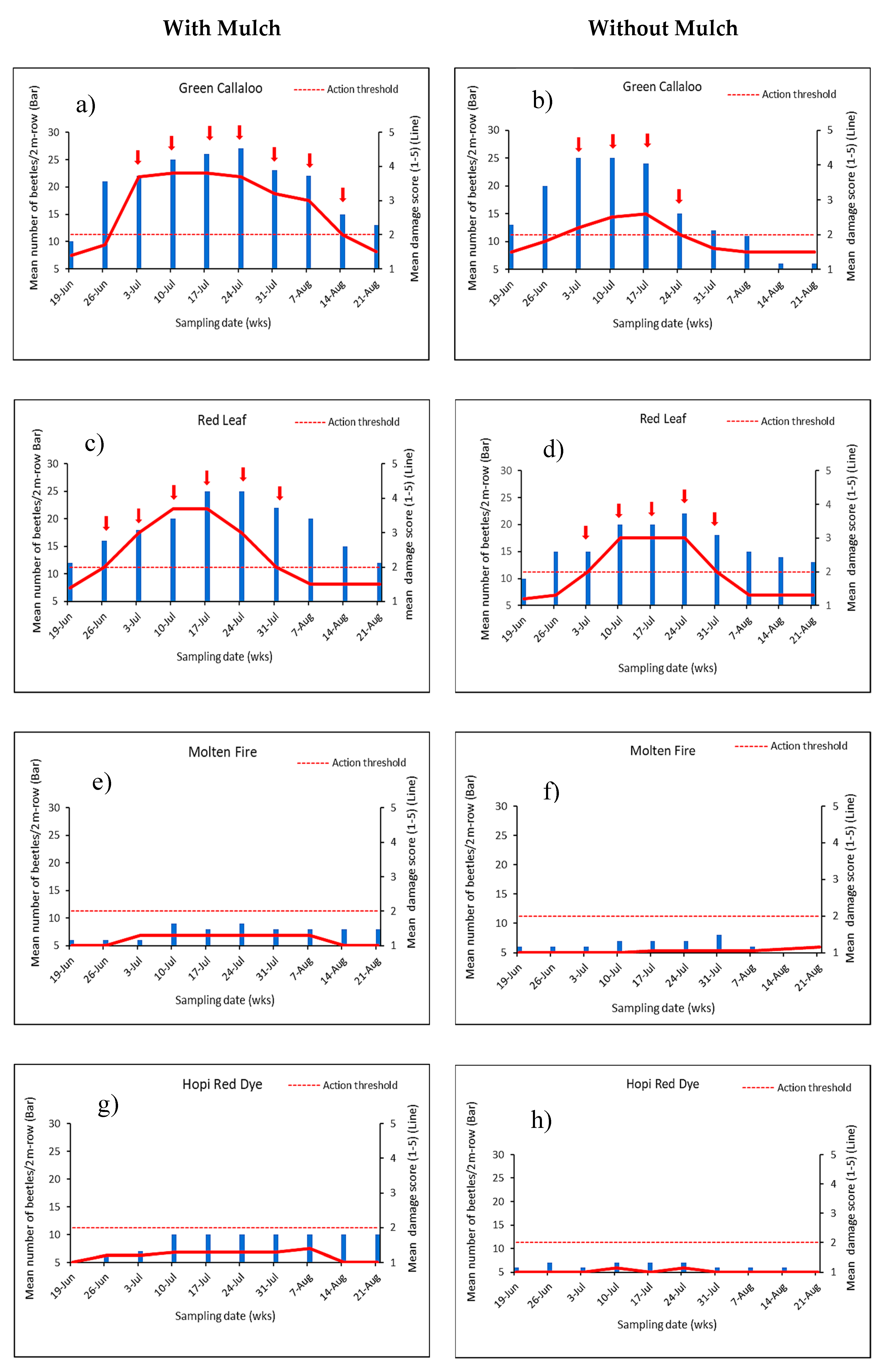
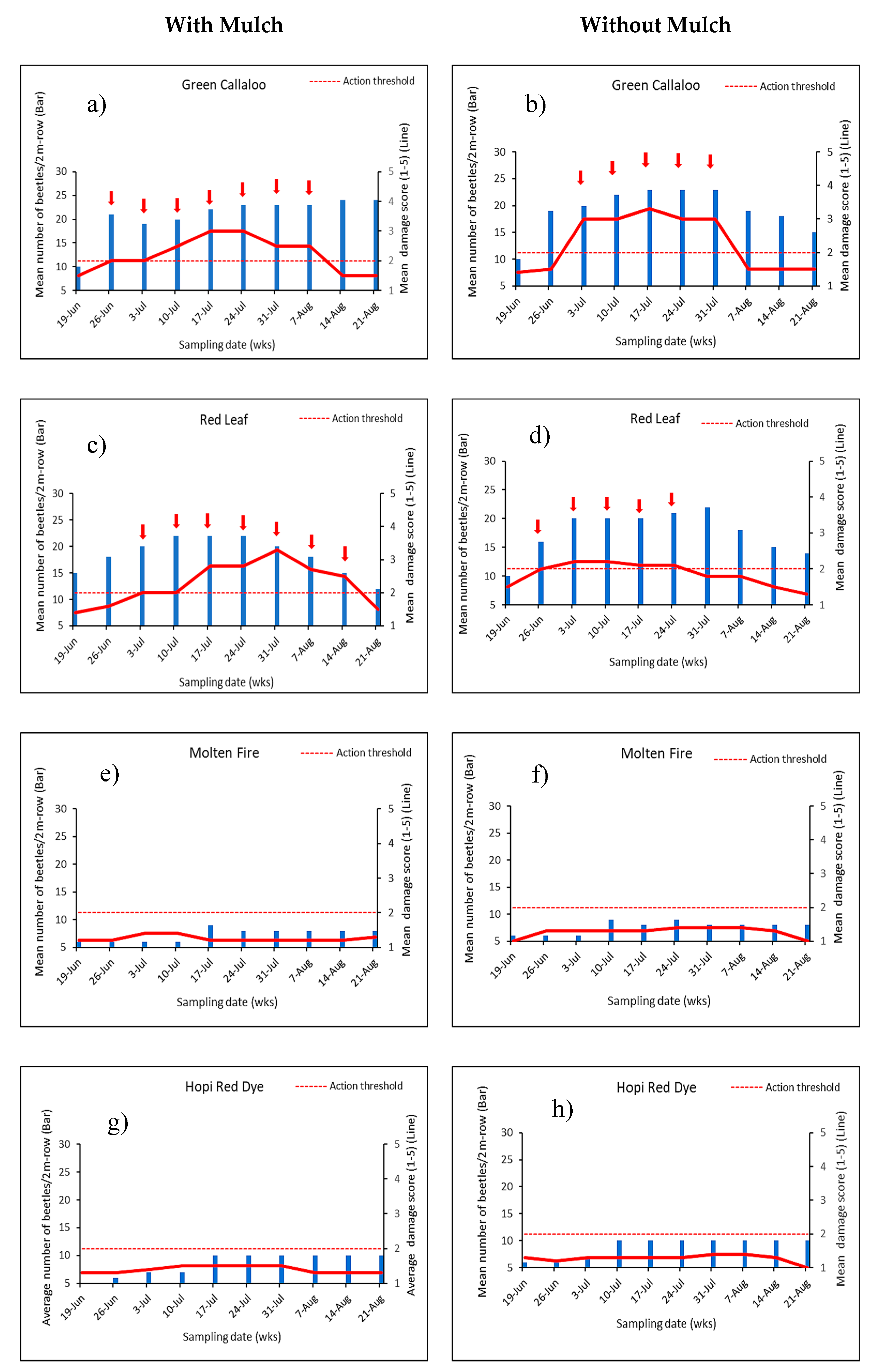
3.2. Feeding Damage by Disonycha Glabrata
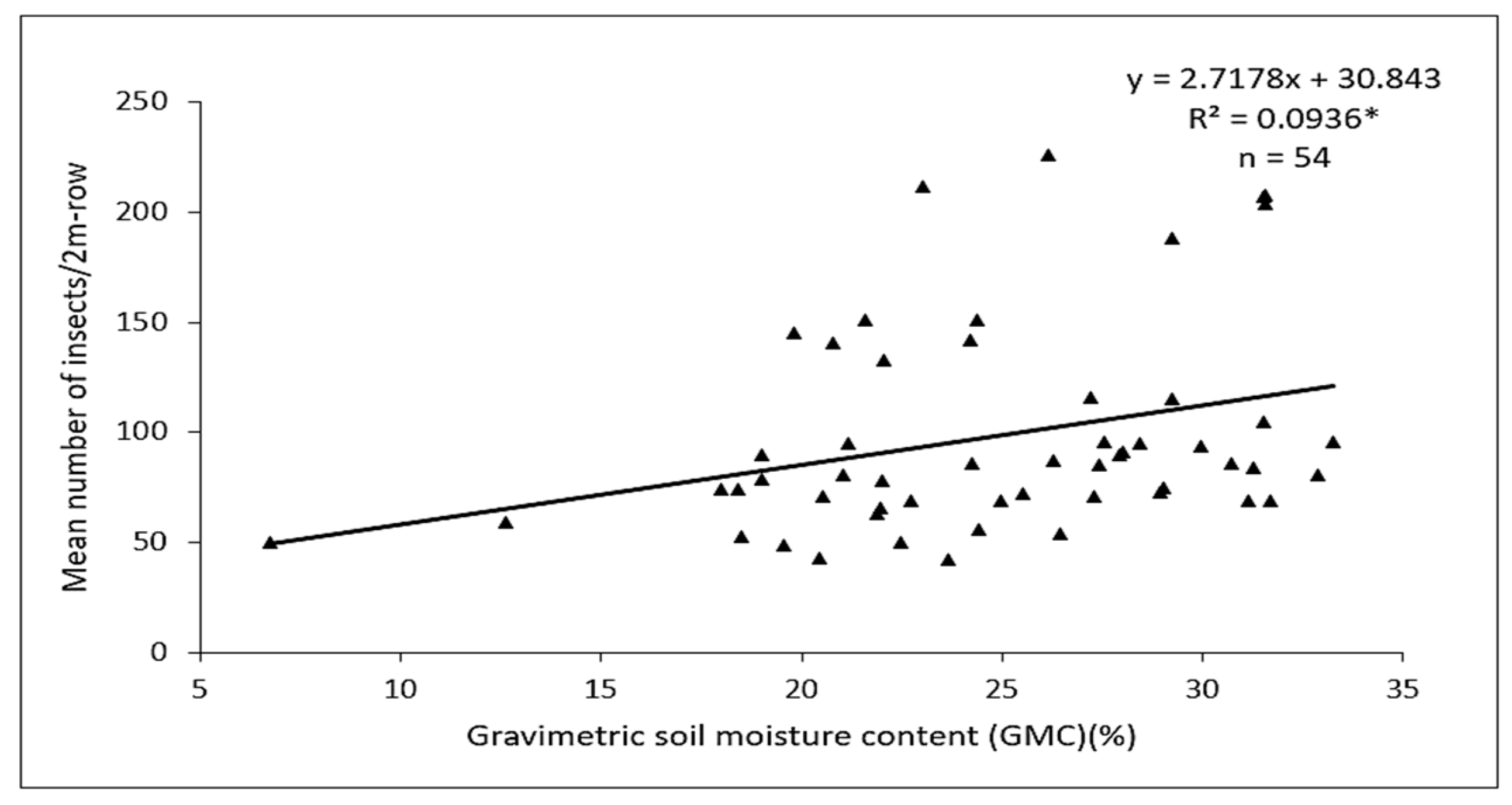
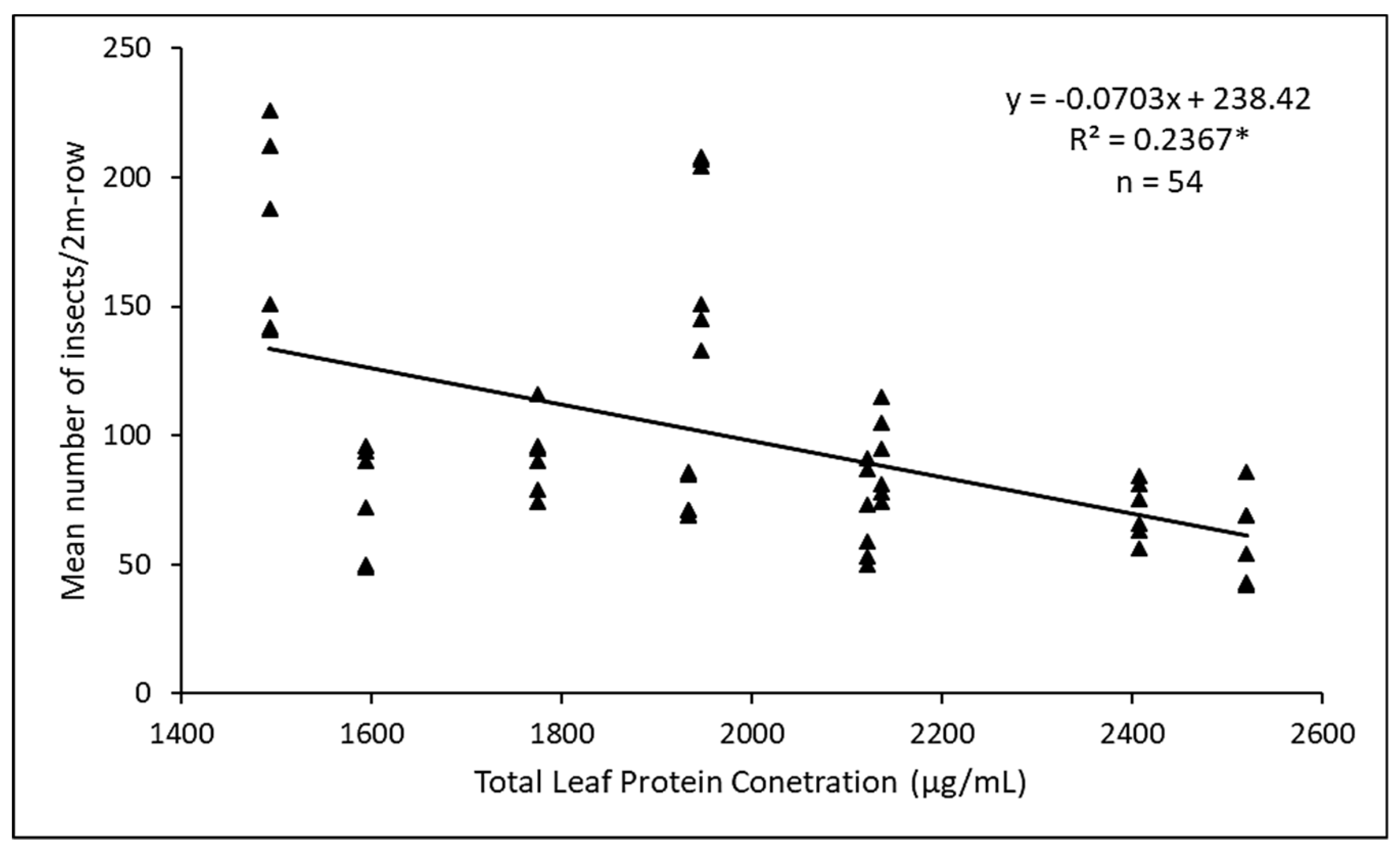
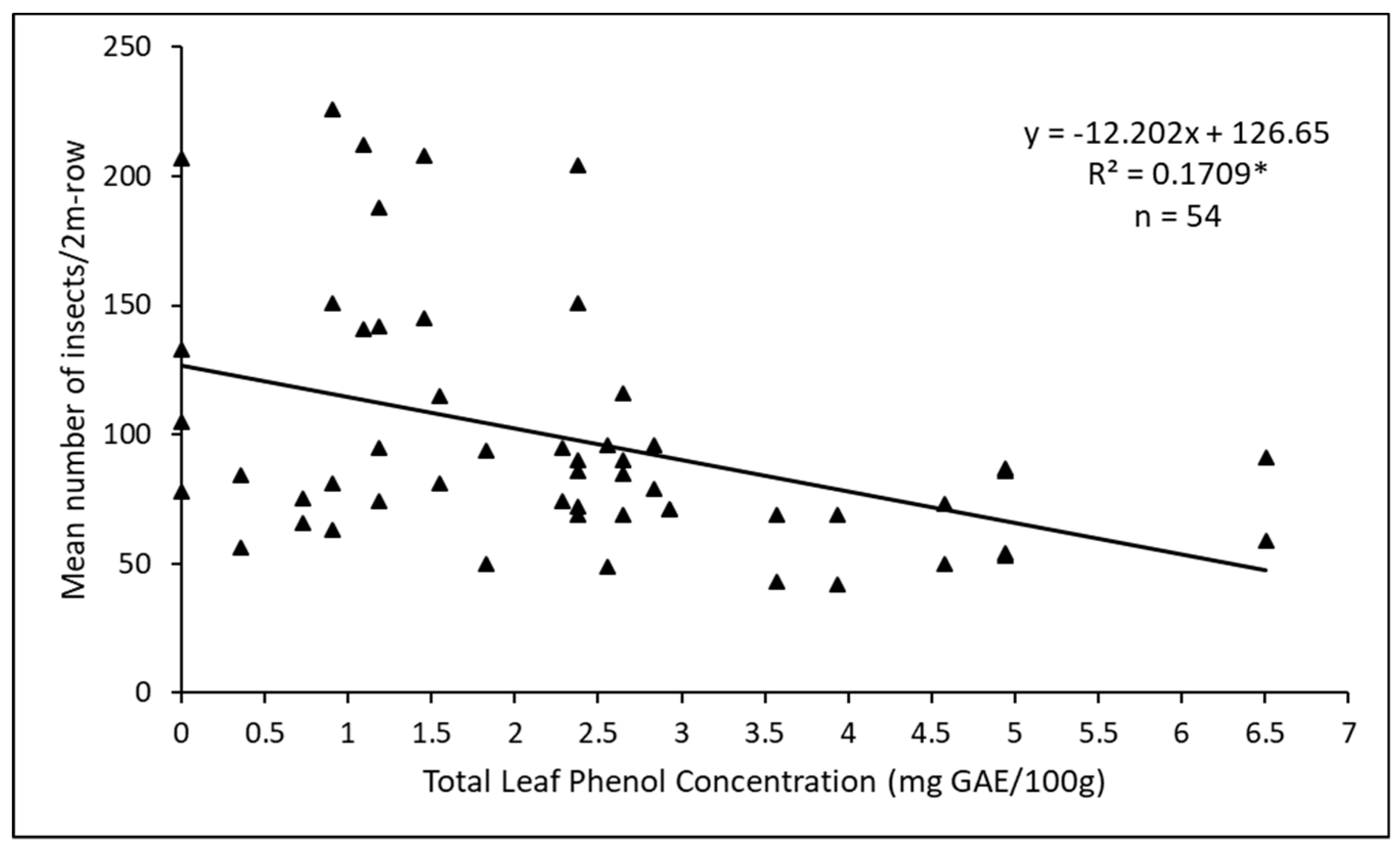
3.3. Factors Influencing Herbivory between Main PlotTreatments
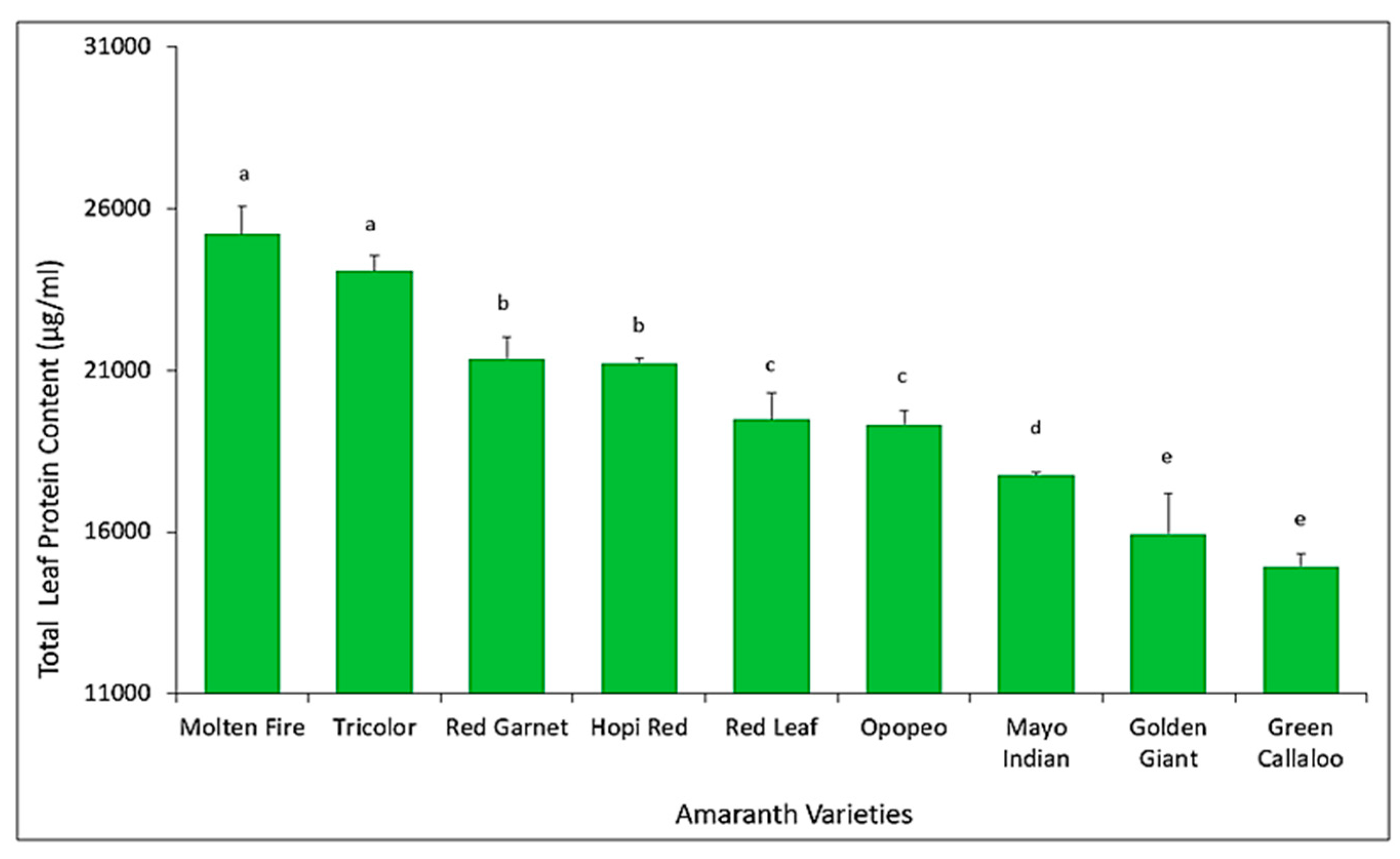
3.4. Quantification of Total Leaf Protein Content among Amaranth Varieties
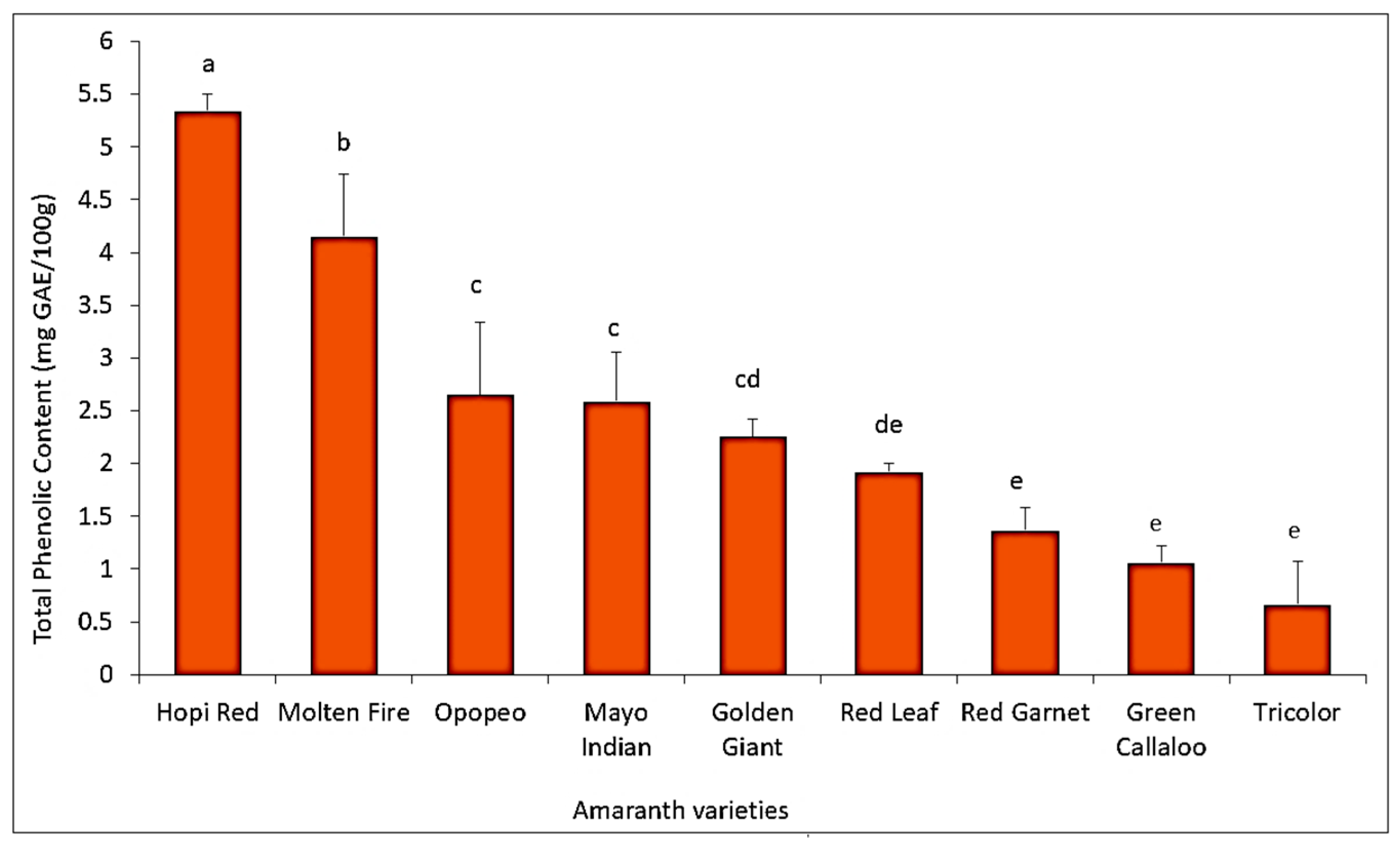
3.5. Quantification of Total Leaf Polyphenol Content among Amaranth Varieties
3.6. Fresh Leaf Yield of Amaranth Varieties
3.7. Dry Grain Yield of Amaranthus spp.
3.8. Overall Ranking of Amaranth Varieties Based on Agronomic Performance
4. Discussion
4.1. Insect Diversity and Population on Amaranthus spp.
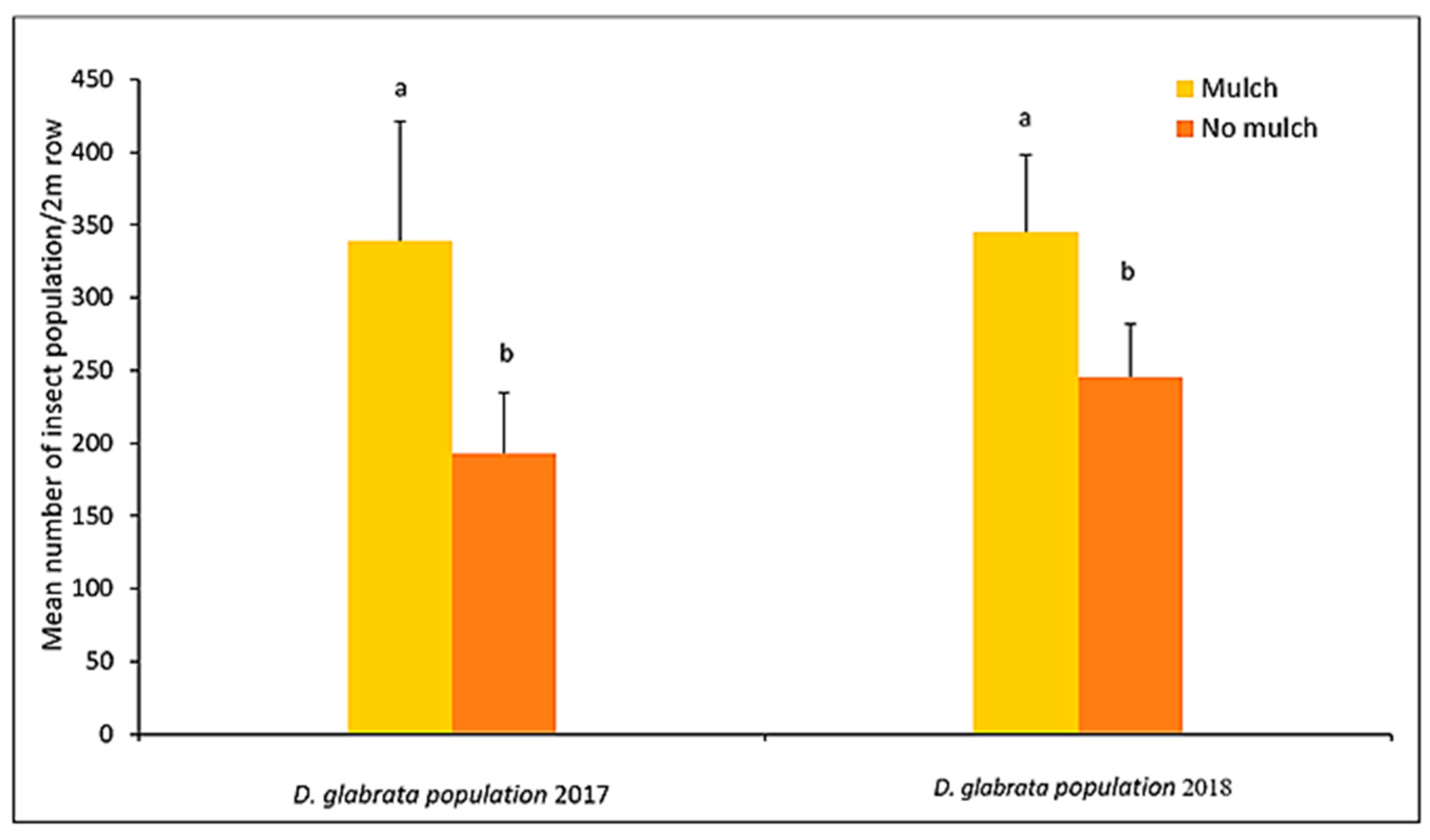
4.2. The Role of Leaf Mulch on D. glabrata Population
4.3. Herbivory on Cultivated Amaranthus spp.
4.4. Effects of Organic Mulch on Fresh Leaf and Dry Grain Yield of Amaranthus spp.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNDESA. The Revision, Key Findings and Advance Tables. 2015. Available online: https://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html (accessed on 20 August 2018).
- Oberholtzer, L.; Dimitri, C.; Pressman, A. Urban Agriculture in the United States: Baseline Findings of a Nationwide Survey. Available online: https://attra.ncat.org/product/urban-agriculture-in-the-united-states-baseline-findings-of-a-nationwide-survey/ (accessed on 19 July 2019).
- USDA-ERS. Organic Production. Data Source. USDA Economic Research Service. 2013. Available online: http://www.ers.usda.gov/data-products/organic-production.aspx#.VDbWc97P6S0 (accessed on 12 October 2018).
- La Rosa, D.; Barbarossa, L.; Privitera, R.; Martinico, F. Agriculture and the city: A method for sustainable planning of new forms of agriculture in urban contexts. Land Use Policy 2014, 41, 290–303. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Cooley, D.; Futrell, S.; Garling, L.; Gershuny, G.; Moyer, J.; Rajotte, E.G.; Seaman, A.J.; Young, S.L. Organic Agriculture and Integrated Pest Management, a Synergistic Partnership to Improve Sustainable Agriculture and Food Systems. 2015. Available online: https://organicipmwg.files.wordpress.com/2015/07/white-paper.pdf (accessed on 15 July 2019).
- Gill, K.H.; McSorley, R.; Goyal, G.; Webb, E.S. Mulch as a potential management strategy for lesser cornstalk borer, Elasmopalpus lignosellus (Insecta: Lepidoptera: Pyralidae), in bush bean (Phaseolus vulgaris). Fla. Entomol. 2010, 93, 183–190. [Google Scholar] [CrossRef]
- Gill, H.K.; Mcsorley, R.; Branham, M. Effect of organic mulches on soil surface insects and other arthropods. Fla. Entomol. 2011, 94, 226–232. [Google Scholar] [CrossRef]
- MacHardy, W.E. Current Status of IPM in apple orchards. J Crop Prot. 2000, 19, 801–806. [Google Scholar] [CrossRef]
- Nottingham, L.B.; Dively, G.P.; Schultz, P.B.; Herbert, D.A.; Kuhar, T.P. Natural history, ecology, and management of the mexican bean beetle (Coleoptera: Coccinellidae) in the United States. J. Integr. Pest Manag. 2016, 7, 2. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Rice, M.E. Entomology and Pest Management, 6th ed.; Prentice Hall: New York, NJ, USA, 2009; pp. 255–284. ISBN 978-1-4786-2285-7. [Google Scholar]
- Coolong, T. Mulches for weed management. In Weed Control; Price, A., Ed.; Intech Open: London, UK, 2012; p. 276. ISBN 978-953-51-0159-8. [Google Scholar]
- Piotr, B.; Piotr, S. Organic and non-organic mulches—Impact on environmental conditions, yield, and quality of Cucurbitaceae. Folia Hortic. 2019, 31, 129–145. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Effects of ground cover (straw and compost) on the abundance of natural enemies and soil macro invertebrates in vineyards. Agric. For. Entomol. 2007, 9, 173–179. [Google Scholar] [CrossRef]
- Addison, P.; Baauw, A.H.; Groenewald, G.A. An investigation of the effects of mulch layers on soil-dwelling arthropod assemblages in vineyards. S. Afr. J. Enol. Vitic. 2013, 34, 266–271. [Google Scholar] [CrossRef]
- Guenat, S. Assessing the Effects of Agroforestry Practices on Biological Control Potential in Kale (Brassica oleracea acephala) Plantations in Western Kenya; Publication of the Swedish Academy of Agricultural Sciences: Uppsala, Sweden, 2014; Available online: https://stud.epsilon.slu.se/7256/1/guenat_s_140909.pdf (accessed on 15 January 2020).
- Hellqvist, S. Mulching with grass clippings in cauliflower: Effects on yield and brassica root flies (Delia spp.). Int. J. Pest Manag. 1996, 42, 36–46. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Thewes, U.; Thies, C.; Tschamthe, T. Aphid suppression by natural enemies in mulched cereals. Entomol. Exp. Appl. 2004, 113, 87–93. [Google Scholar] [CrossRef]
- Bredenhand, E.; Van Hoorn, A.; May, F.; Ferriera, T.; Johnson, S. Evaluation of techniques for monitoring banded fruit weevil, Phlyctinus callosus (Schoenherr) (Coleoptera, Curculionidae) infestation in blueberry orchards. Afr. Entomol. 2010, 18, 205–209. [Google Scholar] [CrossRef]
- Hanada, T. The Effect of Mulching and Row COVERS on Vegetable Production. Bulletin of Chugoku Agr. Exp. Stn, Japan. Available online: http://www.fftc.agnet.org/htmlarea_file/library/20110801145616/eb332.pdf (accessed on 15 January 2020).
- USDA-NASS. 2017 Census of Agriculture: North Carolina State and County Data. Volume 1, Geographical Area Series, Part 33. 2019. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Full_Report/Volume_1,_Chapter_1_State_Level/North_Carolina/ncv1.pdf (accessed on 15 August 2019).
- Brenner, D.; Baltensperger, D.; Kulakow, P.; Lehmann, J.; Myers, R.; Slabbert, M.; Sleugh, B. Genetic resources and breeding of amaranthus. Plant Breed. Rev. 2000, 19, 227–285. [Google Scholar] [CrossRef]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.A.J.; Deleu, J.L.; Delcour, J.A. Proteins of Amaranth (Amaranthus spp.), Buckwheat (Fagopyrum spp.), and Quinoa (Chenopodium spp.): A Food Science and Technology Perspective. Compr. Rev. Food Sci. Food Saf. 2017, 16, 39–58. [Google Scholar] [CrossRef]
- Ezeh, A.E.; Ogbedegbe, A.B.O.; Ogbedegbe, S.A. Insect pest occurrence on cultivated Amaranthus Spp. in Benin City, Edo State, Nigeria. J. Appl. Sci. Environ. Manag. 2015, 19, 335–339. [Google Scholar] [CrossRef]
- Niveyro, S.L.; Mortensen, A.G.; Fomsgaard, I.S.; Salvo, A. Differences among five amaranth varieties (Amaranthus spp.) regarding secondary metabolites and foliar herbivory by chewing insects in the field. Arthropod Plant Interact. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Blake, D.H. Revision of the beetles of the genus Disonycha occurring in America north of Mexico. Proc. US Natl. Mus. 1933, 82, 1–66. [Google Scholar] [CrossRef]
- Blake, D.H. Revision of the vittate species of the chrysomelid beetle genus Disonycha from the Americas south of the United States. Proc. US Natl. Mus. 1955, 104, 1–86. [Google Scholar] [CrossRef][Green Version]
- Tisler, A.M. Feeding in the pigweed flea beetle, Disonycha glabrata Fab. (Coleoptera: Chrysomelidae), on Amaranthus retroflexus. Va. J. Sci. 1990, 41, 243–245. [Google Scholar]
- Hemenway, R.; Whitcomb, W.H. The life history of Disonycha glabrata (Coleoptera: Chrysomelidae). J. Kansas Entomol. Soc. 1968, 41, 174–178. [Google Scholar]
- Gill, H.K.; Goyal, G. Organic mulches: An innovative pest management strategy. Popular Kheti 2014, 2, 118–123. Available online: http://popularkheti.info/documents/2014-1/PK-2-1-22-118-123.pdf (accessed on 12 June 2019).
- Othim, S.T.O.; Kahuthia-Gathu, R.; Akutse, K.S.; Foba, C.N.; Fiaboe, K.K.M. Seasonal occurrence of amaranth Lepidopteran defoliators and effect of attractants and amaranth lines in their management. J. Appl. Entomol. 2018, 142, 637–645. [Google Scholar] [CrossRef]
- Othim, S.T.O.; Ramasamy, S.; Kahuthia-Gathu, R.; Dubois, T.; Ekesi, S.; Fiaboe, K.K.M. Expression of resistance in Amaranthus spp. (Caryophyllales: Amaranthaceae): Effects of selected accessions on the behavior and biology of the amaranth leaf-webber, Spoladea recurvalis (Lepidoptera: Crambidae). Insects 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Kemble, J.M.; Meadows, I.M.; Jennings, K.M.; Walgenbach, J.F. SouthEastern U.S 2017 Southeastern Vegetable Handbook, 18th ed.; Farm Journal Media: Philadelphia, PA, USA, 2017; p. 49. [Google Scholar]
- Zou, Y.; van Telgen, M.D.; Chen, J.; Xiao, H.; de Kraker, J.; Bianchi, F.J.; van der Werf, W. Modification and Application of a Leaf Blower-vac for Field Sampling of Arthropods. J. Vis Exp. 2016, 114. [Google Scholar] [CrossRef] [PubMed]
- Adjei-Fremah, S.; Jackai, L.E.; Worku, M. Analysis of phenolic content and antioxidant properties of selected cowpea varieties tested in bovine peripheral blood. Am. J. Anim. Vet. Sci. 2015, 10, 235–245. [Google Scholar] [CrossRef]
- Obanla, T.; Adjei-Fremah, S.; Gyawali, R.; Zimmerman, T.; Worku, M.; Ibrahim, S.A. Effects of Long-Term Exposure to Aspirin on Growth, Functionality and Protein Profile of Lactobacillus rhamnosus (LGG) (ATCC 53103). J. Food Res. 2016, 5, 46–54. [Google Scholar] [CrossRef][Green Version]
- Worku, M.; Abdalla, A.; Adjei-Fremah, S.; Ismail, H. The impact of diet on expression of genes involved in innate immunity in goat blood. J. Agric. Sci. 2016, 8, 1. [Google Scholar] [CrossRef][Green Version]
- Karlton-Senaye, B.; Adjei-Fremah, S.; Worku, M.; Williams, L. Synergistic Effect of Polysaccharide Gums and Antimicrobial Agents on Susceptibility and Protein Expression of Select Pathogenic Microorganisms in Milk. J. Food Res. 2018, 7, 35–53. [Google Scholar] [CrossRef][Green Version]
- Aderolu, I.A.; Omooloye, A.A.; Okelana, F.A. Occurrence, Abundance and Control of the major insect pests associated with amaranths in Ibadan, Nigeria. Entomol. Ornithol. Herpetol. 2013, 2, 112. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Software Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Smith, J.D.; Dinssa, F.F.; Anderson, R.F.; Su, F.; Srinivasan, R. Identification of major insect pests of Amaranthus spp. and germplasm screening for insect resistance in Tanzania. Int. J. Trop. Insect Sci. 2018, 38, 261–273. [Google Scholar] [CrossRef]
- Stegmaier, C.E. Insects Associated with Rough Pigweed, Amaranthus retroflexus. Master’s Thesis, Kansas State College of Agriculture and Applied Science, Manhattan, Kansas, 1950. [Google Scholar]
- Garman, H. Habits of Disonycha glabrator. J. Agric. Sci. 1981, 5, 143–145. [Google Scholar]
- Lopez-Olguin, J.F.; Garcia, A.A.; Huato, M.A.D.; Lara, M.H.; Saenz-de-cabezon, F.J.; Perez-Moreno, I.; Marco-Mancebon, V. Insect occurrence and losses due to phytophagous species in the amaranth (Amaranthus hypocondriacus L.) in Puebla, Mexico. Afr. J. Agric. Res. 2011, 6, 5924–5929. [Google Scholar] [CrossRef]
- Vogt, G.B.; McGuire, J.U.; Cushman, A.D. (Coleóptera: Chrysomelidae) and Their Amaranthaceous Hosts. USDA Technical Bulletin No. 1593, 1979. Available online: https://naldc.nal.usda.gov/download/CAT80727487/PDF (accessed on 14 June 2019).
- Kagali, R.; Muya, S.M.; Kioko, E.; Oseimo, Z. Insect Abundance and Diversity on Cultivated Amaranthus Spp. (Amaranthacea) in Meru County, Kenya. Am. Int. J. Contemp. Res. 2013, 3, 7. [Google Scholar]
- Brown, M.W.; Tworkoski, T. Pest management benefits of compost mulch in apple orchards. Agric. Ecosyst. Environ. 2004, 103, 465–472. [Google Scholar] [CrossRef]
- Mochiah, M.B.; Baidoo, P.K.; Acheampong, G. Effects of mulching materials on agronomic characteristics, pests of pepper (Capsicum annuum L.) and their natural enemies’ population. Agric. Biol. J. N. Am. 2012, 3, 253–261. [Google Scholar] [CrossRef]
- Packard, A. Color-Preference in Insects. J. N. Y. Entomol. Soc. 1903, 11, 132–137. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Talpur, K.H.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Johnson, J.M.; Hough-Goldstein, J.A.; Vangessel, M.J. Effects of straw mulch on pest insects, predators, and weeds in watermelons and potatoes. Environ. Entomol. 2004, 33, 1632–1643. [Google Scholar] [CrossRef]
- Stoner, K.A.; Ferrandino, L.F.J.; Gent, M.P.N.; Elmer, W.H.; Lamondia, A.A. Effects of straw mulch, spent mushroom compost, and fumigation on the density of Colorado Potato Beetles (Coleoptera: Chrysomelidae) in Potatoes. J. Econ. Entomol. 1996, 89, 1267–1280. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Karageorgou, P.; Manetas, Y. The importance of being red when young: Field evidence that anthocyanins in young leaves of Quercus coccifera afford considerable protection against insect consumers in addition to a slight protection against excess light. Tree Physiol. 2006, 26, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Dadakova, E. Rutin and total quercetin content in Amaranth (Amaranthus spp.). Plant Foods Hum. Nutr. 2009, 64, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.A.; Briones-Cerecero, E.P.; Mendoza-Hernández, G.; De León-Rodríguez, A.; Barba de la Rosa, A.P. Proteomic analysis of amaranth (Amaranthus hypochondriacus L.) leaves under drought stress. Int. J. Plant. Sci. 2009, 170, 990–998. [Google Scholar] [CrossRef]
- Rahman, A.; Yahataa, H.; Miahb, G.; Ahamedb, T.; Begumc, M.N. Effectiveness of tree leaf mulch comparing with conventional mulches on common bean at different irrigation levels: Growth, yield, water use efficiency and weed infestation. Arch. Agron. Soil Sci. 2008, 54, 331–342. [Google Scholar] [CrossRef]
| Variety1 | Species (Type 2) | Plant Architectural Traits | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amaranthus | Origin | Growth Habit 3 | Maturity 4 | Leaf Color 5 | Leaf Shape 6 | Leaf Pubescence 7 | Branch Type 8 | Inflorescence Type 9 Color 10 | |
| TR | tricolor (V) | Asia | 1 | 30–50 | 2 | 3 | 1 | 1 | n/a n/a |
| MF | tricolor (V) | Asia | 1 | 30–50 | 3 | 3 | 1 | 1 | n/a n/a |
| GC | viridis (V/G) | China | 1 | 90–120 | 1 | 2 | 1 | 3 | 1 1 |
| HR-D | hypochondiacus (V/G) | USA | 2 | 65–75 | 2 | 1 | 1 | 2 | 1 2 |
| GG | cruentus (G/V) | USA | 1 | 98–110 | 1 | 2 | 1 | 2 | 2 1 |
| OP | cruentus (G/V) | Mexico | 1 | 62–75 | 3 | 3 | 1 | 4 | 2 3 |
| RG | cruentus (G/V) | Mexico | 1 | 75–110 | 3 | 3 | 1 | 4 | 2 3 |
| RL | cruentus (G/V) | Asia | 1 | 50 | 2 | 4 | 1 | 4 | 3 1 |
| MI | cruentus (G/V) | Mexico | 1 | 90 | 2 | 2 | 1 | 3 | 2 3 |
| Order | Family | Scientific Name | Common Name 1 | Number of Insects 2017 | Number of Insects 2018 | ||
|---|---|---|---|---|---|---|---|
| Mulch | No Mulch | Mulch | No Mulch | ||||
| Hemiptera | Blissidae | Blissus leucopterus | Chinch Bug | 19 | 9 | 19 | 12 |
| Cicadellidae | Graminella nigrifrons | Leafhopper | 500 | 300 | 400 | 157 | |
| Graphocephala sp. | 50 | 30 | 50 | 30 | |||
| Miridae | Lygus sp | Tarnish Plant Bug | 115 | 108 | 100 | 98 | |
| Cercopidae | Prosapia bicincta | Spittle Bug | 30 | 4 | 21 | 18 | |
| Membracidae | Ceresa sp. | Treehopper | 62 | 18 | 50 | 20 | |
| Acanaloniidae | Acanalonia servillei | Planthopper | 39 | 7 | 30 | 27 | |
| Aphididae | Aphis nerii | Aphid | 660 | 278 | 472 | 192 | |
| Coreoidea | Cletus spp. | Horned Coreid Bug | 11 | 6 | 20 | 27 | |
| Pentatomidae | Cosmopepla lintneriana | Twice-stabbed Stink Bug | 23 | 3 | 34 | 8 | |
| Coleoptera | Coccinelidae | Coleomegilla maculate+ | Spotted Lady Beetle | 88 | 109 | 60 | 85 |
| Harmonia axyridis+ | Multicolored Asian Lady Beetle | 25 | 28 | 30 | 20 | ||
| Carabidae | Lebia analis + | Ground Beetle | 44 | 29 | 50 | 20 | |
| Chrysomelidae | Disonycha glabrata | Pigweed Flea Beetle | 980 | 558 | 850 | 508 | |
| Deloyata guttata | Tortoise Beetle | 19 | 17 | 22 | 22 | ||
| Diabrotica undercimpunctata | Spotted Cucumber Beetle | 20 | 31 | 20 | 31 | ||
| Acalymma vittatum | Stripped Cucumber Beetle | 20 | 24 | 2 | 24 | ||
| Curculinodae | Rhyssomatus aequalis | Amaranthus Weevil | 14 | 20 | 12 | 25 | |
| Hypolixus truncatulus | 10 | 18 | 8 | 15 | |||
| Myleridae | Collops quadrimaculatus | Soft-winged Flower Beetle | 11 | 10 | 20 | 20 | |
| Elateridae | Megapenthes insignis | Click Beetle | 10 | 0 | 19 | 30 | |
| Diptera | Agromyzidae | Agromyza spp. | Leaf miner | 100 | 0 | 98 | 80 |
| Do lichopodidae | Condylostylus sp.+ | Long Legged Fly | 102 | 120 | 82 | 200 | |
| Sarcophagidae | Ravinia sp. º | Flesh Fly | 30 | 26 | 52 | 0 | |
| Lepidoptera | Hespiridae | Hylephila phyleus | Skipper | 11 | 12 | 11 | 21 |
| Pieridae | Pieris rapae | Small Cabbage Butterfly | 3 | 5 | 3 | 15 | |
| Hymenoptera | Vespidae | Ancistrocerus sp.º | Yellow Jacket Wasp | 12 | 10 | 12 | 11 |
| Euodynerus sp. | Brown Paper Wasp | 10 | 9 | 10 | 30 | ||
| Pachodynerus erynnis | Hornet | 5 | 1 | 5 | 0 | ||
| Halictidae | Augochloropsis metallica − | Sweat Bee | 10 | 0 | 12 | 24 | |
| Orthoptera | Acrididae | Chortophaga viridifasciata | Green Grasshopper | 8 | 6 | 20 | 0 |
| Total | 3038.0 ± 42.7 | 1796.0 ± 22.3 | 2594.0 ± 35.5 | 1649.0 ± 19.7 | |||
| Shannon Weaver Diversity Index (H′) | 2.3 | 2.1 | 2.3 | 2.0 | |||
| Index of Evenness (E) | 0.23 | 0.19 | 0.67 | 0.59 | |||
| Amaranth Variety | Damage Scores 1 (2017) | Damage Scores 1 (2018) | ||
|---|---|---|---|---|
| Mulch | No Mulch | Mulch | No Mulch | |
| Tricolor (TR) | 1.4 ± 0.09 cA | 1.3 ± 0.14 cB | 1.4 ± 0.08 cbA | 1.3 ± 0.15 cbB |
| Green Callaloo (GC) | 3.7 ± 0.24 aA | 2.2 ± 0.12 aB | 3.8 ± 0.12 aA | 2.4 ± 0.13 aB |
| Hopi Red Dye (HR-D) | 1.3 ± 0.07 bA | 1.3 ± 0.09 bB | 1.2 ± 0.05 cbA | 1.1 ± 0.07 cbB |
| Golden Giant (GG) | 1.6 ± 0.19 bA | 1.6 ± 0.19 bB | 1.5 ± 0.07 cbA | 1.2 ± 0.10 cbB |
| Opopeo (OP) | 1.6 ± 0.15 bA | 1.0 ± 0.03 bB | 1.6 ± 0.03 bA | 1.5 ± 0.03 bB |
| Molten Fire (MF) | 1.0 ± 0.03 cA | 1.2 ± 0.03 cB | 1.2 ± 0.03 cA | 1.0 ± 0.03 cB |
| Red Garnet (RG) | 1.5 ± 0.03 bA | 1.5 ± 0.02 bB | 1.8 ± 0.03 cbA | 1.2 ± 0.03 cbB |
| Red Leaf (RL) | 3.6 ± 0.25 bA | 2.3 ± 0.06 bB | 3.8 ± 0.20 aA | 1.5 ± 0.03 aB |
| Mayo Indian (MI) | 1.5 ± 0.04 aA | 2.0 ± 0.06 aB | 1.5 ± 0.17 bA | 1.5 ± 0.09 bB |
| Amaranth Variety 1 | Mean Fresh Leaf Yield 1 (Kg/ha) (2017) | Change in Yield Due to Mulching | Mean Fresh Leaf Yield 1 (Kg/ha) (2018) | Change in Yield Due to Mulching | ||
|---|---|---|---|---|---|---|
| Mulch | No Mulch | (%) | Mulch | No Mulch | (%) | |
| TR | 9298.6 ± 901.9 eA | 10911.4 ± 950.6 eB | −14.8 ± 3.9 | 6388.9 ± 734.9 aA | 7496.9 ± 819.0 bB | −14.8 ± 2.9 |
| GC | 9651.4 ± 1277.2 deA | 13103.8 ± 908.6 deB | −26.3 ±15.8 | 4333.3 ± 693.9 aA | 9215.6 ± 1033.5 bB | −53.0 ± 3.7 |
| HR-D | 13985.8 ± 1200.9 cdA | 17790.9 ± 1640.3 cdB | −21.4 ±21.0 | 9666.7 ± 787.6 aA | 11012.6 ± 669.5 bB | −12.2 ± 1.9 |
| GG | 14892.9 ± 226.8 bA | 21369.2 ± 643.5 bB | −30.3 ± 3.0 | 12166.7 ± 1205.7 aA | 14237.7 ± 2336.0 bB | −14.5 ± 6.1 |
| OP | 19050.9 ± 1209.6 aA | 23612.0 ± 1034.1 aB | −19.3 ± 1.7 | 12333.3 ± 838.9 aA | 13960.6 ± 1340.7 bB | −11.7 ± 2.8 |
| MF | 9323.8 ± 504.0 cdA | 14666.1 ± 615.7 cdB | −36.4 ± 4.3 | 8277.8 ± 1010.7 aA | 9240.7 ± 990.8 bB | −10.4 ± 1.6 |
| RG | 15422.1 ± 1229.1 bA | 20865.2 ± 1442.3 bB | −26.1 ± 1.1 | 12231.3 ± 846.8 aA | 13542.2 ± 1458.7 bB | −9.7 ± 3.7 |
| RL | 12070.6 ± 995.6 cA | 16455.3 ± 1019.3 cB | −26.6 ±10.3 | 5000.0 ± 1734.7 aA | 9400.2 ± 1918.3 bB | −46.8 ± 7.5 |
| MI | 20361.2 ± 613.1 aA | 26610.7 ± 1930.9 aB | −23.4 ± 6.1 | 1413.70 ± 891.3 aA | 1569.3 ± 857.9 bB | −9.9 ± 0.8 |
| Amaranth Variety 1 | Mean Dry Grain Yield 1 (Kg/ha) (2017) | Change in Yield Due to Mulching | Mean Dry Grain Yield 1 (Kg/ha) (2018) | Change in Yield Due to Mulching | ||
|---|---|---|---|---|---|---|
| Mulch | No Mulch | (%) | Mulch | No Mulch | (%) | |
| GC | 222.2 ± 55.6 aA | 444.4 ± 111.1 aB | −50.0 ± 0.0 | 1000.0 ± 384.9 cA | 1333.3 ± 192.5 bB | −25.0 ± 0.0 |
| HR-D | 333.3 ± 96.2 abA | 555.6 ± 147.0 abB | −40.0 ± 0.0 | 1277.8 ± 618.6 cA | 833.3 ± 419.0 cB | 53.3± 0.0 |
| GG | 1833.3 ± 96.2 abA | 1944.4 ± 200.3 abB | −5.7 ± 0.1 | 1777.8 ± 111.1 bA | 2055.6 ± 242.2 bB | −13.5 ± 0.0 |
| OP | 1888.9 ± 147.0 abA | 1944.40 ± 147.0 abB | −2.9 ± 0.0 | 1722.2 ± 55.6B bA | 1722.2 ± 55.6 bB | 0.0 ± 0.0 |
| RG | 1388.9 ± 277.8 abA | 1777.8 ± 111.1 abB | −21.9 ± 0.3 | 2500.0 ± 481.1 bA | 1611.1 ± 200.3 bB | 55.2± 0.0 |
| MI | 2555.6 ± 337.9 bA | 3388.9 ± 55.6 bB | −24.6 ± 0.4 | 3111.1 ± 309.3 aA | 3611.1 ± 419.4 aB | −13.9 ± 0.0 |
| Amaranth Varieties | Damage Score 1 | Insect Population 1 | Yield 1 (Kg/ha) | Yield Reduction Due to Mulching 1 % | Total Sum of Rank | Final Rank | |||
|---|---|---|---|---|---|---|---|---|---|
| M 2 | NM 3 | M 2 | NM 3 | M 2 | NM 3 | ||||
| Molten fire (MF) | 1 | 1 | 1 | 2 | 7 | 7 | 2 | 21 | 1 |
| Red Garnet (RG) | 2 | 3 | 6 | 7 | 3 | 4 | 2 | 27 | 2 |
| Hopi Red Dye (HR-D) | 2 | 3 | 3 | 2 | 6 | 5 | 7 | 28 | 3 |
| Opopeo (OP) | 5 | 5 | 3 | 7 | 2 | 3 | 6 | 31 | 4 |
| Mayo Indian (MI) | 5 | 5 | 7 | 7 | 2 | 1 | 4 | 31 | 4 |
| Golden Giant (GG) | 5 | 4 | 7 | 6 | 2 | 3 | 4 | 31 | 4 |
| Tricolor (TR) | 3 | 3 | 3 | 4 | 8 | 9 | 6 | 32 | 5 |
| Red Leaf (RL) | 5 | 5 | 7 | 6 | 7 | 6 | 6 | 42 | 6 |
| Green Callaloo (GC) | 7 | 6 | 9 | 9 | 8 | 7 | 9 | 55 | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorsah, R.V.; Dingha, B.N.; Gyawaly, S.; Fremah, S.A.; Sharma, H.; Bhowmik, A.; Worku, M.; Jackai, L.E. Organic Mulch Increases Insect Herbivory by the Flea Beetle Species, Disonycha glabrata, on Amaranthus spp. Insects 2020, 11, 162. https://doi.org/10.3390/insects11030162
Vorsah RV, Dingha BN, Gyawaly S, Fremah SA, Sharma H, Bhowmik A, Worku M, Jackai LE. Organic Mulch Increases Insect Herbivory by the Flea Beetle Species, Disonycha glabrata, on Amaranthus spp. Insects. 2020; 11(3):162. https://doi.org/10.3390/insects11030162
Chicago/Turabian StyleVorsah, Roger V., Beatrice N. Dingha, Sudan Gyawaly, Sarah A. Fremah, Harmandeep Sharma, Arnab Bhowmik, Mulumebet Worku, and Louis E. Jackai. 2020. "Organic Mulch Increases Insect Herbivory by the Flea Beetle Species, Disonycha glabrata, on Amaranthus spp." Insects 11, no. 3: 162. https://doi.org/10.3390/insects11030162
APA StyleVorsah, R. V., Dingha, B. N., Gyawaly, S., Fremah, S. A., Sharma, H., Bhowmik, A., Worku, M., & Jackai, L. E. (2020). Organic Mulch Increases Insect Herbivory by the Flea Beetle Species, Disonycha glabrata, on Amaranthus spp. Insects, 11(3), 162. https://doi.org/10.3390/insects11030162






