Differences in Mobility and Dispersal Capacity Determine Body Size Clines in Two Common Alpine-Tundra Arthropods
Abstract
1. Introduction
2. Materials and Methods
3. Results
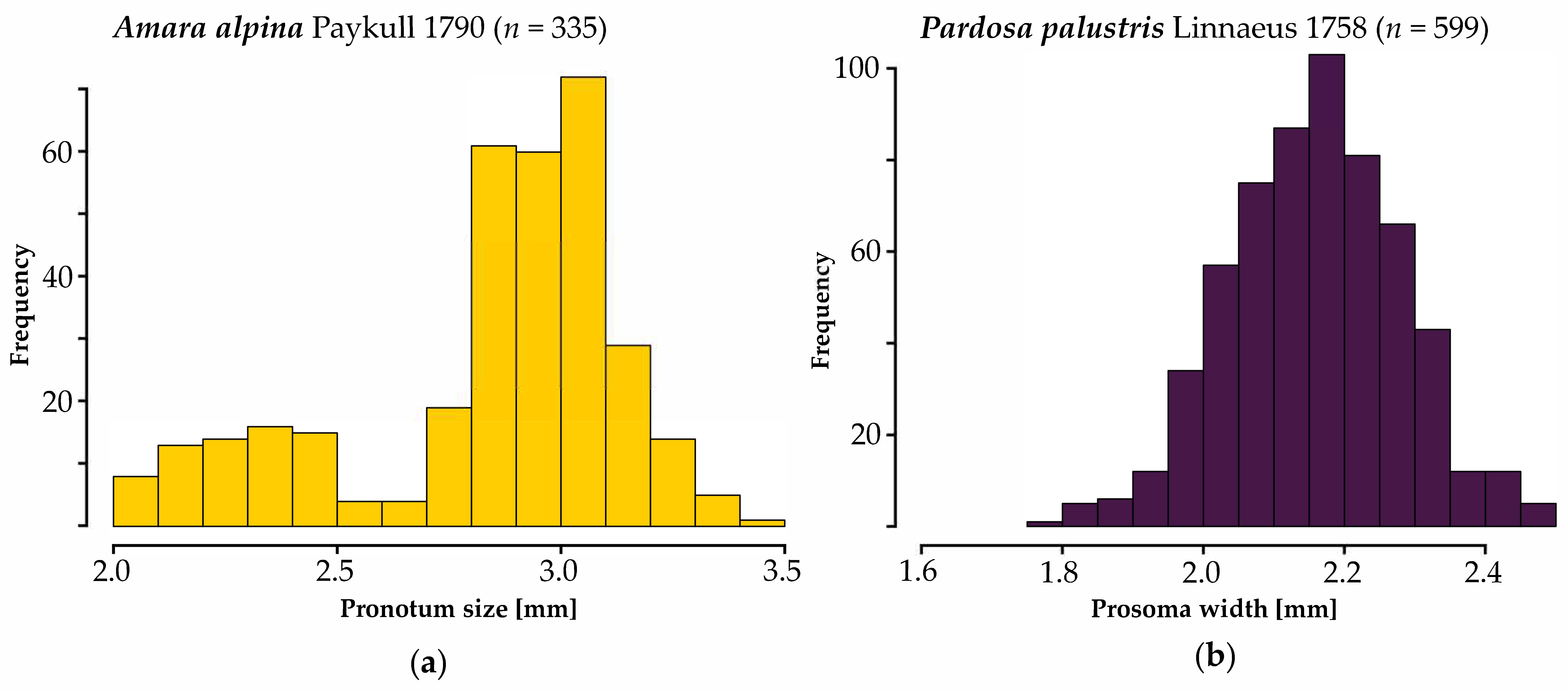
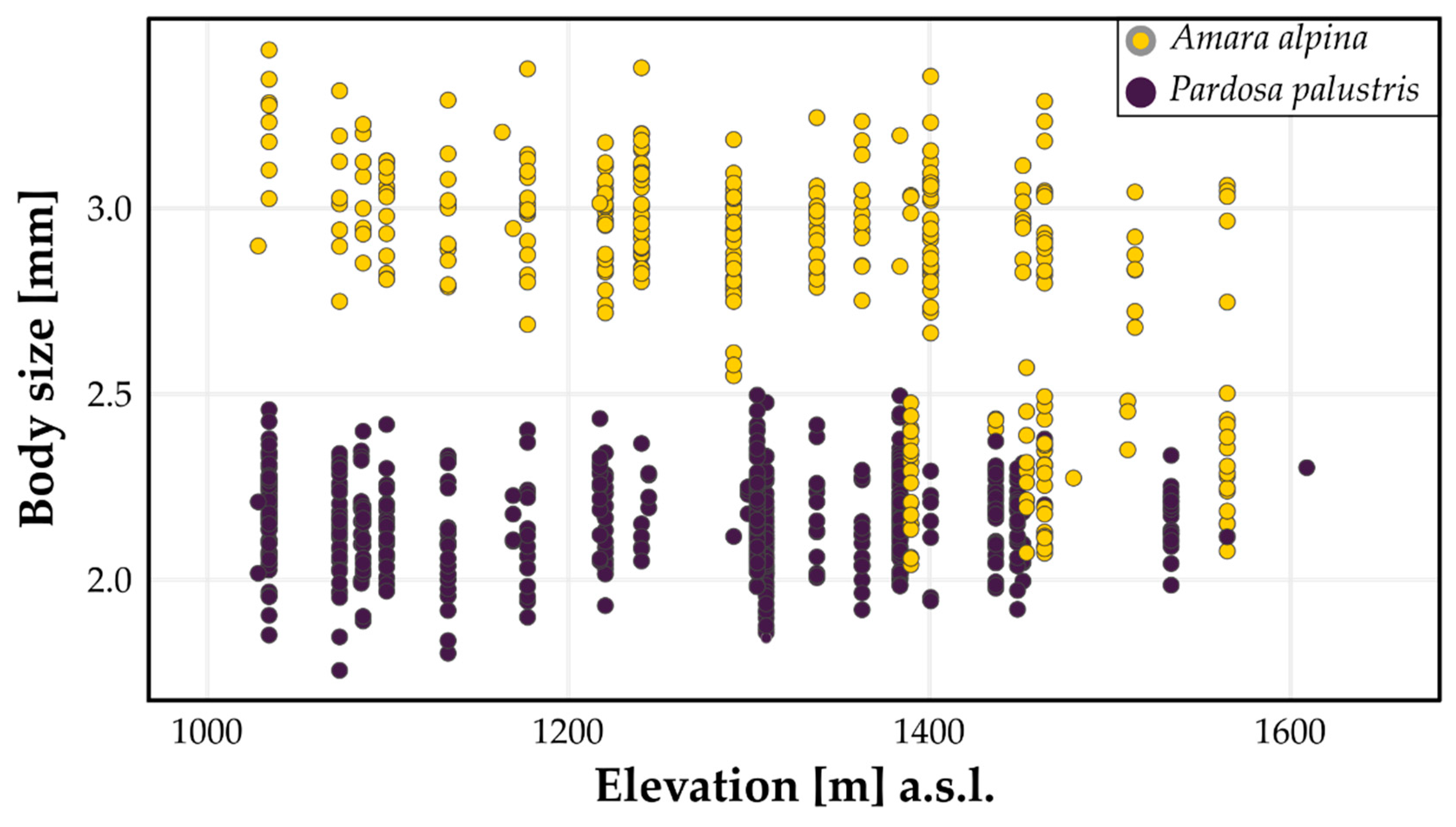
3.1. Body Sizes Per Species
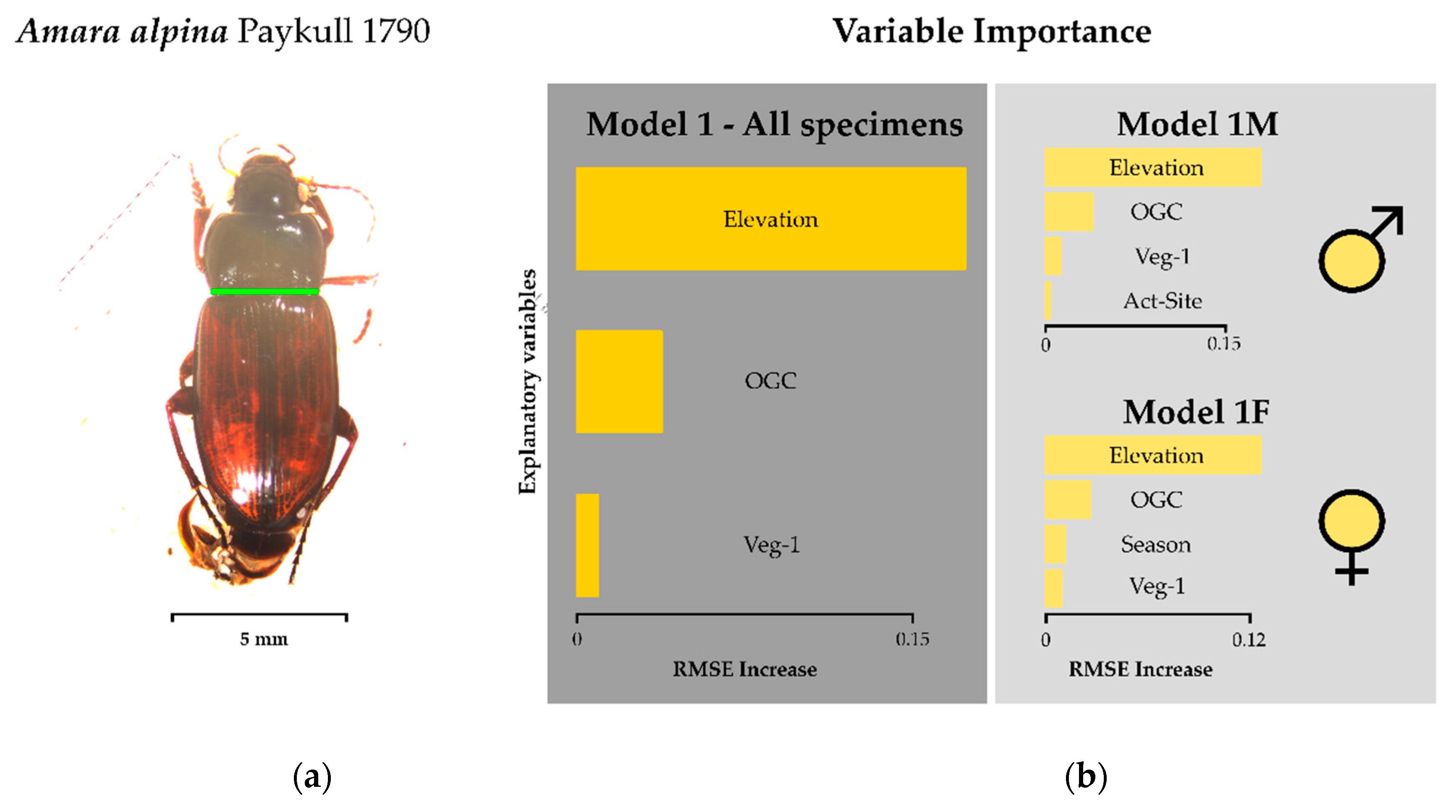
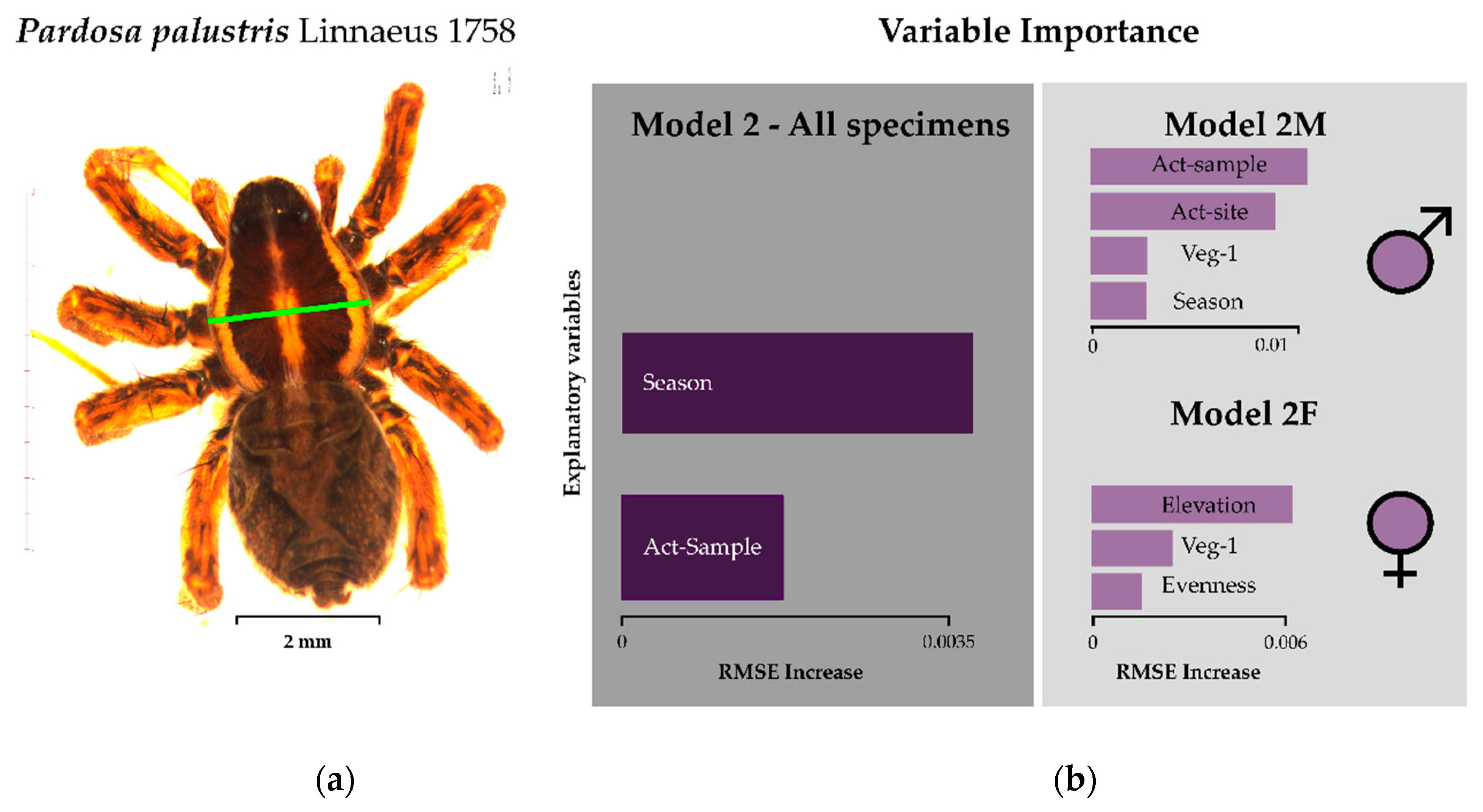
3.2. Environmental Drivers of Body Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- IPCC. Annex I: Atlas of Global and Regional Climate Projections; IPCC: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Löffler, J.; Anschlag, K.; Baker, B.; Finch, O.D.; Diekkrüger, B.; Wundram, D.; Schröder, B.; Pape, R.; Lundberg, A. Mountain ecosystem response to global change. Erdkunde 2011, 65, 189–213. [Google Scholar] [CrossRef]
- Bowden, J.J.; Eskildsen, A.; Hansen, R.R.; Olsen, K.; Kurle, C.M.; Høye, T.T. High-arctic butterflies become smaller with rising temperatures. Biol. Lett. 2015, 11, 20150574. [Google Scholar] [CrossRef] [PubMed]
- Høye, T.T.; Post, E.; Schmidt, N.M.; Trøjelsgaard, K.; Forchhammer, M.C. Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat. Clim. Chang. 2013, 3, 759–763. [Google Scholar] [CrossRef]
- CAFF Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity. Conservation of Arctic Flora and Fauna; Meltofte, H., Ed.; Arctic Council: Akureyri, Iceland, 2013; ISBN 978-9935-431-22-6. [Google Scholar]
- Post, E.; Christensen, T.R.; Ims, R.A.; Jeppesen, E.; Madsen, J.; Mcguire, A.D.; Rysgaard, S. Ecological dynamics across the arctic associated with recent climate change. Science (80-.) 2009, 325, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.A.K.; Alfredsson, M.; Barrio, I.C.; Bowden, J.; Convey, P.; Coulson, S.J.; Culler, L.E.; Dahl, M.T.; Daly, K.M.; Koponen, S.; et al. Circumpolar terrestrial arthropod monitoring: A review of ongoing activities, opportunities and challenges, with a focus on spiders. Ambio 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brandmayr, P.; Pizzolotto, R. Climate change and its impact on epigean and hypogean carabid beetles. Period. Biol. 2016, 118, 147–162. [Google Scholar] [CrossRef]
- Høye, T.T.; Bowden, J.J.; Hansen, O.L.P.; Hansen, R.R.; Henriksen, T.N.; Niebuhr, A.; Skytte, M.G. Elevation modulates how Arctic arthropod communities are structured along local environmental gradients. Polar Biol. 2018, 41, 1555–1565. [Google Scholar] [CrossRef]
- Körner, C. The use of “altitude” in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. Camb. Philos. Soc. 2005, 80, 489–513. [Google Scholar] [CrossRef]
- Levin, S.A. The Problem of Pattern and Scale in Ecology: The Robert H. MacArthur Award Lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Löffler, J. Altitudinal Changes of Ecosystem Dynamics in the Central Norwegian High Mountains. Die Erde 2002, 133, 227–528. [Google Scholar]
- Wundram, D.; Pape, R.; Löffler, J. Alpine soil temperature variability at multiple scales. Arctic, Antarct. Alp. Res. 2010, 42, 117–128. [Google Scholar] [CrossRef]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Høye, T.T.; Post, E.; Meltofte, H.; Schmidt, N.M.; Forchhammer, M.C. Rapid advancement of spring in the High Arctic. Curr. Biol. 2007, 17, 449–451. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Chown, S.L.; Jaco Klok, C. Altitudinal body size clines: Latitudinal effects associated with changing seasonality. Ecography (Cop.) 2003, 26, 445–455. [Google Scholar] [CrossRef]
- Gaston, K.J.; Chown, S.L.; Evans, K.L. Ecogeographical rules: Elements of a synthesis. J. Biogeogr. 2008, 35, 483–500. [Google Scholar] [CrossRef]
- Klok, C.J.; Harrison, J.F. The temperature size rule in arthropods: Independent of macro-environmental variables but size dependent. Integr. Comp. Biol. 2013, 53, 557–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angilletta, M.J.; Dunham, A.E. The Temperature-Size Rule in Ectotherms: Simple Evolutionary Explanations May Not Be General. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Demont, M. Bergmann and converse bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr. Comp. Biol. 2004, 44, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.J.; Hansen, R.R.; Olsen, K.; Høye, T.T. Habitat-specific effects of climate change on a low-mobility Arctic spider species. Polar Biol. 2015, 38, 559–568. [Google Scholar] [CrossRef]
- Hein, N.; Pétillon, J.; Pape, R.; Feilhauer, H.; Vanselow, K.A.; Hein, N. Broad-scale rather than fine-scale environmental variation drives body size in a wandering predator ( Araneae, Lycosidae ). Arctic, Antarct. Alp. Res. 2019, 51, 315–326. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Insect temperature–body size trends common to laboratory, latitudinal and seasonal gradients are not found across altitudes. Funct. Ecol. 2018, 32, 948–957. [Google Scholar] [CrossRef]
- Baars, M.A. Patterns of Movement of Radioactive Carabid Beetles. Oecologia 1979, 44, 125–140. [Google Scholar] [CrossRef]
- Thiele, H.-U. Carabid Beetles in Their Environments. A Study on Habitat Selection by Adaptations in Physiology and Behaviour; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 1977. [Google Scholar]
- Lindroth, C.H. Ground Beetles (Carabidae) of Fennoscandia: A Zoogeographic Study. Part I. Specific Knowledge Regarding the Species; Smithsonian Institution Libraries and National Science Foundation: Washington, DC, USA, 1989. [Google Scholar]
- Assmann, T. (Leuphana University, Lüneburg, Lower Saxony, Germany). Personal communication. 2019. [Google Scholar]
- Lindroth, C.H. The Carabidae (Coleoptera) of Fennoscandia and Denmark; Societas entomologica scandinavica, Ed.; E.J. Brill; Scandinavian Science Press Ltd.: Leiden, Copenhagen, 1985; ISBN 90 04 077278. [Google Scholar]
- Kotze, J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe - from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. Zookeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Eason, R.R.; Eason, R.R. Maternal Care as Exhibited by Wolf Spiders. J. Ark. Acad. Sci. 1964, 18, 13–19. [Google Scholar]
- Moen, A. Nasjonalatlas for Norge: Vegetasjon; Statens kartverk: Hønefoss, Norway, 1998. [Google Scholar]
- Löffler, J. Snow cover dynamics, soil moisture variability and vegetation ecology in high mountain catchments of central Norway. Hydrol. Process. 2005, 19, 2385–2405. [Google Scholar] [CrossRef]
- Dahl, E. Zonation in Arctic and Alpine Tundra and Fellfield Ecobiomes. In Ecosystem Theory and Application; Polunin, N., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 1986; pp. 35–62. [Google Scholar]
- Löffler, J.; Pape, R. Thermal niche predictors of alpine plant species. Ecology 2020, 101, e02891. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, H.; Mayer, R.; Schauemann, J. Ökosystemforschung. Ergebnisse des Sollingprojekts 1966 – 1986; Ulmer-Verlag: Stuttgart, Germany, 1986. [Google Scholar]
- Naujok, J.; Finch, O.-D. Communities and spatio-temporal patterns of epigeic beetles (Coleoptera) in high mountain habitats of the Central Norwegian Scandes, with special emphasis on carabid beetles (Carabidae). Nor. J. Entomol. 2004, 51, 31–55. [Google Scholar]
- Chatzaki, M.; Lymberakis, P.; Markakis, G.; Mylonas, M. The distribution of ground spiders (Araneae, Gnaphosidae) along the altitudinal gradient of Crete, Greece: Species richness, activity and altitudinal range. J. Biogeogr. 2005, 32, 813–831. [Google Scholar] [CrossRef]
- Hein, N.; Feilhauer, H.; Finch, O.D.; Schmidtlein, S.; Löffler, J. Snow cover determines the ecology and biogeography of spiders (araneae) in alpine tundra ecosystems. Erdkunde 2014, 68, 157–172. [Google Scholar] [CrossRef]
- Beckers, N.; Hein, N.; Vanselow, K.A.; Löffler, J. Effects of microclimatic tresholds on the activity-abundance and distribution patterns of alpine Carabidae species. Ann. Zool. Fennici 2018, 55, 25–44. [Google Scholar] [CrossRef]
- Spence, J.R.; Niemelä, J.K. Sampling carabid assemblages with pitfall traps: the madness and the method. Can. Entomol. 1994, 126, 881–894. [Google Scholar] [CrossRef]
- Topping, C.J.; Sunderland, K.D. Limitations to the use of pitfall traps in ecological in a field of spiders studies exemplified by a study of winter wheat. J. Appl. Ecol. 1992, 29, 485–491. [Google Scholar] [CrossRef]
- Woodcock, B.A. Pitfall trapping in ecological studies. In Insect Sampling in Forest Ecosystems; Blackwell Publishing: Malden, MA, USA; Oxford, UK, 2005; ISBN 0-632-05388-7. [Google Scholar]
- Engel, J.; Hertzog, L.; Tiede, J.; Wagg, C.; Ebeling, A.; Briesen, H.; Weisser, W.W. Pitfall trap sampling bias depends on body mass, temperature, and trap number: insights from an individual-based model. Ecosphere 2017, 8, e01790. [Google Scholar] [CrossRef]
- Hågvar, S.; Østbye, E.; Melåen, J. Pit-fall catches of surface-active arthropods in some high mountain habitats at Finse, south Norway. II. General results at group level, with emphasis on Opiliones, Araneida, and Coleoptera. Nor. J. Entomol. 1978, 25, 195–205. [Google Scholar]
- Uetz, G.W.; Unzicker, J.D. Pitfall Trapping in Ecological Studies of Wandering Spiders. J. Arachnol. 1976, 3, 101–111. [Google Scholar]
- Baars, M.A. Catches in Pitfall Traps in Relation to Mean Densities of Carabid Beetles. Oecologia 1979, 41, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Nentwig, W.; Blick, T.; Bosmans, R.; Gloor, D.; Hänggi, A.; Kropf, C. araneae Version 10. Available online: https://www.araneae.nmbe.ch (accessed on 26 October 2019).
- Kiss, B.; Samu, F. Evaluation of population densities of the common wolf spider Pardosa agrestis (Araneae: Lycosidae) in Hungarian alfalfa fields using mark-recapture. Eur. J. Entomol. 2000, 97, 191–195. [Google Scholar] [CrossRef]
- Rickers, S.; Scheu, S. Cannibalism in Pardosa palustris (Araneae, Lycosidae): effects of alternative prey, habitat structure, and density. Basic Appl. Ecol. 2005, 6, 471–478. [Google Scholar] [CrossRef]
- Wirta, H.K.; Weingartner, E.; Hambäck, P.A.; Roslin, T. Extensive niche overlap among the dominant arthropod predators of the High Arctic. Basic Appl. Ecol. 2014, 16, 86–92. [Google Scholar] [CrossRef]
- Morse, D.H. Distribution, Movement, and Activity Patterns of an Intertidal Wolf Spider Pardosa Lapidicina Population (Araneae, Lycosidae). J. Arachnol. 1997, 25, 1–10. [Google Scholar]
- Morse, D.H. Orientation and Movement of Wolf Spiders Pardosa Lapidicina (Araneae, Lycosidae) in the Intertidal Zone. J. Arachnol. 2002, 30, 601–609. [Google Scholar] [CrossRef]
- Bonte, D.; Lens, L. Heritability of spider ballooning motivation under different wind velocities. Evol. Ecol. Res. 2007, 9, 817–827. [Google Scholar]
- Rickers, S. Regulation of Wolf Spider Populations: The Role of Habitat Structure, Autochthonous and Allochthonous Prey; Technische Universität Darmstadt: Darmstadt, Germany, 2005. [Google Scholar]
- Kiss, B.; Samu, F. Comparison of Autumn and Winter Development of Two Wolf Spider Species (Pardosa, Lycosidae, Araneae) Having Different Life History Patterns. J. Arachnol. 2002, 30, 409–415. [Google Scholar] [CrossRef]
- Steigen, A.L. Energetics in a Population of Pardosa palustris (L.) (Araneae, Lycosidae) on Hardangervidda. In Fennoscandian Tundra Ecosystems. Part 2.; Wielgolaski, F.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 129–144. [Google Scholar]
- Atkinson, D.; Sibly, R.M. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997, 12, 235–239. [Google Scholar] [CrossRef]
- Hein, N.; Feilhauer, H.; Löffler, J.; Finch, O.-D. Elevational Variation of Reproductive Traits in Five Pardosa (Lycosidae) Species. Arctic, Antarct. Alp. Res. 2015, 47, 473–479. [Google Scholar] [CrossRef]
- Hein, N.; Brendel, M.R.; Feilhauer, H.; Finch, O.D.; Löffler, J. Egg size versus egg number trade-off in the alpine-tundra wolf spider, Pardosa palustris (Araneae: Lycosidae). Polar Biol. 2018, 41, 1607–1617. [Google Scholar] [CrossRef]
- Ottesen, P.S. Niche segregation of terrestrial alpine beetles (Coleoptera) in relation to environmental gradients and phenology. J. Biogeogr. 1996, 23, 353–369. [Google Scholar] [CrossRef]
- Hågvar, S.; Pedersen, A. Food Choice of Invertebrates during Early Glacier Foreland Succession. Arctic, Antarct. Alp. Res. 2015, 47, 561–572. [Google Scholar] [CrossRef]
- Talarico, F.; Giglio, A.; Pizzolotto, R.; Brandmayr, P. A synthesis of feeding habits and reproduction rhythm in Italian seed-feeding ground beetles (Coleoptera: Carabidae). Eur. J. Entomol. 2016, 325–336. [Google Scholar] [CrossRef]
- Hågvar, S.; Steen, R.; Flø, D. Ecology of alpine carabid beetles (Coleoptera, Carabidae) in a Norwegian glacier foreland, with a special focus on claw wearing to indicate relative age. Nor. J. Entomol. 2017, 64, 82–111. [Google Scholar]
- Andersen, J. The life cycles of carabid beetles (Coleoptera, Carabidae) in dry, open habitats north of 69°N, Northern Norway. Nor. J. Entomol. 2013, 60, 140–158. [Google Scholar]
- Hågvar, S. (Norwegian University of Life Sciences, Ås. Akershus, Norway). Personal communication. 2020. [Google Scholar]
- Bråten, A.T.; Flø, D.; Hågvar, S.; Hanssen, O.; Mong, C.E.; Aakra, K. Primary Succession of Surface Active Beetles and Spiders in an Alpine Glacier Foreland, Central South Norway. Arctic, Antarct. Alp. Res. 2012, 44, 2–15. [Google Scholar] [CrossRef]
- Vater, A.E. Insect and arachnid colonization on the Storbreen glacier foreland, Jotunheimen, Norway: Persistence of taxa suggests an alternative model of succession. Holocene 2012, 22, 1123–1133. [Google Scholar] [CrossRef]
- Freude, H.; Harde, K.-W.; Lohse, G.A.; Klausnitzer, B. Die Käfer Mitteleuropas; Elsevier; Spektrum Akademischer Verlag: München, Germany, 2012; ISBN 3827415519. [Google Scholar]
- Ameline, C.; Høye, T.T.; Bowden, J.J.; Hansen, R.R.; Hansen, O.L.P.; Puzin, C.; Vernon, P.; Pétillon, J. Elevational variation of body size and reproductive traits in high-latitude wolf spiders (Araneae: Lycosidae). Polar Biol. 2018, 41, 2561–2574. [Google Scholar] [CrossRef]
- Rasband, W. ImageJ; U. S. National Institutes of Health: Bethesda, MD, USA; p. 2016.
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ishiguro, M.; Kitagawa, G. Akaike Information Criterion Statistics; Reidel, D: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Ruß, G.; Brenning, A. Spatial Variable Importance Assessment for Yield Prediction in Precision Agriculture. In Proceedings of the Advances in Intelligent Data Analysis IX; Cohen, P.R., Adams, N.M., Berthold, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; p. 259. [Google Scholar]
- Brenning, A. Spatial cross-validation and bootstrap for the assessment of prediction rules in remote sensing: The R package sperrorest. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; 2012; pp. 5372–5375. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Saska, P.; Honek, A. Temperature and development of central European species of Amara (Coleoptera: Carabidae). Eur. J. Entomol. 2003, 100, 509–515. [Google Scholar] [CrossRef]
- Refseth, D. Ecological analyses of carabid communities- potential use in biological classification for nature conservation. Biol. Conserv. 1980, 17, 131–141. [Google Scholar] [CrossRef]
- König, T.; Kaufmann, R.; Scheu, S. The formation of terrestrial food webs in glacier foreland: Evidence for the pivotal role of decomposer prey and intraguild predation. Pedobiologia (Jena) 2011, 54, 147–152. [Google Scholar] [CrossRef]
- Sota, T. Altitudinal variation in life cycles of carabid beetles: Life-cycle strategy and colonization in Alpine Zones. Arctic, Antarct. Alp. Res. 1996, 28, 441–447. [Google Scholar] [CrossRef]
- Ashworth, A.C. The response of arctic Carabidae (Coleoptera) to climate change based on the fossil record of the Quaternary Period. Ann. Zool. Fennici 1996, 33, 125–131. [Google Scholar]
- Simon, J.; Mahéo, F.; Mieuzet, L.; Buchard, C.; Gauthier, J.; Maurice, D.; Bonhomme, J.; Outreman, Y.; Hullé, M. Life on the Edge: Ecological Genetics of a High Arctic Insect Species and Its Circumpolar Counterpart. Insects 2019, 10, 427. [Google Scholar] [CrossRef]
- Arthofer, W.; Rauch, H.; Thaler-Knoflach, B.; Moder, K.; Muster, C.; Schlick-Steiner, B.C.; Steiner, F.M. How diverse is Mitopus morio? Integrative taxonomy detects cryptic species in a small-scale sample of a widespread harvestman. Mol. Ecol. 2013, 22, 3850–3863. [Google Scholar] [CrossRef]
- Ameline, C.; Puzin, C.; Bowden, J.J.; Lambeets, K.; Vernon, P.; Pétillon, J. Habitat specialization and climate affect arthropod fitness: A comparison of generalist vs. specialist spider species in Arctic and temperate biomes. Biol. J. Linn. Soc. 2017, 121, 592–599. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed]
- Turin, H.; den Boer, P.J. Changes in the distribution of carabid beetles in The Netherlands since 1880. II. Isolation of habitats and long-term time trends in the occurence of carabid species with different powers of dispersal (Coleoptera, Carabidae). Biol. Conserv. 1988, 44, 179–200. [Google Scholar] [CrossRef]
- Nolte, D.; Boutaud, E.; Kotze, D.J.; Schuldt, A.; Assmann, T. Habitat specialization, distribution range size and body size drive extinction risk in carabid beetles. Biodivers. Conserv. 2019, 28, 1267–1283. [Google Scholar] [CrossRef]
| Variable | Type | Response/Explanatory | Description |
|---|---|---|---|
| Size [mm] 1 | - | Response | Pronotum width in Amara alpina, prosoma width in Pardosa palustris |
| Elevation [m] a.s.l. | Spatial | Fixed effect | ~1030–1618 m a.s.l. |
| Topography | Spatial | Fixed effect | Four positions (ridge, depression, south-facing slopes, north-facing slopes) |
| Season | Temporal | Fixed effect | Day of snowmelt (at each site) |
| Veg-1 | Biotic | Fixed effect | Abs. number of plant species per site |
| Evenness | Biotic | Fixed effect | Evenness of plant species frequency per site |
| Open Ground Cover (OGC) | Biotic | Fixed effect | Percent of open ground per site |
| Act-Site | Biotic | Fixed effect | Activity–abundance 1 of the respective species at each site through the season |
| Act-Sample | Biotic | Fixed effect | Activity–abundance 1 of the respective species for each sample within the season |
| 1 | Site | - | Random effect | UTM coordinates of the site |
| 1 | Sex | - | Random effect | Sex of the specimen (only used in models using both sexes, i.e., all specimens of a species) |
| Model | Type | Response | Predictor Variable | AIC | RMSE | R2m | R2c | |
|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||
| Model 1 1 | Baseline | All | All variables | - | −935.0 | 0.24 | - | - |
| Final | All | Elevation + Veg-1 + OGC | 1 | Site 1 | Sex | −74.5 | 0.23 | 0.3 | 0.64 | |
| Model 1F1 | Baseline | Females | All variables | - | −395.1 | 0.24 | - | - |
| Final | Females | Elevation + Veg-1 + OGC + Season | 1 | Site | −1.8 | 0.3 | 0.36 | 0.53 | |
| Model 1M1 | Baseline | Males | All variables | - | −550.1 | 0.23 | - | - |
| Final | Males | Elevation + Veg-1 + OGC + Act-sample | 1 | Site | −47.7 | 0.28 | 0.35 | 0.65 | |
| Model | Type | Response | Predictor Variable | AIC | RMSE | R2m | R2c | |
|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||
| Model 2 1 | Baseline | All | All variables | - | −2530.8 | 0.12 | - | - |
| Final | All | Season + Act-sample | 1 | Site 1 | Sex | −838.5 | 0.13 | 0.02 | 0.1 | |
| Model 2F1 | Baseline | Females | All variables | - | −1112.8 | 0.14 | - | - |
| Final | Females | Veg-1 + Evenness + Season | 1 | Site | −313.93 | 0.14 | 0.03 | 0.07 | |
| Model 2M1 | Baseline | Males | All variables | - | −1439.2 | 0.1 | - | - |
| Final | Males | Veg-1 + Season + Act-sample + Act-site | 1 | Site | −548.9 | 0.15 | 0.06 | 0.16 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckers, N.; Hein, N.; Anneser, A.; Vanselow, K.A.; Löffler, J. Differences in Mobility and Dispersal Capacity Determine Body Size Clines in Two Common Alpine-Tundra Arthropods. Insects 2020, 11, 74. https://doi.org/10.3390/insects11020074
Beckers N, Hein N, Anneser A, Vanselow KA, Löffler J. Differences in Mobility and Dispersal Capacity Determine Body Size Clines in Two Common Alpine-Tundra Arthropods. Insects. 2020; 11(2):74. https://doi.org/10.3390/insects11020074
Chicago/Turabian StyleBeckers, Niklas, Nils Hein, Alessa Anneser, Kim A. Vanselow, and Jörg Löffler. 2020. "Differences in Mobility and Dispersal Capacity Determine Body Size Clines in Two Common Alpine-Tundra Arthropods" Insects 11, no. 2: 74. https://doi.org/10.3390/insects11020074
APA StyleBeckers, N., Hein, N., Anneser, A., Vanselow, K. A., & Löffler, J. (2020). Differences in Mobility and Dispersal Capacity Determine Body Size Clines in Two Common Alpine-Tundra Arthropods. Insects, 11(2), 74. https://doi.org/10.3390/insects11020074




