The Ant Who Cried Wolf? Short-Term Repeated Exposure to Alarm Pheromone Reduces Behavioral Response in Argentine Ants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ant Colony Collection
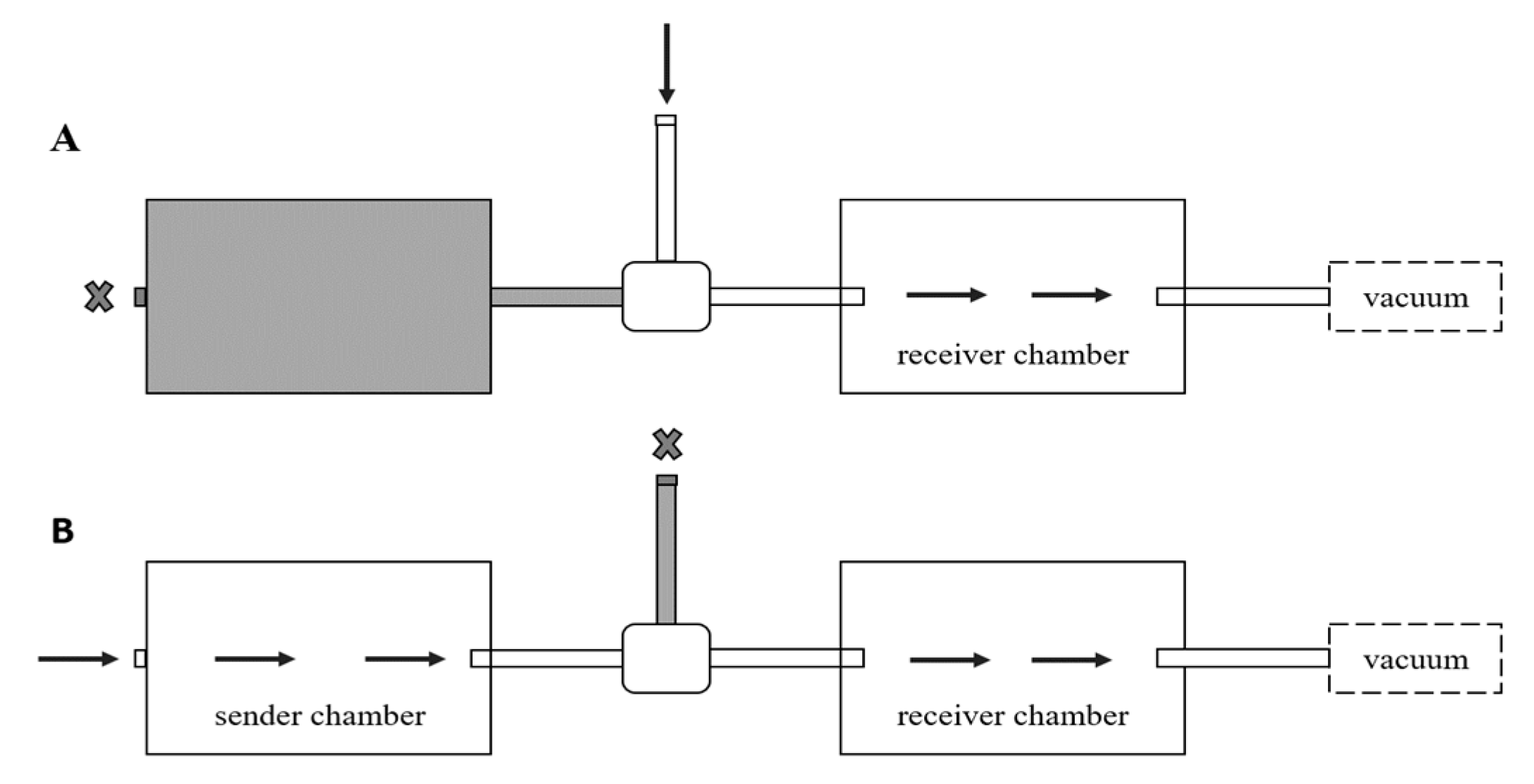
2.2. Olfactometer
2.3. Defining Alarm Behaviors
2.4. Testing Effect of Alarm Pheromone
2.4.1. Single Exposure, Live Ant Pheromone
2.4.2. Single Exposure, Synthetic Pheromone
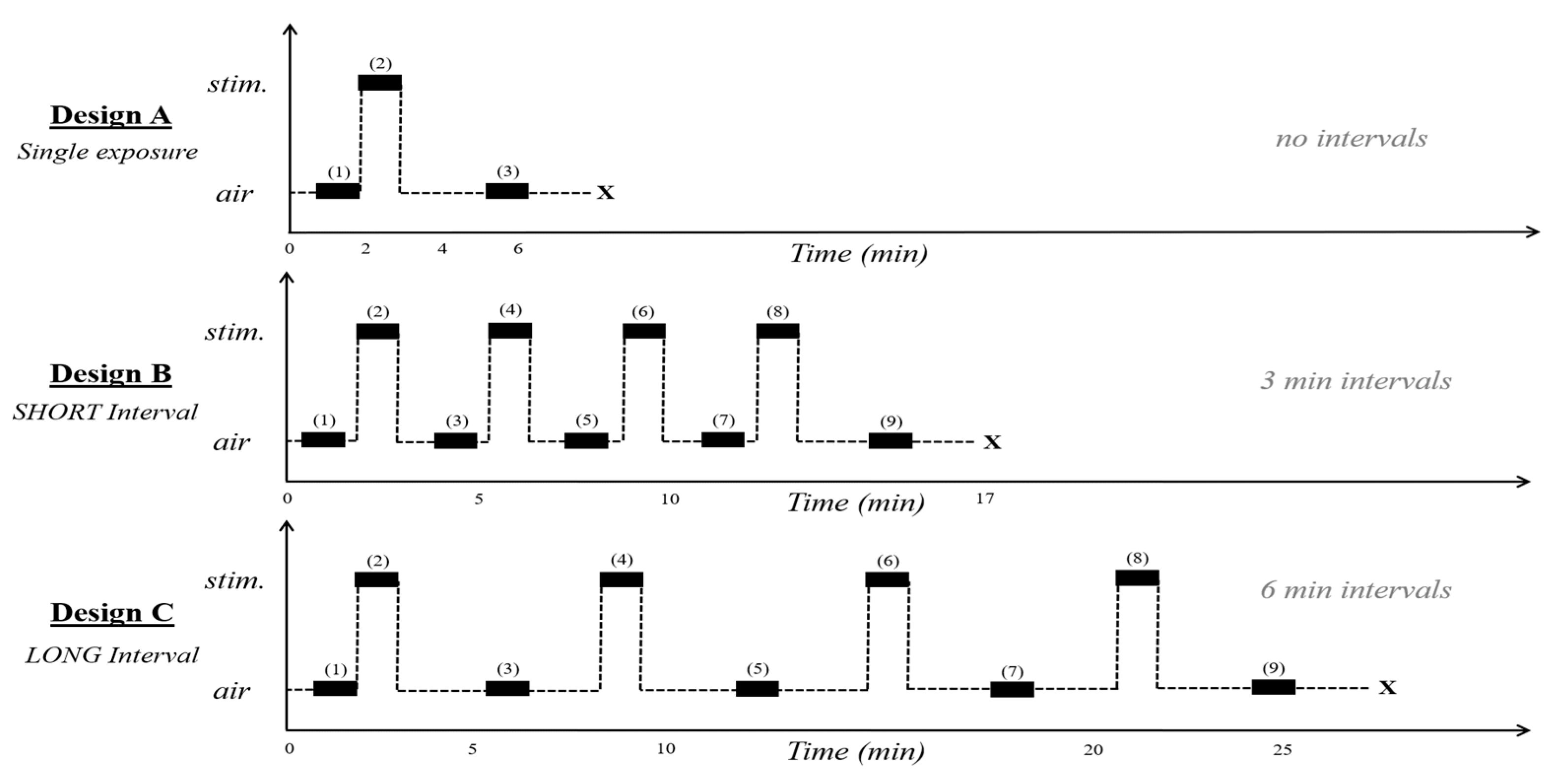
2.5. Repeated Exposures to Alarm Pheromone
2.5.1. Short Intervals
2.5.2. Long Intervals
2.6. Data Collection and Analysis
3. Results
3.1. Single Exposure
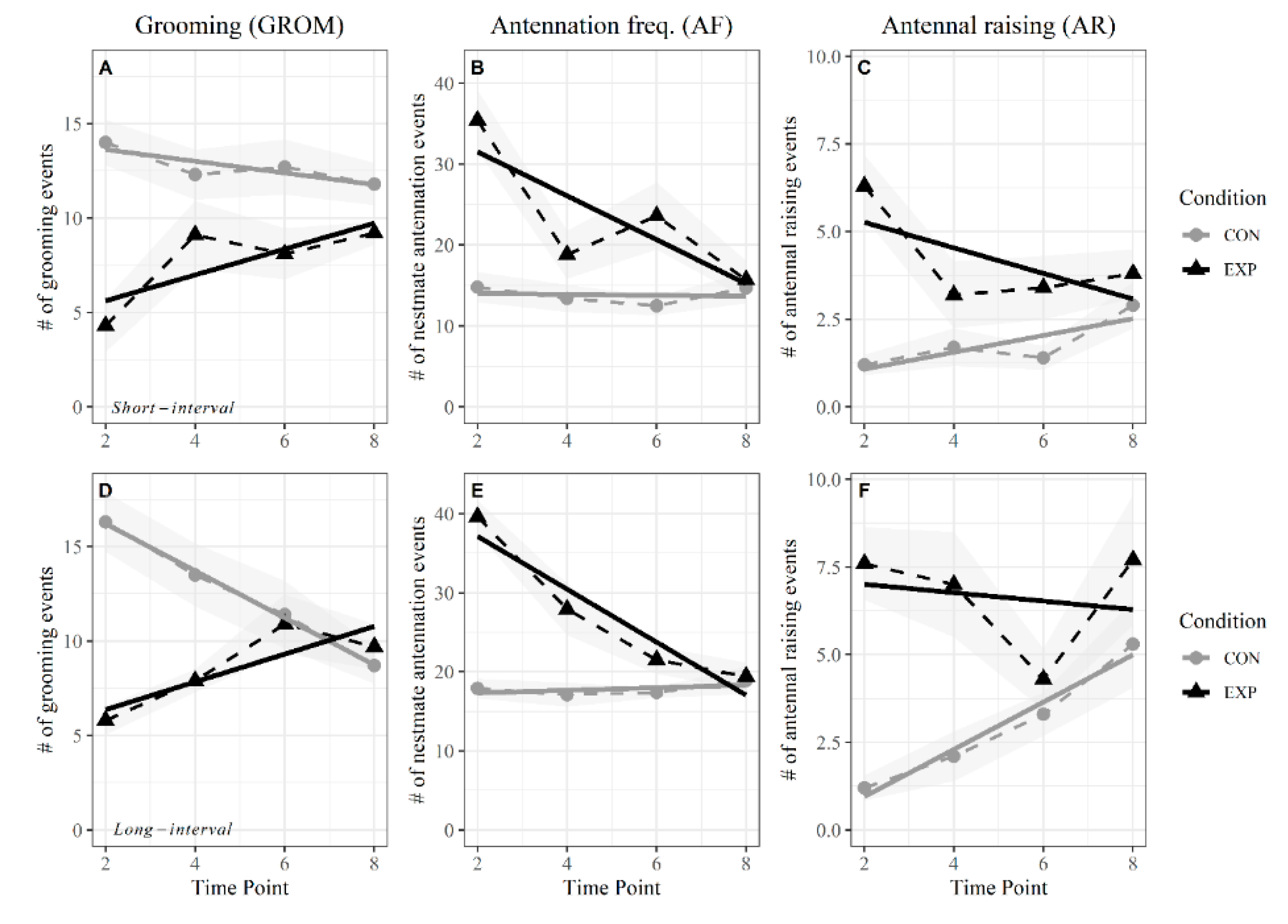
3.2. Short Intervals
3.3. Long Intervals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Czaczkes, T.J.; Grüter, C.; Ratnieks, F.L.W. Trail Pheromones: An Integrative View of Their Role in Social Insect Colony Organization. Annu. Rev. Entomol. 2015, 60, 581–599. [Google Scholar] [CrossRef]
- Brandt, M.; van Wilgenburg, E.; Sulc, R.; Shea, K.J.; Tsutsui, N.D. The scent of supercolonies: The discovery, synthesis and behavioural verification of ant colony recognition cues. BMC Biol. 2009, 7, 71. [Google Scholar] [CrossRef]
- Greenberg, L.; Johnson, C.A.; Trager, J.C.; Mcelfresh, J.S.; Rodstein, J.; Millar, J.G. Sex Attractant Pheromones of Virgin Queens of Sympatric Slave-Making Ant Species in the Genus Polyergus, and their Possible Roles in Reproductive Isolation. J. Chem. Ecol. 2018, 44, 547–555. [Google Scholar] [CrossRef]
- Singer, T.L. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Keller, L.; Nonacs, P. The role of queen pheromones in social insects: Queen control or queen signal? Anim. Behav. 1993, 45, 787–794. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Menzel, F.; Nehring, V.; Schmitt, T. Ecology and Evolution of Communication in Social Insects. Cell 2016, 164, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.J.; Gordon, D.M. Cuticular hydrocarbons inform task decisions. Nature 2003, 423, 32. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.C.; Goulson, D.; Allen, J.A. Repellent scent-marking of flowers by a guild of foraging bumblebees (Bombus spp.). Behav. Ecol. Sociobiol. 1998, 43, 317–326. [Google Scholar] [CrossRef]
- Den Boer, S.P.A.; Duchateau, M.J.H.M. A larval hunger signal in the bumblebee Bombus terrestris. Insectes Soc. 2006, 53, 369–373. [Google Scholar] [CrossRef][Green Version]
- Welzel, K.F.; Lee, S.H.; Dossey, A.T.; Chauhan, K.R.; Choe, D. Verification of Argentine ant defensive compounds and their behavioral effects on heterospecific competitors and conspecific nestmates. Sci. Rep. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990; ISBN 9780674040755. [Google Scholar]
- Dalton, P. Psychophysical and Behavioral Characteristics of Olfactory Adaptation. Chem. Senses 2000, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, R.W. Olfactory adaptation and odour likeness. J. Physiol. 1956, 133, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Torres, C.W.; Brandt, M.; Tsutsui, N.D. The role of cuticular hydrocarbons as chemical cues for nestmate recognition in the invasive Argentine ant (Linepithema humile). Insectes Soc. 2007, 54, 363–373. [Google Scholar] [CrossRef]
- Choe, D.H.; Villafuerte, D.B.; Tsutsui, N.D. Trail Pheromone of the Argentine Ant, Linepithema humile (Mayr) (Hymenoptera: Formicidae). PLoS ONE 2012, 7, e45016. [Google Scholar] [CrossRef]
- Minks, A.K. Mating Disruption of the Codling Moth. Insect Pheromone Res. 1997, 372–376. [Google Scholar] [CrossRef]
- Baker, T.C. Chemical control of behaviour. In Comprehensive Insect Physiology, Biochemistry, and Pharmacology; Kerkut, G.A., Gilbert, L.S., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 621–672. [Google Scholar]
- Maruthadurai, R.; Ramesh, R. Mass trapping of red palm weevil and rhinoceros beetle in coconut with aggregation pheromone. Indian J. Entomol. 2020, 82, 439–443. [Google Scholar] [CrossRef]
- Kirk, W. The aggregation pheromones of thrips (Thysanoptera) and their potential for pest management. Int. J. Trop. Insect Sci. 2017, 37, 41–49. [Google Scholar] [CrossRef]
- Twick, I.; Lee, J.A.; Ramaswami, M. Olfactory Habituation in Drosophila—Odor Encoding and its Plasticity in the Antennal Lobe. Prog. Brain Res. 2014, 208, 3–38. [Google Scholar] [CrossRef]
- Larkin, A.; Karak, S.; Priya, R.; Das, A.; Ayyub, C.; Ito, K.; Rodrigues, V.; Ramaswami, M. Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learn. Mem. 2010, 17, 645–653. [Google Scholar] [CrossRef]
- De Vos, M.; Cheng, W.Y.; Summers, H.E.; Raguso, R.A.; Jander, G. Alarm pheromone habituation in Myzus persicae has fitness consequences and causes extensive gene expression changes. Proc. Natl. Acad. Sci. USA 2010, 107, 14673–14678. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, C.M.; Birgiolas, J.; McHugh, C.; Roubik, D.; Wcislo, W.; Smith, B. Colony-level non-associative plasticity of alarm responses in the stingless honey bee Tetragonisca angustula. Behav. Ecol. Sociobiol. 2008, 72, 58. [Google Scholar] [CrossRef] [PubMed]
- Regnier, F.E.; Wilson, E.O. The alarm-defence system of the ant Acanthomyops claviger. J. Insect Physiol. 1968, 14, 955–970. [Google Scholar] [CrossRef]
- Löfqvist, J. Formic acid and saturated hydrocarbons as alarm pheromones for the ant Formica rufa. J. Insect Physiol. 1976, 22, 1331–1346. [Google Scholar] [CrossRef]
- Theraulaz, G.; Bonabeau, E.; Deneubourg, J.L. Response threshold reinforcement and division of labour in insect societies. Proc. R. Soc. B Biol. Sci. 1998, 265, 327–332. [Google Scholar] [CrossRef]
- Asztalos, Z.; Arora, N.; Tully, T. Olfactory jump reflex habituation in Drosophila and effects of classical conditioning mutations. J. Neurogenet. 2007, 21, 1–18. [Google Scholar] [CrossRef]
- Sunamura, E.; Suzuki, S.; Nishisue, K.; Sakamoto, H.; Otsuka, M.; Utsumi, Y.; Mochizuki, F.; Fukumoto, T.; Ishikawa, Y.; Terayama, M.; et al. Combined use of a synthetic trail pheromone and insecticidal bait provides effective control of an invasive ant. Pest. Manag. Sci. 2011, 67, 1230–1236. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nishishue, K.; Sunamura, E.; Suzuki, S.; Sakamoto, H.; Fukumoto, T.; Terayama, M.; Tatsuki, S. Trail-following disruption in the invasive Argentine ant with a synthetic trail pheromone component (Z)-9-hexadecenal. Sociobiology 2009, 54, 139–152. [Google Scholar]
- Tatsuki, S.; Terayama, M.; Tanaka, Y.; Fukumoto, T. Behavior-Disrupting Agent and Behavior Disrupting Method of Argentine Ant. U.S. Patent 8,278,360, 2 October 2012. [Google Scholar]
- Suckling, D.M.; Stringer, L.D.; Corn, J.E. Argentine Ant Trail Pheromone Disruption is Mediated by Trail Concentration. J. Chem. Ecol. 2011, 37, 1143. [Google Scholar] [CrossRef]
- Nishishue, K.; Sunamura, E.; Tanaka, Y.; Sakamoto, H.; Suzuki, S.; Fukumoto, T.; Terayama, M.; Tatsuki, S. Long-term field trial to control the invasive argentine ant (Hymenoptera: Formicidae) with synthetic trail pheromone. J. Econ. Entomol. 2010, 103, 1784–1789. [Google Scholar] [CrossRef]
- Hamzah, H.; Rixson, D.; Paul-Taylor, J.; Doan, H.; Dadswell, C.; Roffe, G.; Sridhar, A.; Hobday, C.; Wedd, C.; Duren, T.; et al. Inclusion and release of ant alarm pheromones from metal–organic frameworks. Dalton Trans. 2020, 49, 10334–10338. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.; Goulson, D. The use of alarm pheromones to enhance bait harvest by grass-cutting ants. Bull. Entomol. Res. 2002, 92, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.; Howse, P.; Vilela, E.; Knapp, J.; Goulson, D. Field Evaluation of Potential of Alarm Pheromone Compounds to Enhance Baits for Control of Grass-Cutting Ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2002, 95, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.O.H.; Howse, P.E.; Vilela, E.F.; Goulson, D. The response of grass-cutting ants to natural and synthetic versions of their alarm pheromone. Physiol. Entomol. 2001, 26, 165–172. [Google Scholar] [CrossRef]

| Behavior (Short Intervals) | Df | Sum.sq | Mean.sq. | F. Value | Pr|>F| | ||
| Grooming | Error: Trial | Condition | 1 | 505.00 | 505.00 | 13.81 | 0.00158 |
| Residuals | 18 | 658.40 | 36.60 | ||||
| Error: Within | Timepoint | 3 | 29.60 | 9.88 | 0.89 | 0.45 | |
| Condition: Timepoint | 3 | 156.20 | 52.08 | 4.17 | 0.00541 | ||
| Residuals | 54 | 596.90 | 11.05 | ||||
| Antennation freq. | Error: Trial | Condition | 1 | 1814.00 | 1814.50 | 14.82 | 0.00117 |
| Residuals | 18 | 2203.00 | 122.40 | ||||
| Error: Within | Timepoint | 3 | 1207.00 | 402.40 | 7.63 | 0.000244 | |
| Condition: Timepoint | 3 | 1074.00 | 358.00 | 6.78 | 0.000578 | ||
| Residuals | 54 | 2850.00 | 52.80 | ||||
| Antennal raising | Error: Trial | Condition | 1 | 112.80 | 112.81 | 10.55 | 0.00446 |
| Residuals | 18 | 192.40 | 10.69 | ||||
| Error: Within | Timepoint | 3 | 26.94 | 8.98 | 3.03 | 0.03732 | |
| Condition: Timepoint | 3 | 52.54 | 17.51 | 5.90 | 0.00147 | ||
| Residuals | 54 | 160.27 | 2.97 | ||||
| Behavior (Long Intervals) | Df | Sum.sq. | Mean.sq. | F. Value | Pr|>F| | ||
| Grooming | Error: Trial | Condition | 1 | 304.20 | 304.20 | 10.82 | 0.00408 |
| Residuals | 18 | 506.30 | 28.13 | ||||
| Error: Within | Timepoint | 3 | 49.10 | 16.35 | 1.15 | 0.337 | |
| Condition: Timepoint | 3 | 410.10 | 136.70 | 9.63 | 3.41 × 10−6 | ||
| Residuals | 54 | 766.40 | 14.19 | ||||
| Antennation freq. | Error: Trial | Condition | 1 | 1720.50 | 1720.50 | 35.41 | 1.2 × 10−5 |
| Residuals | 18 | 874.60 | 48.60 | ||||
| Error: Within | Timepoint | 3 | 1192.00 | 397.20 | 12.81 | 1.96 × 10−6 | |
| Condition: Timepoint | 3 | 1302.00 | 434.10 | 14 | 7.21 × 10−7 | ||
| Residuals | 54 | 1675.00 | 31.00 | ||||
| Antennal raising | Error: Trial | Condition | 1 | 270.10 | 270.11 | 9.44 | 0.00657 |
| Residuals | 18 | 515.30 | 28.63 | ||||
| Error: Within | Timepoint | 3 | 82.20 | 27.41 | 3.82 | 0.0149 | |
| Condition: Timepoint | 3 | 88.50 | 29.51 | 4.11 | 0.0107 | ||
| Residuals | 54 | 388.00 | 7.19 | ||||
| Behavior | Interval | Condition | Slope | Time pt. int. | Stim pt. int. |
|---|---|---|---|---|---|
| Grooming | Short | Control | −0.31 | 10.05 | 5 |
| Short | Experiment | 0.68 | |||
| Long | Control | −1.20 | 7.15 | 4 | |
| Long | Experiment | 0.73 | |||
| Antennation freq. | Short | Control | −0.06 | 8.58 | 5 |
| Short | Experiment | −2.71 | |||
| Long | Control | 0.16 | 7.63 | 4 | |
| Long | Experiment | −3.35 | |||
| Antennal raising | Short | Control | 0.24 | 9.92 | 5 |
| Short | Experiment | −0.36 | |||
| Long | Control | 0.67 | 9.68 | 5 | |
| Long | Experiment | −0.12 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccaro, J.J.; Whyte, B.A.; Tsutsui, N.D. The Ant Who Cried Wolf? Short-Term Repeated Exposure to Alarm Pheromone Reduces Behavioral Response in Argentine Ants. Insects 2020, 11, 871. https://doi.org/10.3390/insects11120871
Maccaro JJ, Whyte BA, Tsutsui ND. The Ant Who Cried Wolf? Short-Term Repeated Exposure to Alarm Pheromone Reduces Behavioral Response in Argentine Ants. Insects. 2020; 11(12):871. https://doi.org/10.3390/insects11120871
Chicago/Turabian StyleMaccaro, Jessica J., Brian A. Whyte, and Neil D. Tsutsui. 2020. "The Ant Who Cried Wolf? Short-Term Repeated Exposure to Alarm Pheromone Reduces Behavioral Response in Argentine Ants" Insects 11, no. 12: 871. https://doi.org/10.3390/insects11120871
APA StyleMaccaro, J. J., Whyte, B. A., & Tsutsui, N. D. (2020). The Ant Who Cried Wolf? Short-Term Repeated Exposure to Alarm Pheromone Reduces Behavioral Response in Argentine Ants. Insects, 11(12), 871. https://doi.org/10.3390/insects11120871





