Simple Summary
Mythimna loreyi is a serious pest of grain crops and reduces yields in maize plantations. M. loreyi is a native species in East Asia for a long time. However, this species has recently emerged as a migration (or invasive from other Asian countries) pest of some cereal crops in Korea. Little is known about its basic biology, ecology, and it is difficult to identify the morphologically similar species, Mythimna separate, which occur at the cornfield in the larvae stage. Species diagnosis methods for invasive pests have been developed and utilized for this reason. Currently, the molecular biology method for diagnosing M. loreyi species is only using the mtCO1 universal primer (LCO1490, HCO2198) and process PCR and sequencing to compare the degree of homology. However, this method requires a lot of time and effort. In this study, we developed the LAMP (loop-mediated isothermal amplification) assay for rapid, simple, and effective species diagnosis. By analyzing the mitochondrial (mt) genome, the species-specific sequence was found at the coding region of the NADH dehydrogenase subunit 5 gene. A broad range of DNA concentration was workable in LAMP assay, in which the minimum detectable DNA concentration was 100 pg. DNA releasing method was applied, which took five minutes of incubation at 95 °C without the DNA extraction process, and only some pieces of tissue from larvae and adult samples were needed. The incidence of invasive pests is gradually diversifying. Therefore, this simple and accurate LAMP assay is possibly used in the intensive field monitoring for invasive pests and integrated management of Mythimna loreyi.
Abstract
The Mythimna loreyi (Duponchel) is one of the well-known invasive noctuid pests in Africa, Australia, and many Asian countries. However, it is difficult to identify the invasive and morphologically similar species, Mythimna separate, which occur at the cornfield in the larvae stage. Currently, the molecular biology method for diagnosing M. loreyi species is only using the mtCO1 universal primer (LCO1490, HCO2198), which requires a lot of time and effort, such as DNA extraction, PCR, electrophoresis, and sequencing. In this study, the LAMP assay was developed for rapid, simple, effective species identification. By analyzing the mitochondrial genome, the species-specific sequence was found at the coding region of the NADH dehydrogenase subunit 5 gene. Based on this unique sequence, four LAMP primers and two loop primers were designed. The F3 and B3 primers were able to diagnose species-specific, in general, and multiplex PCR and specifically reacted within the inner primers in LAMP assay. The optimal incubation condition of the LAMP assay was 61 °C for 60 min with four LAMP primers, though additional loop primers, BF and LF, did not significantly shorten the amplification time. The broad range of DNA concentration was workable in LAMP assay, in which the minimum detectable DNA concentration was 100 pg. DNA releasing method was applied, which took five minutes of incubation at 95 °C without the DNA extraction process. Only some pieces of tissue of larvae and adult samples were needed to extract DNA. The incidence of invasive pests is gradually diversifying. Therefore, this simple and accurate LAMP assay is possibly applied in the intensive field monitoring for invasive pests and integrated management of Mythimna loreyi.
1. Introduction
The Mythimna loreyi (Duponchel) (often called the cosmopolitan) is a noctuid pest of grain crops found in Africa, Australia, the Near East, and the Middle East and undergoes multiple generations per year [1,2,3,4]. M. loreyi feeds on various host plants, including rice, wheat, maize, sugarcane, barley, sorghum, and others, which have a large effect on female fecundity. The fecundity of female moths is greatest when the larvae feed on maize in Egypt [5]. Since M. loreyi is facilitated to breed, some researchers focus on the identification of products secreted by the adult corpora allata [6]. Not only the physiological understanding of this species [7,8,9,10] but also ecology-based developmental characteristics [11] and flight performance [12] have been studied. For the biological control, M. loreyi densovirus (MIDNVs) was isolated in Egypt and characterized [13,14]. The outbreak of this pest and the damage to crops have been proliferated, particularly in some Asian countries. In Japan, M. loreyi typically occurs together with M. separate (Walker) that has significant negative effects on crop production [15]. Besides, M. loreyi has begun to occur and damage host plants together with M. separate [16].
During a couple of years in 2019 and 2020, there have been reports that cornfield has been damaged by the larvae of M. loreyi in Korea. This indicates that there is a possibility that M. loreyi can change into a sporadic pest, which can cause serious damage to crops. As per these cases, M. loreyi is occasionally damaged with its sister species, M. separate. However, it is difficult to distinguish two species at the cornfield in the larvae stage. Only one molecular diagnostic tool has been studied to distinguish M. loreyi, which is based on the sequencing of part of the mitochondrial COI gene [17]. However, only one mutation exists within the 658 bp amplicon. Moreover, high sequence similarity has been shown between M. loreyi and M. separata, which indicates the limitation of diagnosis of this pest.
In this study, we developed a simpler technique, termed loop-mediated isothermal amplification assay (LAMP). It is also widely used for the rapid and accurate identification of pest species [18,19,20]. The LAMP is a rapid, simple, effective, and specific amplification of DNA compared to real-time PCR based on the mitochondrial gene. It is performed under isothermal conditions that require a set of four primers, a strand-displacing DNA polymerase, and a water bath or heat block to maintain the temperature at about 65 °C following a one-time denaturation at 95 °C [21] or one-step incubation at about 65 °C [22].
Following the first infestation of M. loreyi in Korea, there is great demand from agricultural research, extension services, and farmers for diagnostic methods for these species. Therefore, we present a LAMP-based method for specimens collected in Korea and other sequences from GenBank. This method should be useful in assisting the effective pest management of M. loreyi.
2. Materials and Methods
2.1. Sample Collection and Mitochondrial Genome Sequencing
The larval stage of Mythimna loreyi Korean populations was collected from Hadong (35°02′17″ N, 127°47′12″ E) in a cornfield, 2019. Some larvae reared in the lab for morphological conformation in the adult stage, and the genomic DNA of several of the individual larva was directly extracted with DNAzol (Molecular Research Center, Cincinnati, OH, USA) and quantified by Nanodrop (NanoDrop Technologies, Wilmington, DE, USA). Besides, the genomic DNA of over 20 larvae or adults was extracted (population pooled genomic DNA). Universal primers (LCO1490 and HCO2198) were used with M. loreyi’s individual and pooled genomic DNA as templates in 20 μL PCR reaction containing 1 U TOYOBO KOD—FX TaqTM (Toyobo Life Science, Osaka, Japan), 2X buffer (with 15 mM MgCl2), 0.2 mM each dNTP, 0.5 μM each primer, and 100 ng genomic DNA [23]. The PCR products were directly sequenced (chromatogram) to verify the nucleotide polymorphism, and no mutation was found in intraspecies. For mitochondrial genome sequencing, the Miseq platform was used, and more than 1 Gb was sequenced. To assemble these data, the CLC Assembly Cell package (version 4.2.1) was used. After trimming raw data using CLC quality trim (ver. 4.21), the assembly was accomplished using the CLC de novo assembler with dnaLCW. Assembled sequences were confirmed by BLASTZ [24]. The GeSeq program was used for annotation [25], and the result was manually checked based on the alignment of other Noctuidae species mitochondrial genomes using MEGA 7 [26].
2.2. Phylogenetic Analysis and Primer Design
Molecular phylogenetic analysis of mitochondrion genomes was inferred by using the maximum likelihood method implemented by MEGA 7 with bootstrapping [26,27]. Mitochondrial genome sequences of other Noctuidae species were downloaded from GenBank, NCBI. For comparative analysis, mitochondrial genomes were aligned using mVISTA [28,29]. Based on the global alignment result, partial sequences were re-aligned for LAMP primer design using PrimerExplorer V5.
2.3. LAMP and PCR
WarmStart® LAMP Kit (New England Biolabs, Ipswich, UK) was used for the LAMP assay. The general protocol of LAMP was performed following the manufacture’s guidelines in a 25 μL reaction mixture. For the general PCR, TOYOBO KOD—FX TaqTM (Toyobo Life Science, Osaka, Japan) was used in this study. Appropriate primers were used with the following PCR amplification protocol: a 2 min denaturing step at 94 °C and a PCR amplification cycle consisting of denaturing at 94 °C for 20 s, annealing at 60 °C for 20 s, and extension at 68 °C for 30 s, which was repeated 35 times. The amplified DNA fragments were separated using 1.5% agarose gel electrophoresis and visualized with SYBR green (Life Technologies, Grand Island, NY, USA). The larval stage of M. loreyi samples, which were collected from Hadong (Korea) in a cornfield (2019), was used. Pheromone traps were used for adults’ sample collection, such as M. separate, Agrotis segetum, Spodoptera frugiperda, Spodoptera exigua, Spodoptera litura, and Helicoverpa armigera. Traps were set in Pyeongchang (37°40′53″ N, 128°43′49″ E), Hongchen (37°43′35″ N, 128°24′33″ E), and Gangneung (37°36′56″ N, 128°45′59″ E) [30]. DNA samples were prepared using DNAzol (Molecular Research Center, Cincinnati, OH, USA) from trapped adults. We used universal primers (LCO1490 and HCO2198) with each DNA sample and pooled genomic DNA as templates in a 20 μL PCR reaction containing 1 U TOYOBO KOD—FX TaqTM (Toyobo Life Science, Osaka, Japan), 2X buffer (with 15 mM MgCl2), 0.2 mM each dNTP, 0.5 μM each primer, and 100 ng genomic DNA [23]. The PCR products were directly sequenced to verify the mutation of each population using RAW_F3S and RAW_B3 primer set (MACROGEN). Three biological DNA samples that were collected from the fields were used in each LAMP and PCR to validate the reliability of the LAMP condition.
3. Results
3.1. Mitochondrial Genome Sequencing and Primer Design
A 15,320 bp of mitochondrial genome verified after trimming from about 2.2 Gb (7,312,504 reads) nucleotide sequences information was obtained through Miseq. The mitochondrial genome of Mythimna loreyi was assembled (GenBank MT506351). The mitochondrial genome included 13 protein-coding genes: NADH dehydrogenase components (complex I, ND), cytochrome oxidase subunits (complex VI, COX), cytochrome oxidase b (CYPB) and two ATP synthases, and two ribosomal RNA genes and 22 transfer RNAs (Supplementary Figure S1).
As a result of MegaBLAST, the most homologous species was an allied species, Mythimna separate, which showed 93.7% similarity. The genus of Spodoptera [31], such as Spodoptera exigua, S. litura, S. frugiperda, which are possibly found together with M. loreyi in the cornfields, showed about 89 to 90% homology based on the mitochondrial genome sequence. Agrotis segetum [32], which, in particular, occurs and damage at the corn seedling stage, showed 90.6% similarity to M. loreyi based on mitochondrial genome sequence (data not shown).
The phylogenetic relationship between 15 mitochondrial genomes of 14 species was examined (Figure 1A) to verify a specific nucleotide sequence that only M. loreyi possessed among the related species with high gene similarity or a morphologically similar pest. The result of the phylogenetic relationship was mostly similar to the megaBLAST result. Based on the mVISTA alignment results, conserved regions among Noctuidae species and variable regions were observed (Figure 1B). By combining the two results, the partial sequence in eight species of nine ND5 mitochondrial genome was re-aligned to design the specific primer of the M. loreyi. Finally, four essential primers and two loop primers were designed (Figure 2 and Table 1).
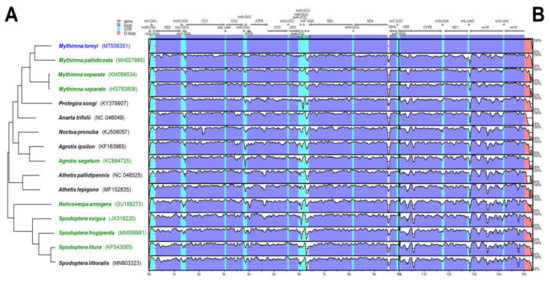
Figure 1.
Comparison of entire mitochondrial genomes of some Noctuidae pests, including newly sequenced Mythimna loreyi. (A) Phylogenetic relationship inferred using maximum likelihood under MEGA7. (B) Schematic diagram of the genes and their flanking regions, showing the sequence diversity in mVISTA. UTR, D-loop denotes untranslated region and displacement-loop, respectively. Eight green colored mitochondrial genome sequences were re-aligned for primer design with that of the target species, M. loreyi (Figure 2).
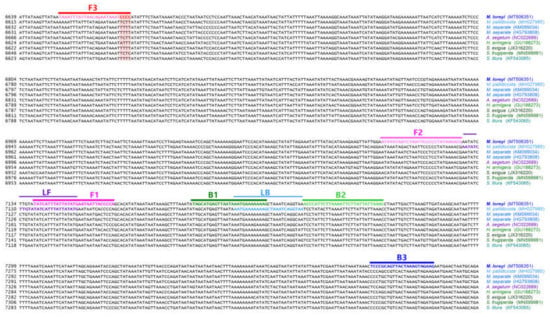
Figure 2.
Location of primers and primer binding regions on partial sequence of some Noctuidae pests’ mtDNA for species identification of M. loreyi. Inner primer, FIP, consists of F1c (complementary sequences of F1) and F2. Another inner primer, BIP, is also composed of B1 and B2c (complementary sequences of B2). Essential four LAMP primers (F3, FIP, BIP, and B3) generate the dumbbell structure, and two loop primers, LF and LB, possibly accelerate the LAMP (loop-mediated isothermal amplification) reaction. Used primer information is documented in Table 1.

Table 1.
Primer list for LAMP (loop-mediated isothermal amplification) and PCR in this study.
Among the six primers, F3 is the specific primer that enables the diagnosis of M. loreyi, and only M. loreyi had the CCCC sequences in four priming regions, which are marked in the red box. A total of four populations that were collected from different regions had the same nucleotide sequence. Therefore, it could be sufficiently used for species diagnosis. The basic species diagnosis primer production strategy is the same as the previously reported species diagnosis development method of S. frugiperda [33]. We targeted the open reading frame (ORF) region because the ORF region is more intraspecies conserved than the non-coding region.
3.2. Diagnostic LAMP and PCR
As previously reported, the sensitivity of LAMP may vary depending on the temperature and reaction time [33]. Therefore, the reaction was performed at 65, 63, and 61 °C to find the optimal reaction conditions (Figure 3).
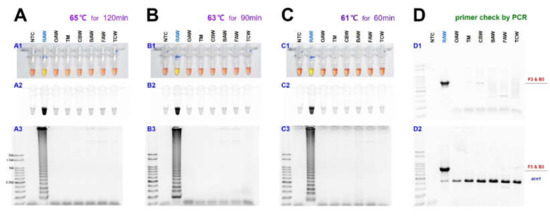
Figure 3.
The sensitivity of the LAMP assay results in three temperature conditions, such as 65, 63, and 61 °C, for M. loreyi species detected under (A1, B1, and C1) visible light, (A2, B2, and C2) ultraviolet light with SYBR Green, and (A3, B3, and C3) gel electrophoresis. The original pink color of the reaction mixture turned yellow in a positive reaction when the product was formed but remained pink in negative reactions. (D) Conventional and multiplex PCR to distinguish M. loreyi. The 794 bp amplicon was amplified only in M. loreyi, and the conserved partial sequence of ace1 type acetylcholinesterase gene was targeted as an internal reference. Abbreviations are NTC (non-template control), RAW (rice armyworm Mythimna loreyi), OAW (oriental armyworm Mythimna separata), TM (Turnip moth Agrotis segetum), CBW (cotton bollworm Helicoverpa armigera), BAW (beet armyworm Spodoptera exigua), FAW (fall armyworm Spodoptera frugiperda), and TCW (tobacco cutworm Spodoptera litura).
As the amount of template DNA was quantified as 50 ng and reacted at each temperature with 25 µL reaction volume, the diagnosis result was confirmed in 120 min at 65 °C and 90 min at 63 °C. The diagnostic level of reaction did not occur when the reaction performed less than the corresponding time in each temperature. Despite the relatively low temperature, at 61 °C, the diagnostic level of reaction was confirmed in only 60 min, and the false-positive reaction did not appear only once in the results of more than three repetition tests. Despite the relatively low temperature, at 61 °C, the diagnostic level of reaction was confirmed in only 60 min, and the false-positive reaction did not appear only once in three repetition tests (Figure 3C). Under 100 bp sized bands were not the LAMP products and were aggregated primers and generated even in the negative control.
In the LAMP assay, to confirm the diagnosis primer F3, it was reacted with reverse primer B3 through PCR, and the 794 bp of PCR product was identified (Figure 3(D1)). Besides, a species-specific reaction was confirmed, as we performed the multiplex PCR with a universal positive control primer set that targets ace1-type acetylcholinesterase, which can produce a positive reaction in all samples (Figure 3(D2)). The two-loop primers were tested for possible enhancement of the LAMP reaction, as suggested by Nagamine et al. [22].
As a result of reacting two-loop primers with each or two together at 61 °C for 60 min, there was no difference between the reaction result of using only four primers and shortening the reaction time (Figure 4). Even when 10 µL of the reaction solution was verified by electrophoresis after the LAMP reaction, there was no difference in band intensity. It was possible to reliably diagnose up to 100 pg when reacting using four LAMP primers without adding a loop primer (Figure 5A). Therefore, M. loreyi species diagnosis LAMP method that we developed could diagnose under various DNA concentration conditions from 100 ng to 100 pg. To increase the usability in the field, we cut a part of the tissue of the adult antenna or leg and put it in 30 µL distilled water, which reacts at 95 °C for 5 min, securing the template DNA without a separate DNA extraction process (Figure 5(B4)). As we measured each sample of DNA concentration obtained through this DNA releasing method, each sample showed various measurements. The species-specific diagnosis was possible in the same method as using the template DNA, which was obtained through separate DNA extraction as a positive control since it was in the range of LAMP reaction (Figure 5B).
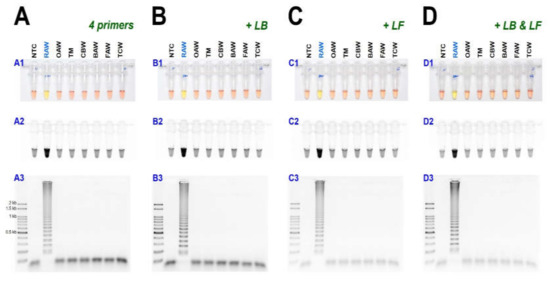
Figure 4.
The LAMP assay results with (A) 4 primers and additional loop primers, (B) loop forward, LF, or (C) loop backward, LB, or (D) two-loop primers, LF and LB under (A1, B1, C1, and D1) visible light, (A2, B2, C2, and D2) ultraviolet light with SYBR Green, and (A3, B3, C3, and D3) gel electrophoresis. Abbreviations are NTC (non-template control), RAW (rice armyworm Mythimna loreyi), OAW (oriental armyworm Mythimna separata), TM (Turnip moth Agrotis segetum), CBW (cotton bollworm Helicoverpa armigera), BAW (beet armyworm Spodoptera exigua), FAW (fall armyworm Spodoptera frugiperda), and TCW (tobacco cutworm Spodoptera litura).
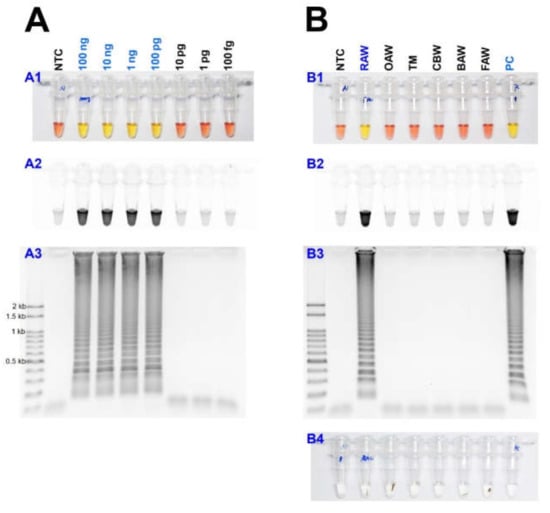
Figure 5.
(A) Identification of the detection limit of genomic DNA in the LAMP assay from 100 ng to 100 fg under (A1 and B1) visible light, (A2 and B2) ultraviolet light with SYBR Green, and (A3 and B3) gel electrophoresis. (B) The sensitivity of the LAMP assay results with the DNA releasing technique from insect tissue. Around 10 mg of the adult leg (or antenna) was incubated at 95 °C for 5 min (B4). Abbreviations are NTC (non-template control), RAW (rice armyworm Mythimna loreyi), OAW (oriental armyworm Mythimna separata), TM (Turnip moth Agrotis segetum), CBW (cotton bollworm Helicoverpa armigera), BAW (beet armyworm Spodoptera exigua), FAW (fall armyworm Spodoptera frugiperda), and PC (positive control, isolated DNA from M. loreyi).
4. Discussion
Invasive pests, such as S. frugiperda, are increasing worldwide due to global warming and climate change [34,35]. Currently, M. loreyi is a serious grain pest in Africa, Australia, the Near East, and the Middle East and undergoes multiple generations per year [1,2,3,4]. Besides, M. loreyi is a species that originates from China and results in damage upon spreading gradually. In Korea, M. loreyi has been reported for a long time and maybe a native pest to Korea. Recently, there are several reports that the larvae of M. loreyi have damaged the cornfield in Korea. There is a possibility that M. loreyi can change into a sporadic pest, which can cause serious damage to crops in Korea.
As with other invasive pests found in the field, they are often similar in morphology to allied species, making the investigation of initial density and pest management difficult. Species diagnosis methods for invasive pests, such as S. frugiperda, have been developed and utilized for this reason [33]. Currently, the molecular biology method for diagnosing M. loreyi species is only using the mtCO1 universal primer (LCO1490, HCO2198) and process PCR and sequencing to compare the degree of homology. However, this method requires a lot of time and effort, such as DNA extraction, PCR, electrophoresis, and sequencing. Therefore, we developed a molecular diagnosis method that could diagnose species within a short time without a separate DNA extraction process, and only a heat block is needed that can control temperature (Figure 5). The basis diagnosis strategy is very similar to the S. frugiperda species diagnosis method [33]. This method can complete all experimental procedures and verify the results within 1 h and 30 min right after obtaining a sample. In this study, the results only specified adult samples, but it is possible to use larvae.
The simplicity, accuracy, and adaptability for high throughput of the LAMP assay are distinct advantages [21,36,37]. Moreover, recently LAMP is utilized in various fields, such as many ecology studies, medical aspects, outside of the lab, and can be applied to diagnose plant viruses in insect body and insecticide-resistant gene mutation [38,39]. Besides, the diagnostic primer used for LAMP can be used for various diagnostic methods because it was possible to apply in general PCR and multiplex PCR (Figure 3). Therefore, it is feasible to diagnose a larger sample with positive control in the form of multiplex PCR, which is sufficiently modified and used in the laboratory. There are advantages and disadvantages to find a species diagnostic marker in the mitochondrial genome as well as in part of the genomic DNA. First of all, the disadvantage is that LAMP primer production is limited because there are many parts of AT-rich. It is complicated to design a primer that requires a minimum of four primers, and primers FIP and BIP are high-performance liquid chromatography (HPLC) purified primers. As an advantage, LAMP has higher amplification and efficiency and sensitivity compared to real-time PCR, and the results can be visually monitored by the naked eye either through turbidity or color change by fluorescent intercalating dye (Syber Green I) [40]. The number of copies is large, and it is possible to diagnose with a small amount of DNA or DNA releasing method, whose amplification can be accomplished with heating block. On the contrary, a species diagnosis marker in the genomic DNA should be found in exon rather than intron because it has a large variation. But it is difficult to develop marker in exon because it is often quite conserved within an allied species. Besides, the DNA releasing method can be used, but the efficiency is low when the copies of the gene are small [41].
5. Conclusions
In this study, a species diagnosis marker was designed within the mitochondrial genome to combine it with a DNA releasing method that is highly applicable to diagnose species in the field. Moreover, a significantly efficient method was developed, which targeted the ace1 gene as a positive control of the species to compare. This simple and accurate diagnosis using LAMP assay could be possibly applied in the intensive field to monitor and for the pest management of M. loreyi.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/11/817/s1, Figure S1: Organization of the mitochondrial genome of Mythimna loreyi from Korea (GenBank MT506351). ND: NADH dehydrogenase components (Complex I) in yellow. COX: cytochrome oxidase subunits (Complex VI) in pink. ATP synthase in green. CYPB: cytochrome oxidase b in purple. Ribosomal RNA genes in red, tRNA genes in blue. Noncoding regions are not colored.
Author Contributions
Conceptualization, H.Y.N. and J.K.; methodology, H.Y.N. and J.K.; software, J.K.; validation, J.K.; formal analysis, J.K.; investigation, H.Y.N. and J.K.; resources, H.Y.N., M.K., H.J.K., and J.K.; data curation, H.Y.N. and J.K.; writing—Original draft preparation, H.Y.N. and J.K.; writing—Review and editing, H.Y.N. and J.K.; visualization, H.Y.N. and J.K.; supervision, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
The Cooperative Research Program supported this study for Agriculture Science and Technology Development (Project No. PJ01509301), the Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calora, F.B. Revision of the species Leucania-complex occurring in the Philippines (Lepidoptera, Noctuidae, Hadeninae). Philipp. Agric. 1966, 50, 633–723. [Google Scholar]
- Chandler, K.J.; Benson, A.J. Evaluation of armyworm infestation in North Queensland surgarcane on crops. Proc. Aust. Soc. Sug. Cane Technol. 1991, 13, 79–82. [Google Scholar]
- Edwards, E.D. A second sugarcane armyworm (Leucania loreyi (Duponchel) from Australia and the identity of L. loreyimima Rungs (Lepidoptera: Noctuidae). J. Aust. Entomol. Soc. 1992, 31, 105–108. [Google Scholar] [CrossRef]
- Ganesha, S.; Rajabale, S. The Mythimna spp. (Lepidoptera: Noctuidae) complex on sugarcane in Mauritius. Proc. S. Afr. Sug. Technol. Ass. 1996, 70, 15–17. [Google Scholar]
- El-Sherif, S.I. On the biology of Leucania loreyi, dup. (Lepidoptera, Noctuidae). J. Appl. Entomol. 1972, 71, 104–111. [Google Scholar] [CrossRef]
- Ho, H.Y.; Tu, M.P.; Chang, C.Y.; Yin, C.M.; Kou, R. Identification of in vitro release products of corpora allata in female and male loreyi leafworms, Leucania loreyi. Experientia 1995, 51, 601–605. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Hsu, E.L.; Chow, Y.S.; Kou, R. Effects of calcium channel antagonists on the corpora allata of adult male loreyi leafworm Mythimna loreyi: Juvenile hormone acids release and intracellular calcium level. Arch. Insect Biochem. Physiol. 2001, 48, 89–99. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Yang, E.C.; Hsu, E.L.; Chow, Y.S.; Kou, R. Voltage-dependent calcium channels in the corpora allata of the adult male loreyi leafworm, Mythimna loreyi. Insect Biochem. Mol. Biol. 2002, 32, 547–557. [Google Scholar] [CrossRef]
- Kou, R. Cholinergic regulation of the corpora allata in adult male loreyi leafworm Mythimna loreyi. Arch. Insect Biochem. Physiol. 2002, 49, 215–224. [Google Scholar] [CrossRef]
- Kou, R.; Chen, S.J. Allatotropic activity in the suboesophageal ganglia and corpora cardiaca of the adult male loreyi leafworm, Mythimna loreyi. Arch. Insect Biochem. Physiol. 2000, 43, 78–86. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, L.; Liu, Y.; Sappington, T.W.; Cheng, Y.; Luo, L.; Jiang, X. Population Projection and Development of the Mythimna loreyi (Lepidoptera: Noctuidae) as Affected by Temperature: Application of an Age-Stage, Two-Sex Life Table. J. Econ. Entomol. 2017, 110, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, Y.; Zhang, L.; Cheng, Y.; Sappington, T.W.; Jiang, X. Effects of Moth Age and Rearing Temperature on the Flight Performance of the Loreyi Leafworm, Mythimna loreyi (Lepidoptera: Noctuidae), in Tethered and Free Flight. J. Econ. Entomol. 2018, 111, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Li, Y.; Fediere, G.; Abol-Ela, S.; Tijssen, P. Lack of infection of vertebrate cells by the densovirus from the maize worm Mythimna loreyi (MlDNV). Virus Res. 2014, 99, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fediere, G.; El-Far, M.; Li, Y.; Bergoin, M.; Tijssen, P. Expression strategy of densonucleosis virus from Mythimna loreyi. Virology 2004, 320, 181–189. [Google Scholar] [CrossRef]
- Hirai, K. The influence of rearing temperature and density on the development of two Leucania species, M. loreyi dup. and L. separata walker (Lepidoptera: Noctuidae). Appl. Ent. Zool. 1975, 10, 234–237. [Google Scholar] [CrossRef]
- Guo, S.J.; Li, S.M.; Ma, L.P.; Zhuo, X.N. Research about biological characteristics and damage laws of Leucania loreyi. J. Henan Agric. Sci. 2003, 9, 37–39. [Google Scholar]
- Jindal, V. DNA barcode reveals occurrence of Mythimna loreyi (Duponchel) in Punjab, India. Indian J. Biotechnol. 2019, 18, 81–84. [Google Scholar]
- Blaser, S.; Diem, H.; von Felten, A.; Gueuning, M.; Andreou, M.; Boonham, N.; Tomlinson, J.; Müller, P.; Utzinger, J.; Frey, J.E.; et al. From laboratory to point of entry: Development and implementation of a loop-mediated isothermal amplification (LAMP)-based genetic identification system to prevent introduction of quarantine insect species. Pest Manag. Sci. 2018, 74, 1504–1512. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Wang, H.Y.; Chen, Y.F.; Ko, C.C. Loop-mediated isothermal amplification for rapid identification of biotypes B and Q of the globally invasive pest Bemisia tabaci, and studying population dynamics. Pest. Manag. Sci. 2012, 68, 1206–1213. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hur, J.H.; Lee, G.S.; Choi, M.Y.; Koh, Y.H. Rapid and highly accurate detection of Drosophila suzukii, spotted wing Drosophila (Diptera: Drosophilidae) by loop-mediated isothermal amplification assays. J. Asia Pac. Entomol. 2016, 19, 1211–1216. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Schwartz, S.; Kent, W.J.; Smit, A.; Zhang, Z.; Baertsch, R.; Hardison, R.C.; Haussler, D.; Miller, W. Human-mouse alignments with BLASTZ. Genome Res. 2003, 13, 103–107. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Wojciechowski, M.F. Improved bootstrap confidence limits in large-scale phylogenies, with an example from Neo-Astragalus (Leguminosae). Syst. Biol. 2000, 49, 671–685. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Patcher, L.S.; Dubchak, I. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, M.; Park, K.J.; Maharjanm, R. Monitoring of four major lepidopteran pests in Korean cornfields and management of Helicoverpa armigera. Entomol. Res. 2018, 48, 308–316. [Google Scholar] [CrossRef]
- CABI Invasive Species Compendium. Available online: https://www.cabiorg/isc/search/index (accessed on 14 August 2020).
- Erasmus, A.; Van Rensburg, J.B.J.; Van den Berg, J. Effects of Bt maize on Agrotis segetum (Lepidoptera: Noctuidae): A pest of maize seedlings. Environ. Entomol. 2010, 39, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kwon, M.; Kim, H.; Yi, H.J.; Hänniger, S.; Unbehend, M.; Heckel, D.G. Development of a simple and accurate molecular tool for Spodoptera frugiperda species identification using LAMP. bioRxiv 2020. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.-F.; Ma, J.; Gao, B.-Y.; Wu, Q.-L.; Chen, A.-D.; Liu, J.; Jiang, Y.-Y.; Zhai, B.-P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2010, 11, e0165632. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and costeffective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef]
- Lee, P.L. DNA amplification in the field: Move over PCR, here comes LAMP. Mol. Ecol. Resour. 2017, 17, 138–141. [Google Scholar] [CrossRef]
- Choi, B.H.; Hur, J.H.; Heckel, D.G.; Kim, J.; Koh, Y.H. Development of a highly accurate and sensitive diagnostic tool for pyrethroid-resistant chimeric P450 CYP337B3 of Helicoverpa armigera using loop-mediated isothermal amplification. Arch. Insect Biochem. Physiol. 2018, 99, e21504. [Google Scholar] [CrossRef]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.V.L.; Morita, K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef]
- Kim, J.; Cha, D.J.; Kwon, M.; Maharjan, R. Potato virus Y (PVY) detection in a single aphid by one-step RT-PCR with boiling technique. Entomol. Res. 2016, 46, 278–285. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).