Modelling Drosophila suzukii Adult Male Populations: A Physiologically Based Approach with Validation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Males Population Modelling
2.2. Development, Fertility and Mortality Rate Functions
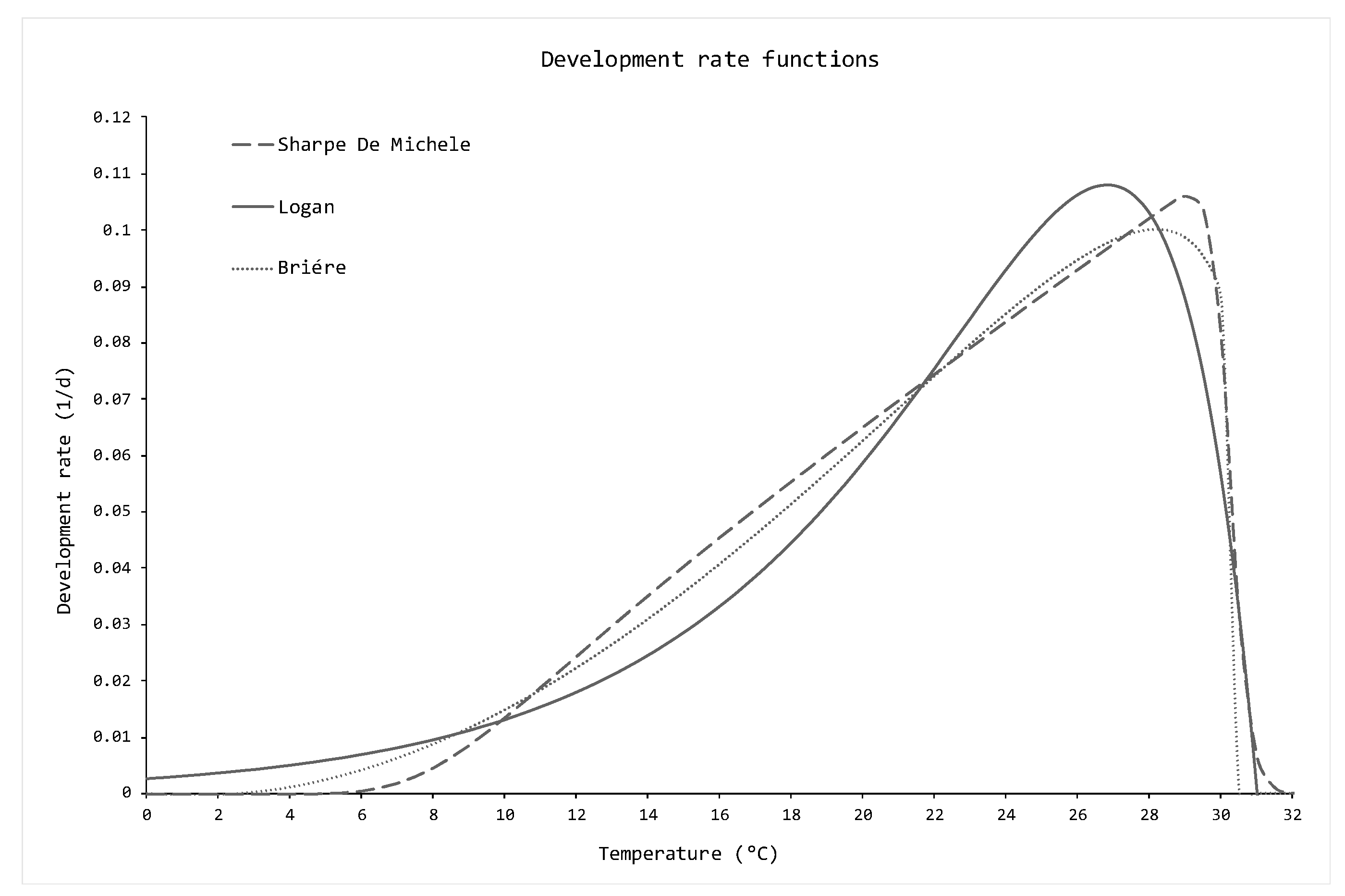
2.2.1. Development Rate Functions
- The Brière development rate function [39]:where is the environmental temperature, and are empirical parameters, and are the upper and lower thermal thresholds, respectively, below and above which the development of the species is theoretically not possible.
- The Logan development rate function [40]:where is the environmental temperature, and are empirical parameters, is the upper thermal threshold above which the development of the species is theoretically not possible, and is the range between the maximum of the function and .
2.2.2. Mortality Rate Function
2.2.3. Fertility Rate Function
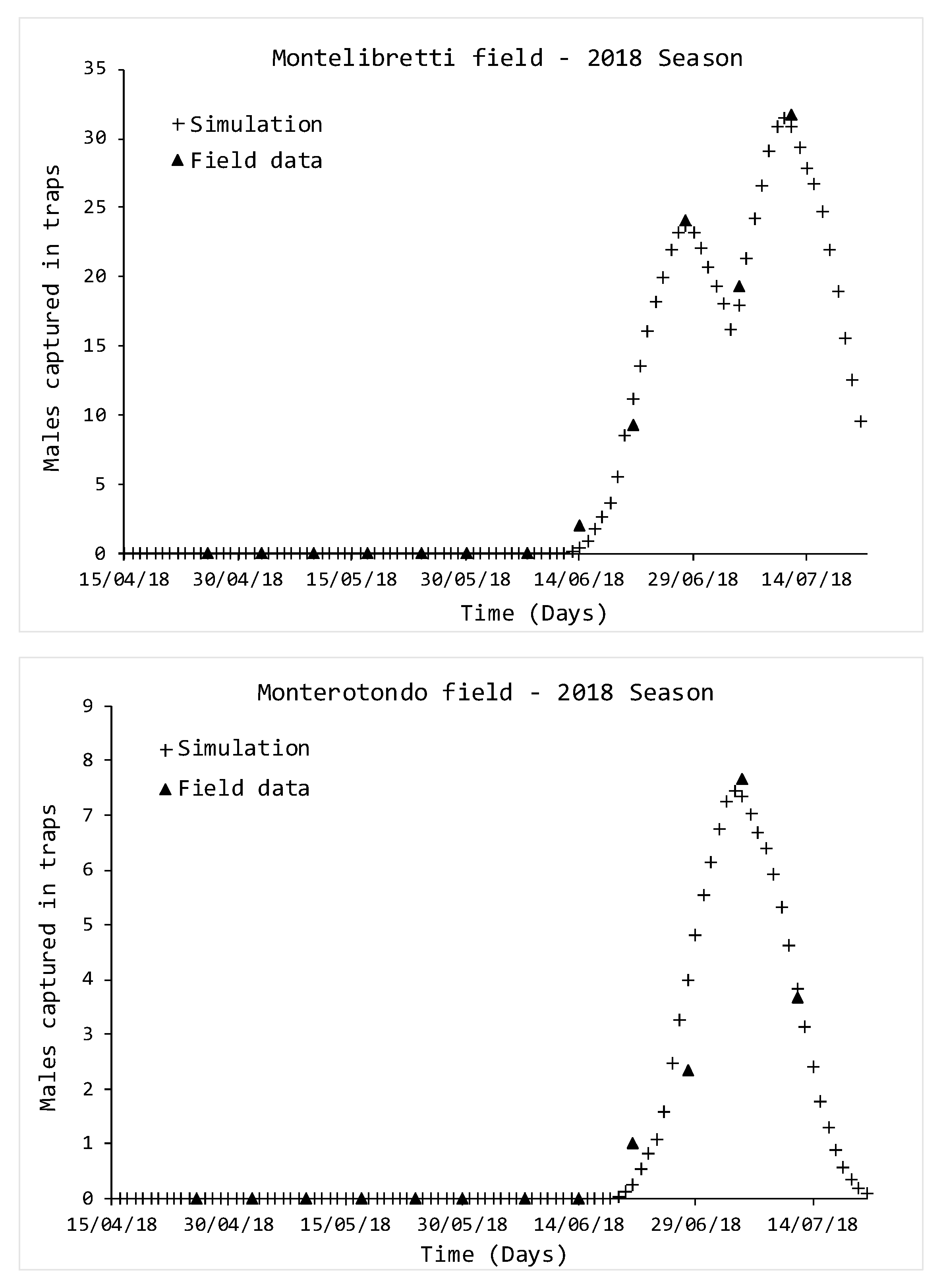
2.3. Data Analysis and Comparison between Simulations and Field Data
2.4. Experimental Design for Model Validation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanzawa, T. Studies on Drosophila suzukii Mats. In Review of Applied Entomology; Wiley: Hoboken, NJ, USA, 1939; Available online: https://www.cabdirect.org/cabdirect/abstract/19410501073 (accessed on 11 October 2020).
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/ (accessed on 11 October 2020).
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Grassi, A.; Giongo, L.; Palmieri, L.; Giongo, L.; Palmieri, L. Drosophila (Sophophora) suzukii (Matsumura) (Diptera: Drosophilidae), new pest of soft fruits in Trentino (North-Italy) and in Europe. IOBC/WPRS Bull. 2011, 70, 121–128. [Google Scholar]
- Ioratti, C.; Frontuto, A.; Grassi, A.; Anfora, G.; Simoni, S. Drosophila suzukii, (Matsumura), una Nuova Specie Invasiva Dannosa Alle Colture di Piccoli Frutti; Un’emergenza fitosanitaria per la fragola e la frutticoltura montana: Drosophila suzukii: Firenze, Italy, 2011; Volume 8, pp. 69–80. [Google Scholar]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted wing Drosophila: Potential economic impact of a newly established pest. Agric. Resour. Econ. Update 2010, 13, 5–8. [Google Scholar]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Tait, G.; Grassi, A.; Pfab, F.; Crava, C.M.; Dalton, D.T.; Magarey, R.; Ometto, L.; Vezzulli, S.; Rossi-Stacconi, M.V.; Gottardello, A.; et al. Large-scale spatial dynamics of Drosophila suzukii in Trentino, Italy. J. Pest Sci. 2018, 91, 1213–1224. [Google Scholar] [CrossRef]
- Lee, J.C.; Wang, X.; Daane, K.M.; Hoelmer, K.A.; Isaacs, R.; Sial, A.A.; Walton, V.M. Biological control of spotted-wing Drosophila (Diptera: Drosophilidae)—Current and pending tactics. J. Integr. Pest Manag. 2019, 10. [Google Scholar] [CrossRef]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef]
- Ryan, G.D.; Emiljanowicz, L.; Wilkinson, F.; Kornya, M.; Newman, J.A. Thermal tolerances of the spotted-wing Drosophila Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2016, 109, 746–752. [Google Scholar] [CrossRef]
- Eben, A.; Reifenrath, M.; Briem, F.; Pink, S.; Vogt, H. Response of Drosophila suzukii (Diptera: Drosophilidae) to extreme heat and dryness. Agric. Forest Entomol. 2018, 20, 113–121. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Cuthbertson, A.; Blackburn, L.; Audsley, N. Efficacy of commercially available invertebrate predators against Drosophila suzukii. Insects 2014, 5, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-G.; Serrato, M.A.; Son, Y.; Walton, V.M.; Hogg, B.N.; Daane, K.M. Thermal performance of two indigenous pupal parasitoids attacking the invasive Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2018, 47, 764–772. [Google Scholar] [CrossRef]
- Gabarra, R.; Riudavets, J.; Rodríguez, G.A.; Pujade-Villar, J.; Arnó, J. Prospects for the biological control of Drosophila suzukii. BioControl 2015, 60, 331–339. [Google Scholar] [CrossRef]
- Hiebert, N.; Carrau, T.; Bartling, M.; Vilcinskas, A.; Lee, K. Identification of entomopathogenic bacteria associated with the invasive pest Drosophila suzukii in infested areas of Germany. J. Invertebr. Pathol. 2020, 173, 107389. [Google Scholar] [CrossRef]
- Ibouh, K.; Oreste, M.; Bubici, G.; Tarasco, E.; Rossi Stacconi, M.V.; Ioriatti, C.; Verrastro, V.; Anfora, G.; Baser, N. Biological control of Drosophila suzukii: Efficacy of parasitoids, entomopathogenic fungi, nematodes and deterrents of oviposition in laboratory assays. Crop Prot. 2019, 125, 104897. [Google Scholar] [CrossRef]
- Wang, X.-G.; Stewart, T.J.; Biondi, A.; Chavez, B.A.; Ingels, C.; Caprile, J.; Grant, J.A.; Walton, V.M.; Daane, K.M. Population dynamics and ecology of Drosophila suzukii in Central California. J. Pest Sci. 2016, 89, 701–712. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tonina, L.; Marini, L.; Duso, C.; Mori, N. Integrated management of Drosophila suzukii in sweet cherry orchards. Entomologia Generalis 2020, 40, 297–305. [Google Scholar] [CrossRef]
- Coop, L. Online Phenology and Degree-Day Model for Agricultural and Decision-Making in the US; Integrated Plant Protection Center, Botany & Plant Pathology Dep. Oregon State University: Corvallis, OR, USA, 2010; Available online: http://uspest.org/risk/models?spp=swd (accessed on 11 October 2020).
- Damus, M. Some Preliminary Results from Climex and Maxent Distribution Modeling of Drosophila suzukii, Version 2; CFIA Plant Health Risk Assessment: Ottawa, ON, Canada, 2009. [Google Scholar]
- Rossini, L.; Severini, M.; Contarini, M.; Speranza, S. A novel modelling approach to describe an insect life cycle vis-à-vis plant protection: Description and application in the case study of Tuta absoluta. Ecol. Model. 2019, 409, 108778. [Google Scholar] [CrossRef]
- Rossini, L.; Severini, M.; Contarini, M.; Speranza, S. EntoSim, a ROOT-based simulator to forecast insects’ life cycle: Description and application in the case of Lobesia botrana. Crop Prot. 2020, 129, 105024. [Google Scholar] [CrossRef]
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Rossini, L.; Contarini, M.; Severini, M.; Talano, D.; Speranza, S. A modelling approach to describe the Anthonomus eugenii (Coleoptera: Curculionidae) life cycle in plant protection: A priori and a posteriori analysis. Fla. Entomol. 2020, 103, 259–263. [Google Scholar] [CrossRef]
- Rossini, L.; Severini, M.; Contarini, M.; Speranza, S. Use of ROOT to build a software optimized for parameter estimation and simulations with Distributed Delay Model. Ecol. Inform. 2019, 50, 184–190. [Google Scholar] [CrossRef]
- Rossini, L.; Speranza, S.; Contarini, M. Distributed Delay Model and Von Foerster’s equation: Different points of view to describe insects’ life cycles with chronological age and physiological time. Ecol. Inform. 2020, 59, 101117. [Google Scholar] [CrossRef]
- Rossini, L.; Contarini, M.; Severini, M.; Speranza, S. Reformulation of the Distributed Delay Model to describe insect pest populations using count variables. Ecol. Model. 2020, 436, 109286. [Google Scholar] [CrossRef]
- Rossini, L.; Contarini, M.; Speranza, S. A novel version of the Von Foerster equation to describe poikilothermic organisms including physiological age and reproduction rate. Ric. Mat. 2020. [Google Scholar] [CrossRef]
- Cern ROOT Cern Web Page. Available online: http://root.cern.ch (accessed on 30 October 2020).
- Brun, R.; Rademakers, F. ROOT—An object oriented data analysis framework. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1997, 389, 81–86. [Google Scholar] [CrossRef]
- Ikemoto, T.; Kiritani, K. Novel method of specifying low and high threshold temperatures using thermodynamic SSI model of insect development. Environ. Entomol. 2019, 48, 479–488. [Google Scholar] [CrossRef]
- Mirhosseini, M.A.; Fathipour, Y.; Reddy, G.V.P. Arthropod development’s response to temperature: A review and new software for modeling. Ann. Entomol. Soc. Am. 2017, 110, 507–520. [Google Scholar] [CrossRef]
- Damos, P.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche 2012, 2012. [Google Scholar] [CrossRef]
- Severini, M.; Gilioli, G. Storia e filosofia dei modelli di simulazione nella difesa delle colture agrarie. Not. Sulla Prot. Delle Piante 2002, 15, 9–29. [Google Scholar]
- Briére, J.-F.; Pracros, P.; Le Roux, A.-Y.; Pierre, J.-S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Sharpe, P.J.H.; DeMichele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Schoolfield, R.M.; Sharpe, P.J.H.; Magnuson, C.E. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 1981, 88, 719–731. [Google Scholar] [CrossRef]
- Wagner, T.L.; Wu, H.-I.; Sharpe, P.J.H.; Coulson, R.N. Modeling distributions of Insect development time: A literature review and application of the weibull function. Ann. Entomol. Soc. Am. 1984, 77, 475–483. [Google Scholar] [CrossRef]
- Harcourt, D.G. Development and use of life tables in study of natural insect populations. Annu. Rev. Entomol. 1969, 14, 175. [Google Scholar] [CrossRef]
- Brun, R.; Rademakers, F. ROOT User’s Guide. Available online: https://root.cern.ch/guides/users-guide (accessed on 30 October 2020).
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef]
- Kinjo, H.; Kunimi, Y.; Nakai, M. Effects of temperature on the reproduction and development of Drosophila suzukii (Diptera: Drosophilidae). Appl. Entomol. Zool. 2014, 49, 297–304. [Google Scholar] [CrossRef]
- Bieri, M.; Baumgärtner, J.; Bianchi, G.; Delucchi, V.; Arx, R. Development and fecundity of pea aphid (Acyrthosiphon pisum Harris) as affected by constant temperatures and by pea varieties. Mitt. Schweiz. Entomol. Ges. 1983, 56, 163–171. [Google Scholar]
- Revadi, S.; Lebreton, S.; Witzgall, P.; Anfora, G.; Dekker, T.; Becher, P. Sexual behavior of Drosophila suzukii. Insects 2015, 6, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Lin, Q.; Zhang, J.; Zhang, F.; Zheng, L.; Yu, Y. Adult reproductive diapause in Drosophila suzukii females. J. Pest Sci. 2016, 89, 679–688. [Google Scholar] [CrossRef]
- Panel, A.; Zeeman, L.; Van der Sluis, B.; Van Elk, P.; Pannebakker, B.; Wertheim, B.; Helsen, H. Overwintered Drosophila suzukii are the main source for infestations of the first fruit crops of the season. Insects 2018, 9, 145. [Google Scholar] [CrossRef]
- Thistlewood, H.M.A.; Gill, P.; Beers, E.H.; Shearer, P.W.; Walsh, D.B.; Rozema, B.M.; Acheampong, S.; Castagnoli, S.; Yee, W.L.; Smytheman, P.; et al. Spatial analysis of seasonal dynamics and overwintering of Drosophila suzukii (Diptera: Drosophilidae) in the Okanagan-Columbia Basin, 2010–2014. Environ. Entomol. 2018, 47, 221–232. [Google Scholar] [CrossRef]


| Parameter | Numerical Value | Parameter | Numerical Value |
|---|---|---|---|
| Rate Function | Parameters | NDF (n) | ||
|---|---|---|---|---|
| Brière | ||||
| Logan | ||||
| Sharpe and De Michele | ||||
| Growing Season | Experimental Field | ||
|---|---|---|---|
| Montelibretti | |||
| Monterotondo | - | - | |
| Montelibretti | |||
| Monterotondo | |||
| Montelibretti | |||
| Monterotondo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, L.; Contarini, M.; Giarruzzo, F.; Assennato, M.; Speranza, S. Modelling Drosophila suzukii Adult Male Populations: A Physiologically Based Approach with Validation. Insects 2020, 11, 751. https://doi.org/10.3390/insects11110751
Rossini L, Contarini M, Giarruzzo F, Assennato M, Speranza S. Modelling Drosophila suzukii Adult Male Populations: A Physiologically Based Approach with Validation. Insects. 2020; 11(11):751. https://doi.org/10.3390/insects11110751
Chicago/Turabian StyleRossini, Luca, Mario Contarini, Federica Giarruzzo, Matteo Assennato, and Stefano Speranza. 2020. "Modelling Drosophila suzukii Adult Male Populations: A Physiologically Based Approach with Validation" Insects 11, no. 11: 751. https://doi.org/10.3390/insects11110751
APA StyleRossini, L., Contarini, M., Giarruzzo, F., Assennato, M., & Speranza, S. (2020). Modelling Drosophila suzukii Adult Male Populations: A Physiologically Based Approach with Validation. Insects, 11(11), 751. https://doi.org/10.3390/insects11110751








