Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Maize and Sorghum in Central Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sentinel Technique to Detect Egg Parasitoids in Cereal Fields
2.3. Parasitoid Identification
2.4. Parasitism of Two Trichogramma Species, Laboratory Test
2.5. Parasitism in Field Cages
3. Results
3.1. Natural Enemies of Egg Masses of S. frugiperda
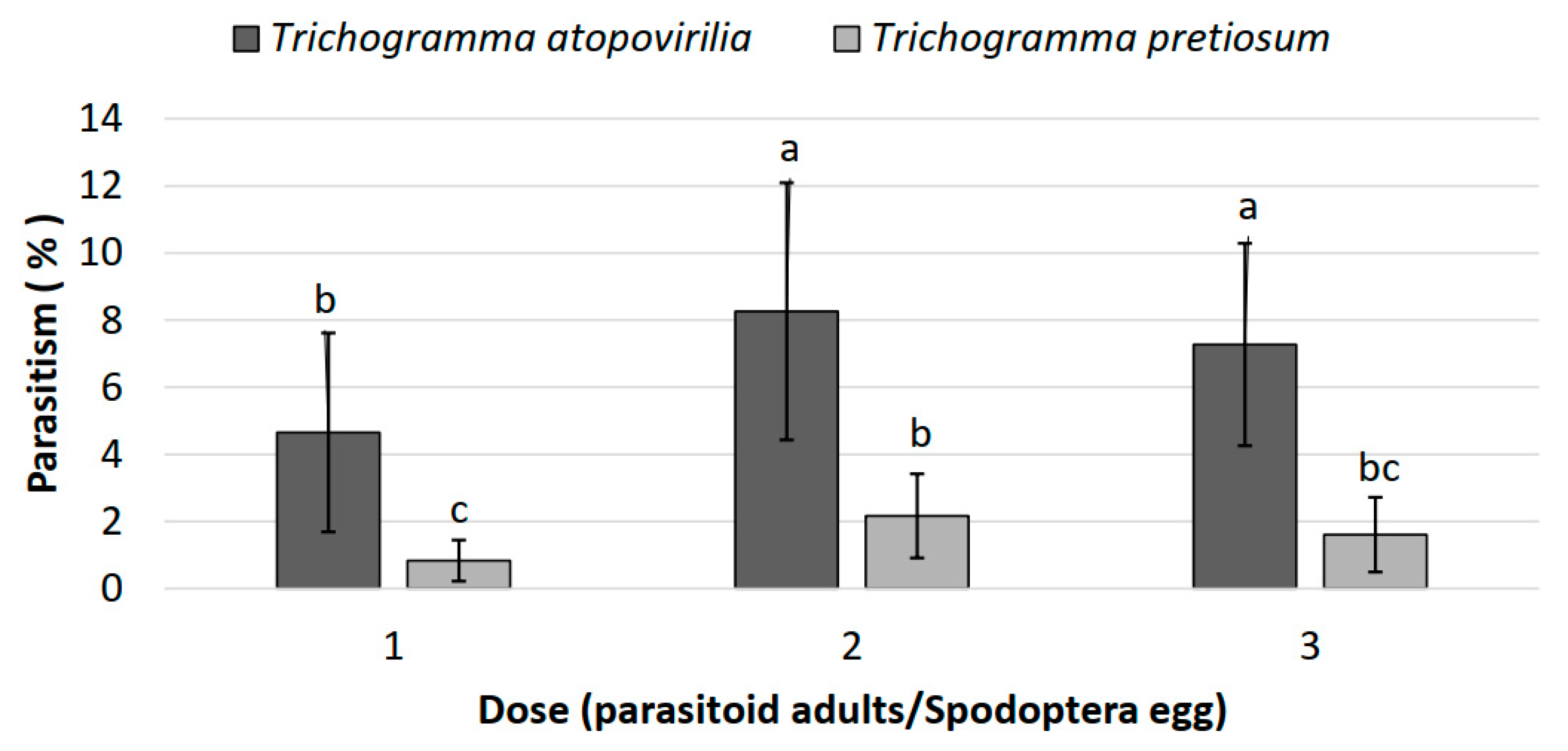
3.2. Parasitism of Two Trichogramma Species in Laboratory Conditions
3.3. Parasitism in Field Cages
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashley, T.R.; Wiseman, B.R.; Davis, F.M.; Andrews, K.L. The fall armyworm: A bibliography. Fla. Entomol. 1989, 72, 152–202. [Google Scholar] [CrossRef]
- Sparks, A.N. A review of the biology of the Fall Armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Chormule, A.; Shejawal, N.; Sharanabasappa, C.M.; Asokan, R.; Swamy, H.M. First report of the fall Armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae) on sugarcane and other crops from Maharashtra, India. JEZS 2019, 7, 114–117. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). First Detection of Fall Armyworm in China; FAO: Italy, Rome, 20 January 2019; Available online: https://www.ippc.int/fr/news/first-detection-of-fall-armyworm-in-china/ (accessed on 20 March 2019).
- International Plant Protection Convention (IPPC). Report on Fall armyworm (Spodoptera frugiperda). In Report on Fall Armyworm (Spodoptera frugiperda) IPPC Official Pest Report, No. GHA-01/4; FAO: Italy, Rome, 31 August 2018; Available online: https://www.ippc.int/es/countries/ghana/pestreports/2018/08/report-on-fall-armyworm-spodoptera-frugiperda-4/ (accessed on 20 March 2019).
- Jaramillo, Á.; Jaramillo, O.; Bustillo, A.E.; Gómez, H. Efecto del gusano cogollero Spodopfera frugiderpa (J.E. Smith) sobre el rendimiento del maíz. RFNA 1989, 42, 25–33. [Google Scholar]
- Luginbill, P. The Fall Army Worm; Technical Bulletin No. 34; US Department of Agriculture: Washington, DC, USA, 1928.
- Blanco, C.A.; Pellegaud, J.G.; Nava-Camberos, U.; Lugo-Barrera, D.; Vega-Aquino, P.; Coello, J.; Terán-Vargas, A.P.; Vargas-Camplis, J. Maize pests in Mexico and challenges for the adoption of integrated pest management programs. J. Integr. Pest Manag. 2014, 5, E1–E9. [Google Scholar] [CrossRef]
- Murúa, G.; Molina-Ochoa, J.; Coviella, C. Population dynamics of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) and its parasitoids in northwestern Argentina. Fla. Entomol. 2006, 89, 175–182. [Google Scholar] [CrossRef]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NB 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Georghiou, G.P.; Mellon, R.B. Pesticide resistance in time and space. In Pest Resistance to Pesticides; Georghiou, G.P., Saito, T., Eds.; Springer: Boston, MA, USA, 1983; pp. 1–46. ISBN 978-1-4684-4466-7. [Google Scholar]
- Yu, S.J. Detection and biochemical characterization of insecticide resistance in fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1992, 85, 675–691. [Google Scholar] [CrossRef]
- Lagunes, T.Á.; Rodríguez, M.J.C.; Loera, B.D.; Juan, C. Susceptibilidad a insecticidas en poblaciones de artrópodos de México. Agrociencia 2009, 43, 173–196. [Google Scholar]
- Diez, R.G.I.; Omoto, C. Herança da resistência de Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) a lambdacialotrina. Neotrop. Entomol. 2001, 30, 311–316. [Google Scholar] [CrossRef]
- Casmuz, A.; Juárez, M.L.; Socías, M.G.; Murúa, M.G.; Prieto, S.; Medina, S.; Willink, E.; Gastaminza, G. Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Argent. 2010, 69, 209–231. [Google Scholar]
- Mangelsdorf, P.C.; Reeves, R.G. The origin of maize. Proc. Natl. Acad. Sci. USA 1938, 24, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Arredondo-Bernal, H.C.; Rodríguez-del-Bosque, L.A. Biological pest control in Mexico. Annu. Rev. Entomol. 2013, 58, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ochoa, J.; Hamm, J.J.; Lezama-Gutiérrez, R.; López-Edwards, M.; González-Ramírez, M.; Pescador-Rubio, A.; Lezama-Gutierrez, R.; Lopez-Edwards, M.; Gonzalez-Ramirez, M. A survey of Fall Armyworm (Lepidoptera: Noctuidae) parasitoids in the Mexican States of Michoacán, Colima, Jalisco, and Tamaulipas. Fla. Entomol. 2001, 84, 31–36. [Google Scholar] [CrossRef]
- Molina-Ochoa, J.; Carpenter, J.E.; Heinrichs, E.A.; Foster, J.E. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: An inventory. Fla. Entomol. 2003, 86, 254–289. [Google Scholar] [CrossRef]
- Bahena, J.F.; Cortez, M.E. Gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). In Casos de Control Biológico en México 2, 1st ed.; Arredondo, B.H.C., Ed.; Biblioteca Básica de Agricultura: Guadalajara, Jalisco, México, 2016; Volume 2, pp. 181–250. [Google Scholar]
- Andrews, K.L. Latin American research on Spodoptera frugiperda (Lepidoptera: Noctuidae). Fla. Entomol. 1988, 71, 630–653. [Google Scholar] [CrossRef]
- Andrews, K.L. Fall Armyworm Symposium: The Whorlworm, Spodoptera Frugiperda, in Central America and Neighboring Areas. Fla. Entomol. 1980, 63, 456–467. [Google Scholar] [CrossRef]
- Cruz, I. A Lagarta do Cartucho na Cultura do Milho; Circular Técnica No. 21; EMBRAPA-CNPMS: Brasília, Brasil, 1995; pp. 1–45. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database; World Wide Web Electronic Publication; Department of Entomology, The Natural History Museum: Cromwell Road, London, UK, 2019; Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 20 May 2019).
- Van Lenteren, J.C.; Bueno, V.H. Augmentative biological control of arthropods in Latin America. BioControl 2003, 48, 123–139. [Google Scholar] [CrossRef]
- Sánchez, S.J.A.; García, G.; Garza, R.A.A.; Valdez, D.K.M.; González, J.F.; Quiroz, M.H.; Rodríguez, C.V.A. Control biológico de las principales plagas de lepidópteros en pastos con Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). In Proceedings of the Memorias del XXII Congreso Nacional de Control Biológico, Texcoco, Estado de México, México, 28–29 Octubre 1999; Rodríguez, L.E., Escobar, A.J.J., Eds.; Sociedad Mexicana de Control Biológico: Montecillo, Estado de México, México, 1999; pp. 183–185. [Google Scholar]
- Toonders, T.J. Evaluación de la efectividad de Trichogramma spp. (Hym.: Trichogrammatidae) en el combate de Spodoptera frugiperda (JE Smith) (Lep.: Noctuidae) y recomendaciones para su uso. In Tesis Para Obtención de Grado de Maestría; Colegio de Postgraduados: Campus Montecillo, Texcoco, Estado de México, 1986. [Google Scholar]
- Armenta, R.; Martinez, A.M.; Chapman, J.W.; Magallanes, R.; Goulson, D.; Caballero, P.; Cave, R.D.; Cisneros, J.; Valle, J.; Castillejos, V.; et al. Impact of a nucleopolyhedrovirus bioinsecticide and selected synthetic insecticides on the abundance of insect natural enemies on maize in Southern Mexico. J. Econ. Entomol. 2003, 96, 649–661. [Google Scholar] [CrossRef]
- Poitout, S.; Bues, R. Elevage de chenilles de vingt-huit espèces de Lépidoptères Noctuidae et de deux espèces d’Arctiidae sur milieu artificiel simple. Particularités de l’élevage selon les espèces. Ann. Zool. Ecol. Anim. 1974, 6, 431–441. [Google Scholar]
- Da Silva, C.S.B.; Vieira, J.M.; Loiácono, M.; Margaría, C.; Parra, J.R.P. Evidence of exploitative competition among egg parasitoids of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Rev. Colomb. Entomol. 2015, 41, 184–186. [Google Scholar]
- Pinto, J.D. Trichogrammatidae. In Annoted Key to the Genera of Nearctic Chalcidoidea (Hymenoptera); Gibson, G.A.P., Huber, J.T., Woolley, J.B., Eds.; NRC Research Press: Ottawa, ON, Canada, 1997; pp. 726–752. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 20 July 2019).
- Jalali, S.K.; Mohanraj, P.; Lakshmi, B.L. Trichogrammatids. In Ecofriendly Pest Management for Food Security; Elsevier: Amsterdam, The Netherlands, 2016; pp. 139–181. ISBN 978-0-12-803265-7. [Google Scholar]
- Rodriguez-del-Bosque, L.A.; Smith, J.W. Biological control of maize and sugarcane stemborers in Mexico: A review. Int. J. Trop. Insect Sci. 1997, 17, 305–314. [Google Scholar] [CrossRef]
- Quijano, V.J.; Canale, P.R.A. La problemática de la inducción de Trichogramma (Hymenoptera Trichogrammatidae) en caña de azúcar. In Proceedings of the XXI Congreso Nacional de Control Biológico, Rio Bravo, Tamaulipas, México, 5–6 Noviembre 1998; Sociedad Mexicana de Control Biológico: Rio Bravo, Tamaulipas, México, 1998. [Google Scholar]
- Hoballah, M.E.; Degen, T.; Bergvinson, D.; Savidan, A.; Tamo, C.; Turlings, T.C.J. Occurrence and direct control potential of parasitoids and predators of the fall armyworm (Lepidoptera: Noctuidae) on maize in the subtropical lowlands of Mexico. Agric. For. Entomol. 2004, 6, 83–88. [Google Scholar] [CrossRef]
- Comité Estatal de Sanidad Vegetal del Estado de Guanajuato (CESAVEG). Programa de Trabajo Campaña Manejo Fitosanitario de Hortalizas a Operar con Recursos del Programa de Sanidad e Inocuidad Agroalimentaria 2017, Componente Sanidad Federalizado, Subcomponente de Sanidad Vegetal, en el Estado de Guanajuato. Available online: https://www.gob.mx/cms/uploads/attachment/file/303342/Manejo_fitosanitario_de_hortalizas.pdf (accessed on 20 July 2019).
- Bueno, A.D.F.; Bueno, R.C.O.D.F.; Parra, J.R.P.; Vieira, S.S. Effects of pesticides used in soybean crops to the egg parasitoid Trichogramma pretiosum. Ciência Rural 2008, 38, 1495–1503. [Google Scholar] [CrossRef]
- Norris, R.F.; Kogan, M. Ecology of interactions between weeds and arthropods. Annu. Rev. Entomol. 2005, 50, 479–503. [Google Scholar] [CrossRef]
- Romero-Sueldo, M.; Virla, E.G. Doru lineare (Dermaptera: Forficulidae), insecto benéfico en cultivos de maíz del norte Argentino: Preferencias alimenticias y tasas de consumo. Bol. Sanid. Veg. 2009, 35, 39–47. [Google Scholar]
- Childers, C.C.; Rock, G.C. Observations on the occurrence and feeding habits of Balaustium pulmani (Acari: Erythraeidae) in North Carolina apple orchards. Int. J. Acarol. 1981, 7, 63–68. [Google Scholar] [CrossRef]
- Beserra, E.B.; Parra, J.R.P. Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) em ovos de Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae). Rev. Bras. Entomol. 2004, 48, 119–126. [Google Scholar] [CrossRef][Green Version]
- Fatouros, N.E.; Dicke, M.; Mumm, R.; Meiners, T.; Hilker, M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 2008, 19, 677–689. [Google Scholar] [CrossRef]
- Lewis, W.J.; Nordlund, D.A.; Gueldner, R.C.; Teal, P.E.A.; Tumlinson, J.H. Kairomones and their use for management of entomophagous insects. J. Chem. Ecol. 1982, 8, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.J.I. Resumen del avance de los programas de control biológico en la región central de la República Mexicana. In Proceedings of the V Reunión Nacional de Control Biológico y Sector Agropecuario Organizado, Ciudad Victoria, Tamaulipas, México, 27–29 Abril 1977; Dirección General de Sanidad Vegetal; Departamento de Control Biológico: Ciudad Victoria, Tamaulipas, México, 1977; pp. 46–54. [Google Scholar]
- Peralta, F.G. Incidencia de insectos benéficos y plaga en maíz y sorgo. In Proceedings of the VIII Reunión Nacional de Control Biológico y Sector Agropecuario Organizado, Manzanillo, Colima, México, 22–25 Abril 1980; Dirección General de Sanidad Vegetal; Departamento de Control Biológico: Manzanillo, Colima, México, 1980; pp. 159–168. [Google Scholar]
- Beserra, E.B.; Parra, J.R.P. Impact of the number of Spodoptera frugiperda egg layers on parasitism by Trichogramma atopovirilia. Sci. Agric. 2005, 62, 190–193. [Google Scholar] [CrossRef][Green Version]
- Beserra, E.B.; Dias, C.T.; Parra, J.R.P. Behavior of Trichogramma atopovirilia Oatman & Platner and T. pretiosum Riley (Hymenoptera: Trichogrammatidae) on Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) egg masses. Braz. J. Biol. 2005, 65, 9–17. [Google Scholar] [PubMed]
- Parra, J.R.P.; Zucchi, R.A. Trichogramma in Brazil: Feasibility of use after twenty years of research. Neotrop. Entomol. 2004, 33, 271–281. [Google Scholar] [CrossRef]

| Site | Locality | Level of Predation in Sentinels | |||
|---|---|---|---|---|---|
| Year | Without Damage | Fully Consumed | Partially Consumed | ||
| 1 | Cortazar, Cortazar | 2017 | 221 | 128 | 247 |
| 2 | INIFAP, Campo Experimental Bajío, Celaya | 2017 | 542 | 39 | 423 |
| 3 | Morales, Comonfort | 2017 | 436 | 72 | 376 |
| 4 | Instituto Tecnológico de Roque, Celaya | 2018 | 191 | 3 | 19 |
| 5 | Roque, Celaya | 2018 | 138 | 3 | 18 |
| 6 | Neutla, Comonfort | 2018 | 80 | 2 | 20 |
| 7 | Empalme Escobedo, Comonfort | 2018 | 131 | 30 | 54 |
| 8 | Jaral del progreso, Jaral del progreso | 2018 | 141 | 13 | 10 |
| 9 | Villadiego, Valle de Santiago | 2018 | 108 | 7 | 1 |
| 10 | El Piloncillo, Acámbaro | 2018 | 211 | 59 | 35 |
| 11 | La Concepción, Acámbaro | 2018 | 194 | 54 | 27 |
| 12 | San Nicolás de la Condesa, Tarimoro | 2018 | 195 | 61 | 34 |
| Total | 2588 | 471 | 1264 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaraleño-Teniente, J.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Bujanos-Muñiz, R.; Rodríguez-Rodríguez, S.E. Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Maize and Sorghum in Central Mexico. Insects 2020, 11, 157. https://doi.org/10.3390/insects11030157
Jaraleño-Teniente J, Lomeli-Flores JR, Rodríguez-Leyva E, Bujanos-Muñiz R, Rodríguez-Rodríguez SE. Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Maize and Sorghum in Central Mexico. Insects. 2020; 11(3):157. https://doi.org/10.3390/insects11030157
Chicago/Turabian StyleJaraleño-Teniente, Jannet, J. Refugio Lomeli-Flores, Esteban Rodríguez-Leyva, Rafael Bujanos-Muñiz, and Susana E. Rodríguez-Rodríguez. 2020. "Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Maize and Sorghum in Central Mexico" Insects 11, no. 3: 157. https://doi.org/10.3390/insects11030157
APA StyleJaraleño-Teniente, J., Lomeli-Flores, J. R., Rodríguez-Leyva, E., Bujanos-Muñiz, R., & Rodríguez-Rodríguez, S. E. (2020). Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Maize and Sorghum in Central Mexico. Insects, 11(3), 157. https://doi.org/10.3390/insects11030157





