Physicochemical Characteristics of Four Limonene-Based Nanoemulsions and Their Larvicidal Properties against Two Mosquito Species, Aedes albopictus and Culex pipiens molestus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
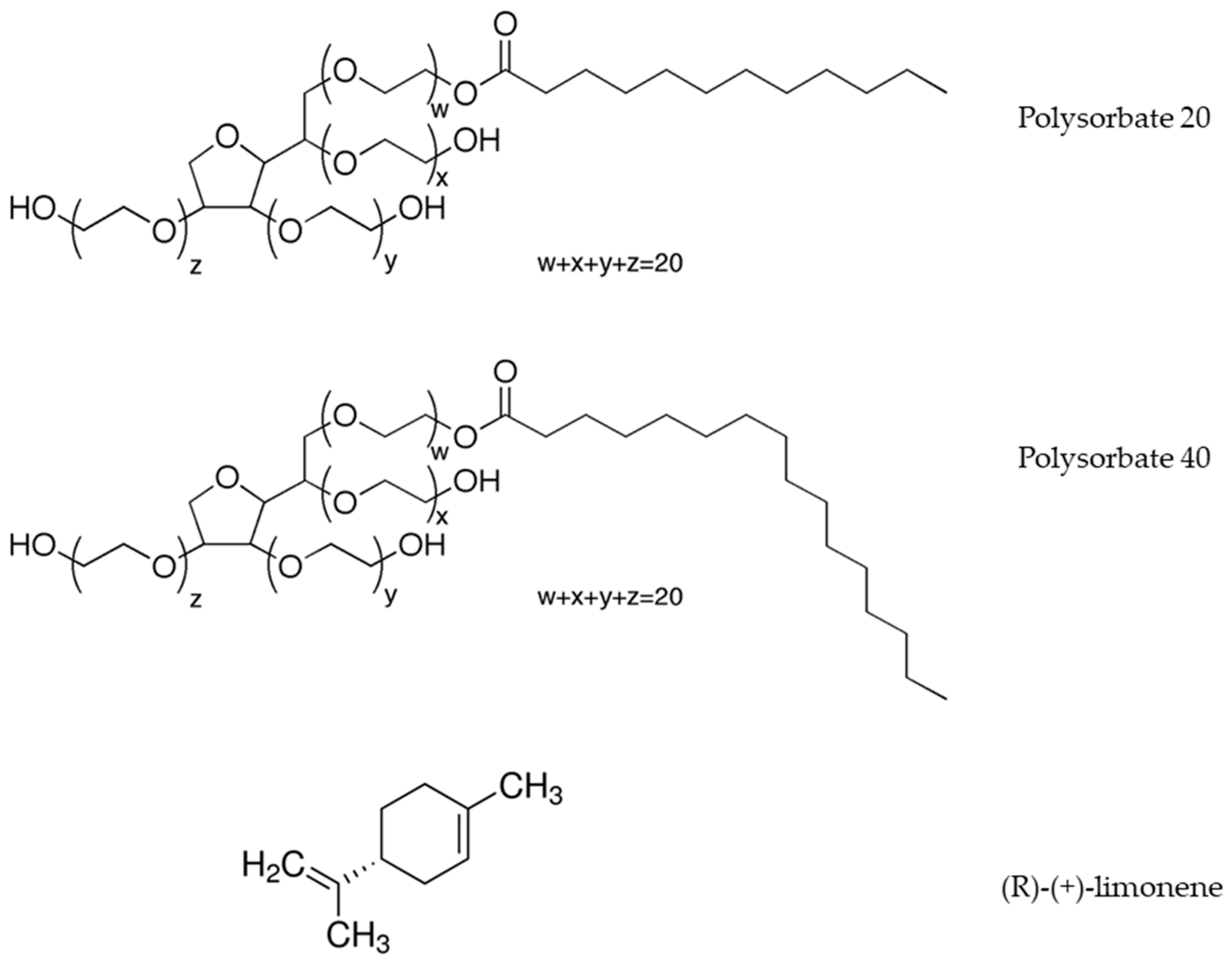
2.1. Chemicals
2.2. Preparation of Nanoemulsions
2.3. Viscosity Measurements
2.4. Dynamic Light Scattering (DLS) Measurements
2.5. EPR Spectroscopy Measurements
2.6. Mosquito Rearing
2.7. Larvicidal Bioassays
2.8. Data Analysis
3. Results and Discussion
3.1. Formation, Stability, Viscosity and Membrane Dynamics of O/W Nanoemulsions
3.2. Insecticidal Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fanun, M. (Ed.) Colloids in drug delivery. In Surfactant Science Series; CRC Press: Boca Raton, FL, USA, 2016; Volume 150. [Google Scholar]
- Jafari, S.M. (Ed.) Nano-Encapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles- Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Kalaitzaki, A.; Papanikolaou, N.E.; Karamaouna, F.; Dourtoglou, V.; Xenakis, A.; Papadimitriou, V. Biocompatible Colloidal Dispersions as Potential Formulations of Natural Pyrethrins: A Structural and Efficacy Study. Langmuir 2015, 31, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Manubolu, M.; Hwang, H.M. Nanostructures: Current uses and future applications in food science. J. Food Drug Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter. 2006, 18, R635. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter. 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. General Aspects of Nanoemulsions and Their Formulation. In Nanoemulsions: Formulation, Applications, and Characterization; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–20. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Demisli, S.; Theochari, I.; Christodoulou, P.; Zervou, M.; Xenakis, A.; Papadimitriou, V. Structure, activity and dynamics of extra virgin olive oil-in-water nanoemulsions loaded with vitamin D3 and calcium citrate. J. Mol. Liq. 2020, 306, 112908. [Google Scholar] [CrossRef]
- Kalaitzaki, A.; Emo, M.; Stébé, M.J.; Xenakis, A.; Papadimitriou, V. Biocompatible nanodispersions as delivery systems of food additives: A structural study. Food Res. Int. 2013, 54, 1448–1454. [Google Scholar] [CrossRef]
- Papadimitriou, V.; Pispas, S.; Syriou, S.; Pournara, A.; Zoumpanioti, M.; Sotiroudis, T.G.; Xenakis, A. Biocompatible microemulsions based on Limonene: Formulation, structure, and applications. Langmuir 2008, 24, 3380–3386. [Google Scholar] [CrossRef]
- Spernath, A.; Yaghmur, A.; Aserin, A.; Hoffman, R.; Garti, N. Food-grade microemulsions based on nonionic emulsifiers: Media to enhance lycopene solubilisation. J. Agric. Food Chem. 2002, 50, 6917–6922. [Google Scholar] [CrossRef]
- Klossek, M.L.; Marcus, J.; Touraud, D.; Kunz, W. Highly water dilutable green microemulsions. Colloids Surf. A 2014, 442, 105–110. [Google Scholar] [CrossRef]
- Lu, W.C.; Huang, D.W.; Wang, C.R.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; Duarte Galhardo de Albuquerque, R.D. Nanoemulsions of essential oils: New tool for control of vector-borne diseases and in vitro effects on some parasitic agents. Medicines 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 577. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Schaner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Bortel, W.V. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector-Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Martins, J.A.J. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem. Inst. Oswaldo Cruz. 2019, 114, e180544. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; Della Torre, A.; Montarsi, F.; Rizzoli, A. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef]
- Bellini, R.; Zeller, H.; Van Bortel, W. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasites Vectors 2014, 7, 323. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Conti, B.; Leonardi, M.; Pistelli, L.; Profeti, R.; Ouerghemmi, I.; Benelli, G. Larvicidal and repellent activity of essential oils from wild and cultivated Ruta chalepensis L. (Rutaceae) against Aedes albopictus Skuse (Diptera: Culicidae), an arbovirus vector. Parasitol. Res. 2013, 112, 991–999. [Google Scholar] [CrossRef]
- Conti, B.; Benelli, G.; Flamini, G.; Cioni, P.L.; Profeti, R.; Ceccarini, L.; Macchia, M.; Canale, A. Larvicidal and repellent activity of Hyptis suaveolens (Lamiaceae) essential oil against the mosquito Aedes albopictus Skuse (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2013–2021. [Google Scholar] [CrossRef]
- Montefuscoli, A.R.; Werdin González, J.O.; Palma, S.D.; Ferrero, A.A.; Fernández Band, B. Design and development of aqueous nanoformulations for mosquito control. Parasitol. Res. 2014, 113, 793–800. [Google Scholar] [CrossRef]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro- and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Loach, N.; Gupta, S.; Mohan, L. Phyto-nanoemulsion: An emerging nano-insecticidal formulation. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100331. [Google Scholar] [CrossRef]
- Michaelakis, A.; Papachristos, D.; Kimbaris, A.; Koliopoulos, G.; Giatropoulos, A.; Polissiou, M.G. Citrus essential oils and four enantiomeric pinenes against Culex pipiens (Diptera: Culicidae). Parasitol. Res. 2009, 105, 769–773. [Google Scholar] [CrossRef]
- Pohlit, A.M.; Rezende, A.R.; Lopes Baltin, E.L.; Lopes, N.P.; Neto, V.F. Plant extracts, isolated phytochemicals, and plant-derived agents which are lethal to arthropod vectors of human tropical diseases—A review. Planta Med. 2011, 77, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakis, A. Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS 14 for Windows Users Guide; SPSS Inc.: Chicago, IL, USA, 2004. [Google Scholar]
- Garti, N.; Avrahami, M.; Aserin, A. Improved solubilization of Celecoxib in U-type nonionic microemulsions and their structural transitions with progressive aqueous dilution. J. Colloid Interface Sci. 2006, 299, 352–365. [Google Scholar] [CrossRef]
- Kalaitzaki, A.; Xenakis, A.; Papadimitriou, V. Highly Water Dilutable Microemulsions: A structural study. Colloid Polym. Sci. 2015, 293, 1111–1119. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Duarte, J.L.; Amado, J.R.R.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Ferreira, A.M.; Souto, R.N.P.; Falcao, D.Q.; Carvalho, J.C.T.; Fernandes, C.P. Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Rev. Bras. Farmacogn. 2015, 25, 189–192. [Google Scholar] [CrossRef]
- Kala, S.; Sogan, N.; Verma, P.; Naik, S.N.; Agarwal, A.; Patanjali, P.K.; Kumar, J. Nanoemulsion of cashew nut shell liquid bio-waste: Mosquito larvicidal activity and insights on possible mode of action. S. Afr. J. Bot. 2019, 127, 293–300. [Google Scholar] [CrossRef]
- Botas, G.D.S.; Cruz, R.A.S.; de Almeida, F.B.; Duarte, J.L.; Araújo, R.S.; Souto, R.N.P.; Ferreira, R.; Carvalho, J.C.T.; Santos, M.G.; Rocha, L.; et al. Baccharis reticularia DC. and Limonene nanoemulsions: Promising larvicidal agents for Aedes aegypti (Diptera: Culicidae) control. Molecules 2017, 22, 1990. [Google Scholar] [CrossRef] [PubMed]
- Azmy, R.M.; El Gohary, G.E.; Mahmoud, D.M.; Salem, D.A.M.; Abdou, M.A.; Salama, M.S. Assessment of larvicidal activity of nanoemulsion from Citrus sinensis essential oil on Culex pipiens L. (Diptera: Culicidae). Egypt J. Aquat. Biol. Fish. 2019, 23, 61–67. [Google Scholar] [CrossRef][Green Version]
- Savić, V.; Todosijević, M.; Ilić, T.; Lukić, M.; Mitsou, E.; Papadimitriou, V.; Avramiotis, S.; Marković, B.; Cekić, N.; Savic, S. Tacrolimus loaded biocompatible lecithin-based microemulsions with improved skin penetration: Structure characterization and in vitro/in vivo performances. Int. J. Pharm. 2017, 529, 491–505. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Nikolic, I.; Mitsou, E.; Damjanovic, A.; Papadimitriou, V.; Antic Stankovic, J.; Stanojevic, B.; Xenakis, A.; Savic, S. Curcumin-loaded low-energy nanoemulsions: Linking EPR spectroscopy-analysed microstructure and antioxidant potential with in vitro evaluated biological activity. J. Mol. Liq. 2020, 301, 112479. [Google Scholar] [CrossRef]
- Theochari, I.; Goulielmaki, M.; Danino, D.; Papadimitriou, V.; Pintzas, A.; Xenakis, A. Drug nanocarriers for cancer chemotherapy based on microemulsions: The case of Vemurafenib analog PLX4720. Colloids Surf. B 2017, 154, 350–356. [Google Scholar] [CrossRef]


| System | ||||
|---|---|---|---|---|
| Ingredients (% w/w) | 1 | 2 | 3 | 4 |
| (R)-(+)-Limonene | 3.8 | 8.9 | 8.7 | 5 |
| Polysorbate 40 | 15.3 | - | - | 12 |
| Polysorbate 20 | - | 36.9 | 39.5 | _ |
| Water | 51.4 | 30.2 | 32.6 | 55 |
| Propylene Glycol | 25.7 | 15.1 | 16.3 | 28 |
| Ethanol | 3.8 | 8.9 | 2.9 | - |
| System | Size (nm) | PdI | Viscosity (cP) |
|---|---|---|---|
| 1 | 288 ± 12 | 0.7 ± 0.03 | 102.7 ± 5.7 |
| 2 | 16 ± 1 | 0.12 ± 0.01 | 9.2 ± 1.3 |
| 3 | 288 ± 17 | 0.16 ± 0.03 | 137.4 ± 27.5 |
| 4 | 57 ± 1 | 0.23 ± 0.01 | 3.7 ± 0.6 |
| System | τR (ns) | S | α′ο (×10−4 T) |
|---|---|---|---|
| 1 | 0.42 ± 0.01 | 0.03 ± 0.00 | 14.79 ± 0.01 |
| 2 | 0.38 ± 0.08 | 0.03 ± 0.00 | 14.76 ± 0.3 |
| 3 | 0.31 ± 0.0 | 0.03 ± 0.00 | 14.74 ± 0.03 |
| 4 | 0.40 ± 0.01 | 0.03 ± 0.00 | 14.73 ± 0.02 |
| Type of Limonene Formulation Applied | Slope (±SE) | LC50 (95% CL) a | LC90 (95% CL) a | x2 | d.f. |
|---|---|---|---|---|---|
| Aedes albopictus | |||||
| System 1 | 7.3 ± 0.7 | 30.6 (28.8–32.4) | 45.9 (42.8–50.5) | 18.260 | 13 |
| System 2 | 5.9 ± 0.6 | 27.1 (23.8–30.6) | 44.5 (38.2–57.6) | 28.585 b | 13 |
| System 3 | 3.9 ± 0.3 | 20.1 (18.2–22.1) | 42.7 (37.2–51.7) | 47.560 b | 28 |
| System 4 | 5.5 ± 0.5 | 29.6 (27.2–31.9) | 50.8 (45.3–60.7) | 42.075 | 23 |
| R-(+)-limonene | 9.7 ± 1.1 | 36.0 (32.6–39.5) | 48.8 (43.6–60.9) | 26.174 b | 10 |
| Cx. pipiens molestus | |||||
| System 1 | 6.4 ± 0.6 | 34.6 (30.7–38.1) | 54.7 (48.5–66.9) | 41.323 b | 16 |
| System 2 | 4.1 ± 0.3 | 33.7 (29.9–37.5) | 69.5 (61.5–80.7) | 23.062 | 15 |
| System 3 | 4.5 ± 0.3 | 23.9 (21.9–26.1) | 46.4 (40.7–55.5) | 46.229 b | 28 |
| System 4 | 5.8 ± 0.5 | 30.9 (28.1–34.1) | 51.9 (44.7–65.5) | 42.075 b | 23 |
| R-(+)-limonene | 4.9 ± 0.5 | 32.2 (27.6–36.7) | 58.2 (50.6–72.4) | 22.705 b | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theochari, I.; Giatropoulos, A.; Papadimitriou, V.; Karras, V.; Balatsos, G.; Papachristos, D.; Michaelakis, A. Physicochemical Characteristics of Four Limonene-Based Nanoemulsions and Their Larvicidal Properties against Two Mosquito Species, Aedes albopictus and Culex pipiens molestus. Insects 2020, 11, 740. https://doi.org/10.3390/insects11110740
Theochari I, Giatropoulos A, Papadimitriou V, Karras V, Balatsos G, Papachristos D, Michaelakis A. Physicochemical Characteristics of Four Limonene-Based Nanoemulsions and Their Larvicidal Properties against Two Mosquito Species, Aedes albopictus and Culex pipiens molestus. Insects. 2020; 11(11):740. https://doi.org/10.3390/insects11110740
Chicago/Turabian StyleTheochari, Ioanna, Athanasios Giatropoulos, Vassiliki Papadimitriou, Vasileios Karras, Georgios Balatsos, Dimitrios Papachristos, and Antonios Michaelakis. 2020. "Physicochemical Characteristics of Four Limonene-Based Nanoemulsions and Their Larvicidal Properties against Two Mosquito Species, Aedes albopictus and Culex pipiens molestus" Insects 11, no. 11: 740. https://doi.org/10.3390/insects11110740
APA StyleTheochari, I., Giatropoulos, A., Papadimitriou, V., Karras, V., Balatsos, G., Papachristos, D., & Michaelakis, A. (2020). Physicochemical Characteristics of Four Limonene-Based Nanoemulsions and Their Larvicidal Properties against Two Mosquito Species, Aedes albopictus and Culex pipiens molestus. Insects, 11(11), 740. https://doi.org/10.3390/insects11110740







