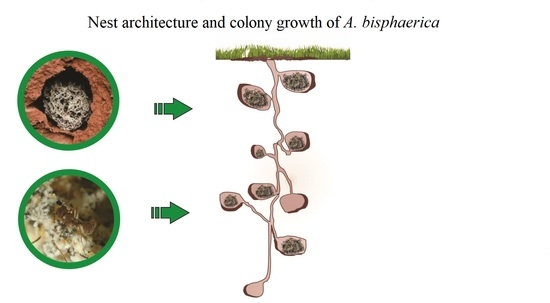
Nest Architecture and Colony Growth of Atta bisphaerica Grass-Cutting Ants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Study Colonies and Nests
2.3. Population and Symbiont Fungus Garden Biomass
2.4. Architecture
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hansell, M. Oxford animal biology series. In Animal Architecture; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Gilbert, S.F. Evolutionary transitions revisited: Holobiont evo-devo. J. Exp. Zool. Part B Mol. Dev. Evol. 2019, 332, 307–314. [Google Scholar] [CrossRef]
- Okano, J.I.; Sasaki, O.; Kano, H. The effects of surface roughness of sediment particles on the respiration of case-bearing caddisfly larvae. Freshw. Sci. 2016, 35, 611–618. [Google Scholar] [CrossRef]
- Rushbrook, B.J.; Head, M.L.; Katsiadaki, I.; Barber, I. Flow regime affects building behaviour and nest structure in sticklebacks. Behav. Ecol. Sociobiol. 2010, 64, 1927–1935. [Google Scholar] [CrossRef]
- Franks, N.R.; Worley, A.; Falkenberg, M.; Sendova-Franks, A.B.; Christensen, K. Digging the optimum pit: Antlions, spirals and spontaneous stratification. Proc. Biol. Sci. 2019, 286, 20190365. [Google Scholar] [CrossRef] [Green Version]
- Cranford, S.W.; Tarakanova, A.; Pugno, N.M.; Buehler, M.J. Nonlinear material behaviour of spider silk yields robust webs. Nature 2012, 482, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.F.; Castro, M.M.; Barbosa, B.C.; Prezoto, F. Variation in nesting behavior of the arboreal Ant Camponotus sericeiventris (Hymenoptera: Formicidae). Fla. Entomol. 2014, 97, 1237–1239. [Google Scholar] [CrossRef]
- Ward, P.S.; Downie, D.A. The ant subfamily Pseudomyrmecinae (Hymenoptera: Formicidae): Phylogeny and evolution of big-eyed arboreal ants. Syst. Entomol. 2005, 30, 310–335. [Google Scholar] [CrossRef]
- Guimarães, I.C.; Pereira, M.C.; Batista, N.R.; Rodrigues, C.A.P.; Antonialli, W.F. The complex nest architecture of the Ponerinae ant Odontomachus chelifer. PLoS ONE 2018, 13, e0189896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschinkel, W.R. The architecture of subterranean ant nests: Beauty and mystery underfoot. J. Bioeconomics 2015, 17, 271–291. [Google Scholar] [CrossRef]
- Forti, L.C.; Camargo, R.S.; Fujihara, R.T.; Lopes, J.F.S. The nest architecture of the ant, Pheidole oxyops Forel, 1908 (Hymenoptera: Formicidae). Insect Sci. 2007, 14, 437–442. [Google Scholar] [CrossRef]
- Moreira, A.A.; Forti, L.C.; Boaretto, M.A.C.; Andrade, A.P.P.; Lopes, J.F.S.; Ramos, V.M. External and internal structure of Atta bisphaerica Forel (Hymenoptera: Formicidae) nests. J. Appl. Entomol. 2004, 128, 204–211. [Google Scholar] [CrossRef]
- Moreira, A.A.; Forti, L.C.; Andrade, A.P.P.; Boaretto, M.A.C.; Lopes, J.F.S. Nest Architecture of Atta laevigata (F. Smith, 1858) (Hymenoptera: Formicidae). Stud. Neotrop. Fauna Environ. 2004, 39, 109–116. [Google Scholar] [CrossRef]
- Forti, L.C.; Andrade, A.P.P.; Camargo, R.S.; Caldato, N.; Moreira, A.A. Discovering the giant nest architecture of grass-cutting ants, Atta capiguara (Hymenoptera, Formicidae). Insects 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Seal, J.N. Scaling of body weight and fat content in fungus-gardening ant queens: Does this explain why leaf-cutting ants found claustrally? Insectes Soc. 2009, 56, 135–141. [Google Scholar] [CrossRef]
- Fujihara, R.T.; Camargo, R.S.; Forti, L.C. Lipid and energy contents in the bodies of queens of Atta sexdens rubropilosa forel (Hymenoptera, formicidae): Pre-and post-nuptial flight. Rev. Bras. Entomol. 2012, 56, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Camargo, R.S.; Forti, L.C. Queen lipid content and nest growth in the leaf cutting ant (Atta sexdens rubropilosa) (Hymenoptera: Formicidae). J. Nat. Hist. 2013, 47, 65–73. [Google Scholar] [CrossRef]
- Autuori, M. Contribuição para o conhecimento da saúva (Atta spp. Hymenoptera -Formicidae) V-Número de formas aladas e redução dos sauveiros iniciais. Arq. Inst. Biol. 1950, 19, 325–331. [Google Scholar]
- Camargo, R.S.; Forti, L.C.; Lopes, J.F.S.; Matos, C.A.O. Growth of populations and fungus gardens of Atta sexdens rubropilosa (Hymenoptera, Formicidae) response to foraged substrates. Sociobiology 2008, 52, 1–11. [Google Scholar]
- Seal, J.N.; Tschinkel, W.R. Food limitation in the fungus-gardening ant, Trachymyrmex septentrionalis. Ecol. Entomol. 2008, 33, 597–607. [Google Scholar] [CrossRef]
- Lopes, J.F.S.; Brugger, M.S.; Menezes, R.B.; Camargo, R.S.; Forti, L.C.; Fourcassié, V. Spatio-temporal dynamics of foraging networks in the grass-cutting ant Atta bisphaerica Forel, 1908 (Formicidae, Attini). PLoS ONE 2016, 11, e0146613. [Google Scholar] [CrossRef]
- Turner, J.S. The Extended Organism: The Physiology of Animal-Built Structures; Harvard University Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Forti, L.C.; Moreira, A.A.; Camargo, R.S.; Caldato, N.; Castellani, M.A. Nest architecture development of grass-cutting ants. Rev. Bras. Entomol. 2017, 62, 46–50. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Tschinkel, W.R. The nest architecture of three species of north Florida Aphaenogaster ants. J. Insect Sci. 2011, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Tschinkel, W.R. The nest architecture of the Florida harvester ant, Pogonomyrmex badius. J. Insect Sci. 2004, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Mariconi, F.A.M. As Saúvas; Ceres: São Paulo, Brasil, 1970. [Google Scholar]
- Statistica for Windows Version 7.0.; Statsoft INC.: Tulsa, OK, USA, 1984–2004.
- Römer, D.; Roces, F. Available space, symbiotic fungus and colony brood influence excavation and lead to the adjustment of nest enlargement in leaf-cutting ants. Insectes Soc. 2015, 62, 401–413. [Google Scholar] [CrossRef]
- Römer, D.; Roces, F. Nest enlargement in leaf-cutting ants: Relocated brood and fungus trigger the excavation of new chambers. PLoS ONE 2014, 9, e97872. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.S.; Forti, L.C. What is the stimulus for the excavation of fungus chamber in leaf-cutting ants? Acta Ethol. 2014, 18, 31–35. [Google Scholar] [CrossRef]
- Cassill, D.; Tschinkel, W.R.; Vinson, S.B. Nest complexity, group size and brood rearing in the fire ant, Solenopsis invicta. Insectes Soc. 2002, 49, 158–163. [Google Scholar] [CrossRef]
- Zamith, A.P.L.; Mariconi, F.A.M.; Castro, U.D.P. Contribuição para o conhecimento da ’’saúva mata pasto" Atta bisphaerica Forel, 1908. An. Esc. Super. Agric. Luiz Queiroz 1961, 18, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Halboth, F.; Roces, F. The construction of ventilation turrets in Atta vollenweideri leaf-cutting ants: Carbon dioxide levels in the nest tunnels, but not airflow or air humidity, influence turret structure. PLoS ONE 2017, 12, e0188162. [Google Scholar] [CrossRef] [Green Version]
- Bershers, S.N.; Traniello, J.F.A. The adaptiveness of worker demography in the Attine ant Trachymyrmex septentrionalis. Ecology 1994, 75, 763–775. [Google Scholar] [CrossRef]
- Kang, Y.; Clark, R.; Makiyama, M.; Fewell, J. Mathematical modeling on obligate mutualism: Interactions between leaf-cutter ants and their fungus garden. J. Theor. Biol. 2011, 289, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Mintzer, A.C. Foundress female weight and cooperative foundation in Atta leaf-cutting ants. In Applied Myrmecology: A World Perspective; Vander Meer, R.K., Jaffe, K., Cedeno, A., Eds.; Westview Press: Boulder, CO, USA, 1990. [Google Scholar]
- Jonkman, J.C.M. The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1893 (Hym.: Formicidae). Z. Angew. Entomol. 1980, 89, 217–246. [Google Scholar] [CrossRef]
- Wilson, E.O. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). I: The overall pattern in A. sexdens. Behav. Ecol. Sociobiol. 1980, 7, 143–156. [Google Scholar] [CrossRef]
- Rosenberg, N.J.; Blad, B.L.; Verma, S.B. Microclimate: The Biological Environment; Wiley-Blackwell: Hoboken, NJ, USA, 1983. [Google Scholar]




| Age (months) | EA (m2) | EH | FC | FCV (L) | Dp (m) | FGB (Kg) | W (×1000) | |
|---|---|---|---|---|---|---|---|---|
| 2 | Mean | 0.020 | 1.00 | 1.00 | 0.161 | 0.18 | 0.005 | 0.121 |
| SD * | 0.0008 | 0.00 | 0.00 | 0.015 | 0.02 | 0.002 | 0.039 | |
| Max. | 0.040 | 1.00 | 1.00 | 0.175 | 0.19 | 0.006 | 0.161 | |
| Min. | 0.032 | 1.00 | 1.00 | 0.144 | 0.15 | 0.003 | 0.083 | |
| 8 | Mean | 0.036 | 1.00 | 2.00 | 0.705 | 0.96 | 0.021 | 0.662 |
| SD * | 0.003 | 0.00 | 0.00 | 0.190 | 0.26 | 0.006 | 0.285 | |
| Max. | 0.021 | 1.00 | 2.00 | 0.859 | 1.25 | 0.026 | 0.970 | |
| Min. | 0.019 | 1.00 | 2.00 | 0.434 | 0.67 | 0.013 | 0.280 | |
| 20 | Mean | 0.220 | 3.25 | 4.50 | 5.822 | 2.27 | 0.107 | 3.315 |
| SD * | 0.150 | 1.50 | 1.91 | 4.465 | 0.79 | 0.083 | 2.242 | |
| Max. | 0.352 | 5.00 | 6.00 | 11.050 | 3.41 | 0.207 | 5.203 | |
| Min. | 0.086 | 2.00 | 2.00 | 0.980 | 1.61 | 0.024 | 0.587 | |
| 32 | Mean | 10.657 | 15.67 | 13.00 | 45.729 | 3.07 | 1.267 | 65.144 |
| SD * | 2.598 | 4.93 | 6.08 | 22.089 | 0.02 | 0.683 | 46.079 | |
| Max. | 13.011 | 19.00 | 20.00 | 71.232 | 3.30 | 2.042 | 117.772 | |
| Min. | 7.869 | 10.00 | 9.00 | 32.596 | 2.70 | 0.752 | 32.047 | |
| 44 | n = 1 | 16.342 | 25.00 | 42.00 | 130.988 | 2.60 | 4.610 | 192.611 |
| 56 | n = 1 | 42.125 | 132.00 | 104.00 | 291.537 | 3.30 | 12.359 | 444.224 |
| Relationships | Models | F (1,14) | p-Value | R2 |
|---|---|---|---|---|
| Workers (W) vs. Fungus biomass | log y = −2.87 + 0.92log x | 918.63 | <0.001 | 0.98 |
| W vs. Fungus chambers (FC) | log y = −2.39 + 0.48log x | 262.81 | <0.001 | 0.94 |
| W vs. Entrance holes | log y = −2.98 + 0.53log x | 144.33 | <0.001 | 0.90 |
| W vs. External area | log y = −0.02 + 1.00log x | 268.14 | <0.001 | 0.95 |
| W vs. Total volume of FC | log y = −0.91 + 0.90log x | 907.24 | <0.001 | 0.98 |
| W vs. Depth | log y = 2.25 + 0.31log x | 26.84 | <0.001 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, A.P.; Camargo, R.d.S.; Andrade Sousa, K.K.; Caldato, N.; Forti, L.C. Nest Architecture and Colony Growth of Atta bisphaerica Grass-Cutting Ants. Insects 2020, 11, 741. https://doi.org/10.3390/insects11110741
Farias AP, Camargo RdS, Andrade Sousa KK, Caldato N, Forti LC. Nest Architecture and Colony Growth of Atta bisphaerica Grass-Cutting Ants. Insects. 2020; 11(11):741. https://doi.org/10.3390/insects11110741
Chicago/Turabian StyleFarias, Adriano Pimentel, Roberto da Silva Camargo, Kátia Kaelly Andrade Sousa, Nadia Caldato, and Luiz Carlos Forti. 2020. "Nest Architecture and Colony Growth of Atta bisphaerica Grass-Cutting Ants" Insects 11, no. 11: 741. https://doi.org/10.3390/insects11110741
APA StyleFarias, A. P., Camargo, R. d. S., Andrade Sousa, K. K., Caldato, N., & Forti, L. C. (2020). Nest Architecture and Colony Growth of Atta bisphaerica Grass-Cutting Ants. Insects, 11(11), 741. https://doi.org/10.3390/insects11110741