Survival and Recovery of the Pine-Tree Lappet Dendrolimus pini When Subjected to Simulated Starvation
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plant Material
2.2. Experimental Design and Measurements
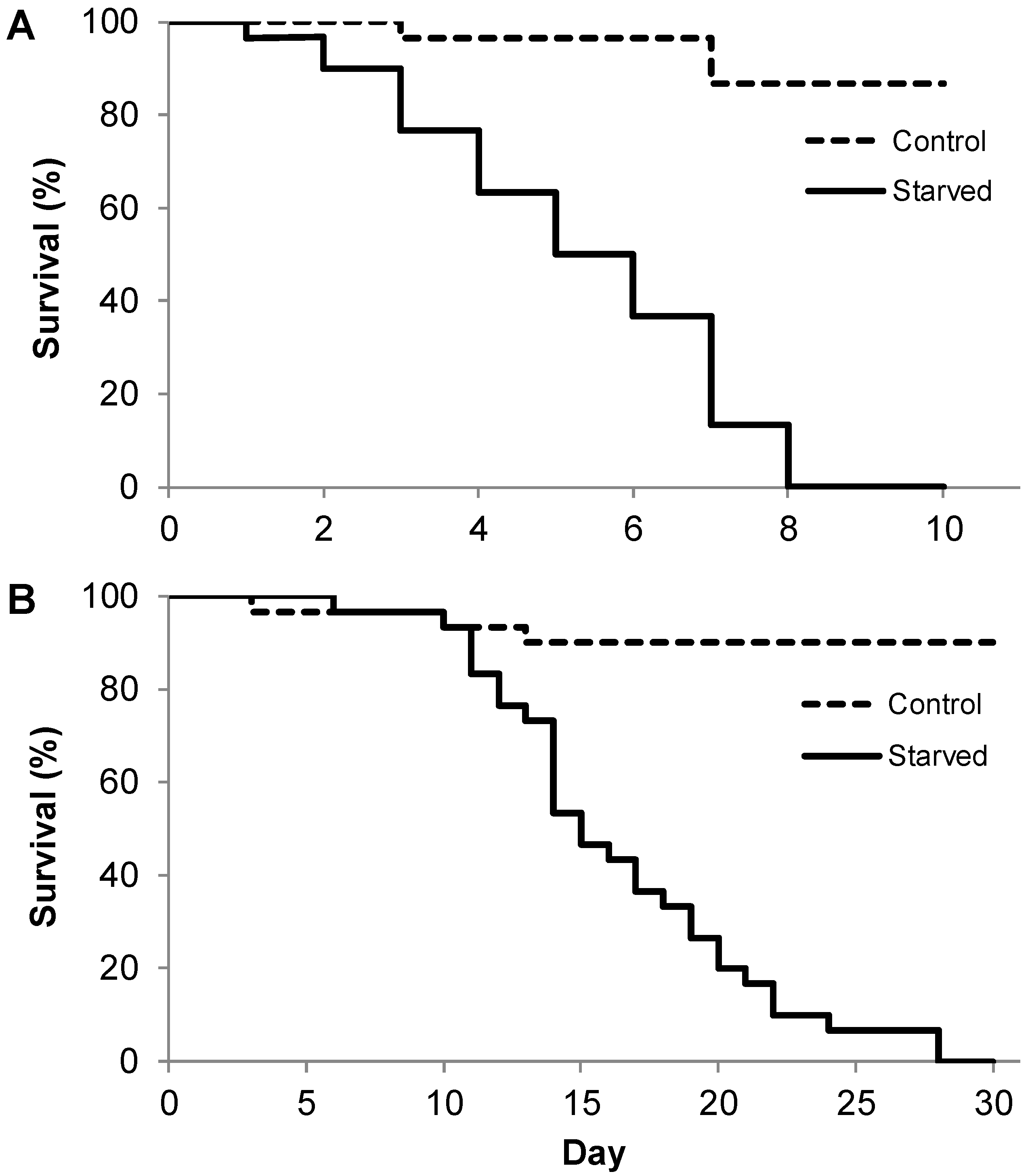
2.2.1. Experiment I: What Is the Maximum Survival of Different Instar Larvae after Total Starvation?
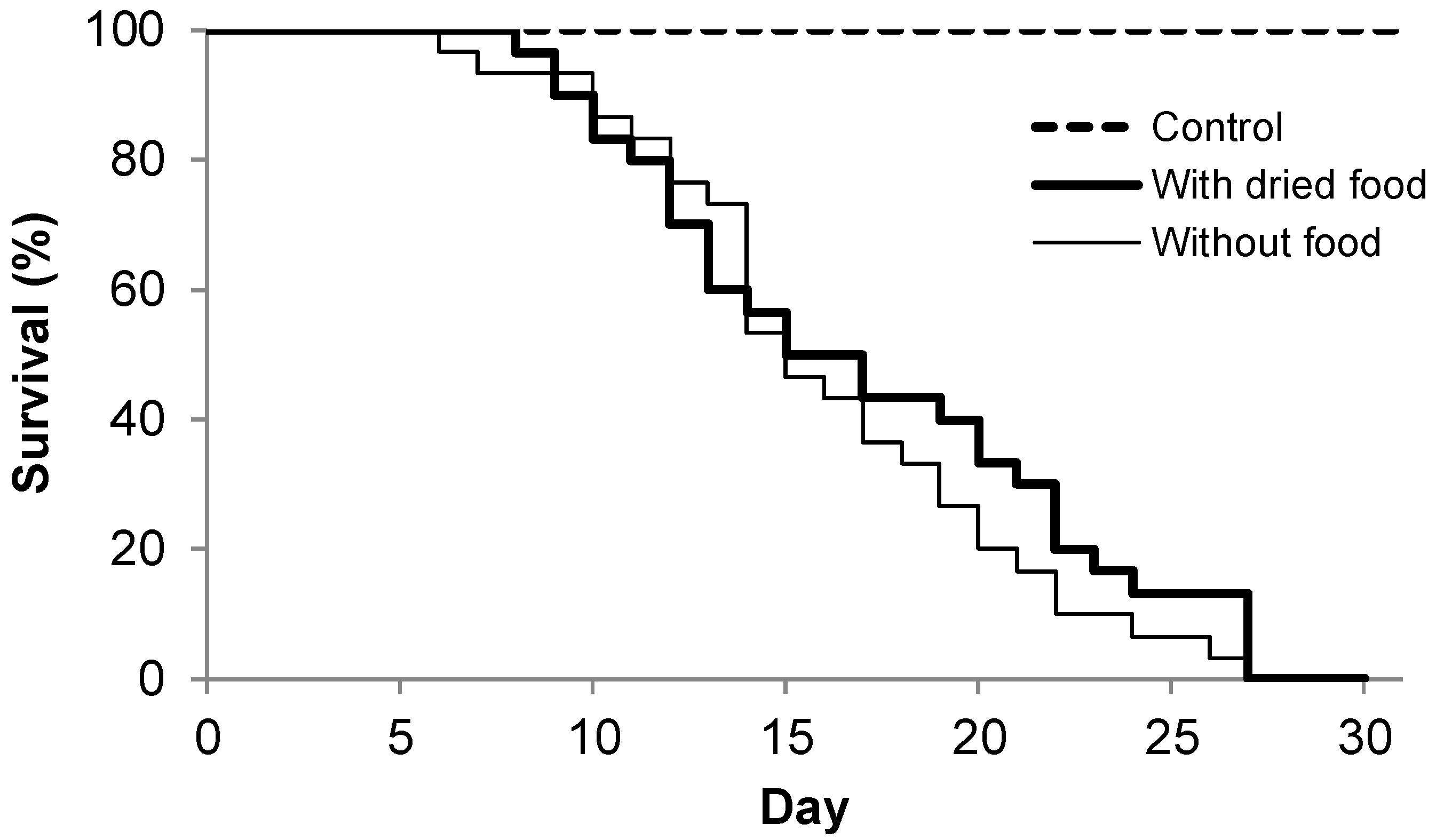
2.2.2. Experiment II: Does Dried Food Extend the Lifespan of the Larvae?
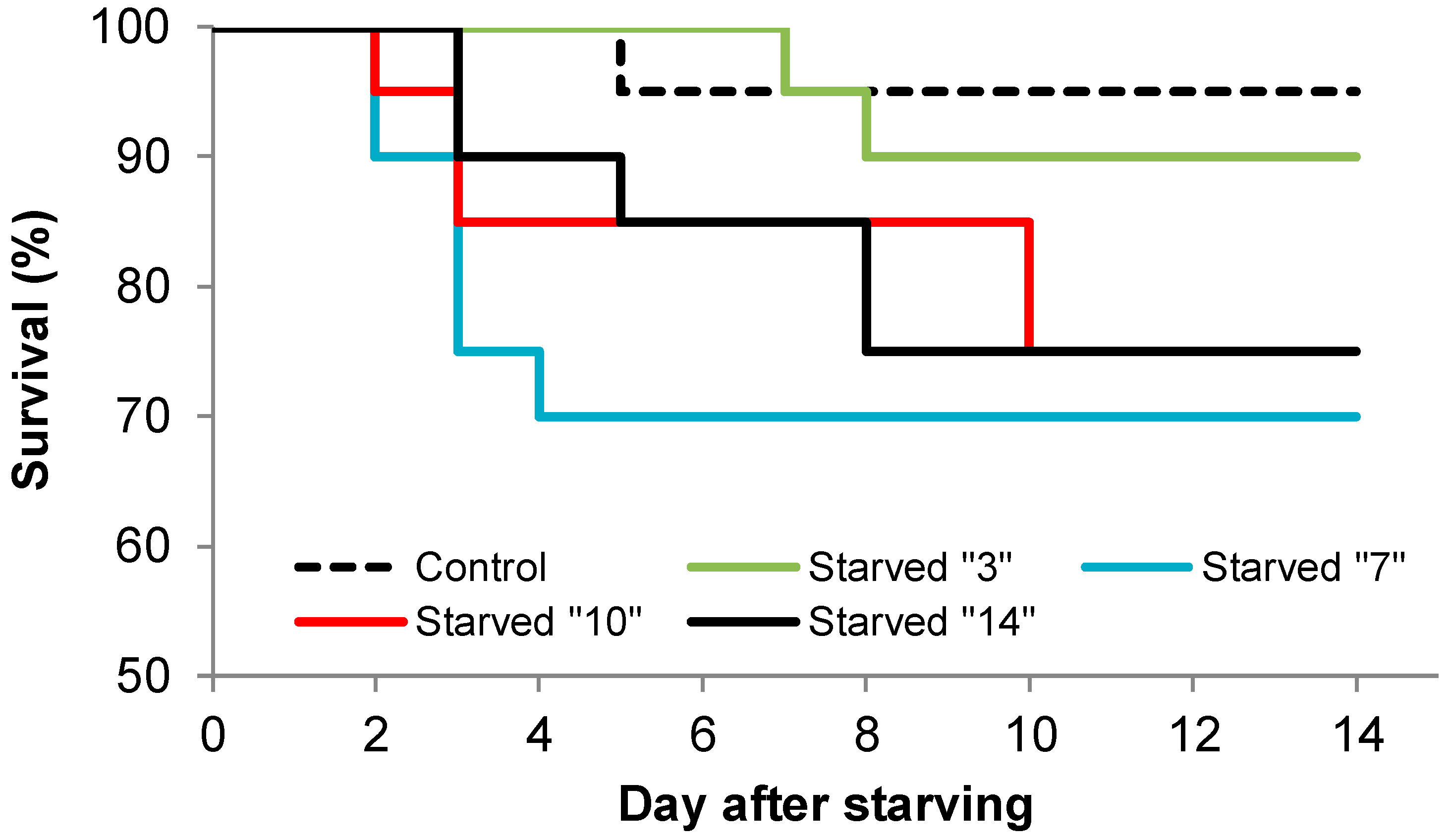
2.2.3. Experiment III: Is There a Possibility of Recovery after Starving Larvae for Various Periods of Time?
2.3. Statistical Analyses
3. Results
4. Discussion
4.1. Lifespan of Larvae During Starvation
4.2. Ability to Recover
4.3. Application Aspects and Future Directions
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kenis, M.; Auger-Rozenberg, M.-A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Perrings, C.; Williamson, M.; Barbier, E.B.; Delfino, D.; Dalmazzone, S.; Shogren, J.; Simmons, P.; Watkinson, A. Biological invasion risk and the public good: An economic perspective. Conserv. Ecol. 2002, 6, 1. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Mooney, H.A. Invasive alien species in an era of globalization. Front. Ecol. Environ. 2007, 5, 199–208. [Google Scholar] [CrossRef]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef]
- Schilthuizen, M.; Santos Pimenta, L.P.; Lammers, Y.; Steenbergen, P.J.; Flohil, M.; Beveridge, N.G.P.; van Duijn, P.T.; Meulblok, M.M.; Sosef, N.; van de Ven, R.; et al. Incorporation of an invasive plant into a native insect herbivore food web. PeerJ 2016, 4, e1954. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Janek, W.; Baraniak, E.; Walczak, U.; Karolewski, P. Changing host plants causes structural differences in the parasitoid complex of the monophagous moth Yponomeuta evonymella, but does not improve survival rate. Insects 2019, 10, 197. [Google Scholar] [CrossRef]
- Rogers, R.; Wallner, C.; Goodwin, B.; Heitland, W.; Weisser, W.W.; Brosius, H.-B. When do people take action? The importance of people’s observation that nature is changing for pro-environmental behavior within the field of impersonal, environmental risk. J. Integr. Environ. Sci. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- D’Costa, L.; Koricheva, J.; Straw, N.; Simmonds, M.S.J. Oviposition patterns and larval damage by the invasive horse-chestnut leaf miner Cameraria ohridella on different species of Aesculus. Ecol. Entomol. 2013, 38, 456–462. [Google Scholar] [CrossRef]
- Prestemon, J.P.; Zhu, S.; Turner, J.A.; Buongiorno, J.; Li, R. Forest product trade impacts of an invasive species: Modeling structure and intervention trade-offs. Agric. Resour. Econ. Rev. 2006, 35, 128–143. [Google Scholar] [CrossRef][Green Version]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Albert, C.; Roiz, D.; Barbet-Massin, M.; Fournier, A.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Wiatrowska, B.; Łukowski, A.; Karolewski, P.; Danielewicz, W. Invasive Spiraea tomentosa: A new host for monophagous Earias clorana? Arthropod. Plant. Interact. 2018, 12, 423–434. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Ray, D.; Peace, A.; Moore, R.; Petr, M.; Grieve, Y.; Convery, C.; Ziesche, T. Improved prediction of the climate-driven outbreaks of Dendrolimus pini in Pinus sylvestris forests. Forestry 2016, 89, 230–244. [Google Scholar] [CrossRef]
- Kirichenko, N.; Flament, J.; Baranchikov, Y.; Grégoire, J.-C. Larval performances and life cycle completion of the Siberian moth, Dendrolimus sibiricus (Lepidoptera: Lasiocampidae), on potential host plants in Europe: A laboratory study on potted trees. Eur. J. For. Res. 2011, 130, 1067–1074. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Möykkynen, T.; Pukkala, T. Modelling of the spread of a potential invasive pest, the Siberian moth (Dendrolimus sibiricus) in Europe. For. Ecosyst. 2014, 1, 10. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.M.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Matsuki, M.; Haugen, D.A.; Hurley, B.P.; Ahumada, R.; Klasmer, P.; Sun, J.; Iede, E.T. Predicting the potential distribution of Sirex noctilio (Hymenoptera: Siricidae), a significant exotic pest of Pinus plantations. Ann. For. Sci. 2006, 63, 119–128. [Google Scholar] [CrossRef]
- Lennox, R.; Choi, K.; Harrison, P.M.; Paterson, J.E.; Peat, T.B.; Ward, T.D.; Cooke, S.J. Improving science-based invasive species management with physiological knowledge, concepts, and tools. Biol. Invasions 2015, 17, 2213–2227. [Google Scholar] [CrossRef]
- Simberloff, D.; Parker, I.M.; Windle, P.N. Introduced species policy, management, and future research needs. Front. Ecol. Environ. 2005, 3, 12–20. [Google Scholar] [CrossRef]
- Bacon, S.J.; Bacher, S.; Aebi, A. Gaps in border controls are related to quarantine alien insect invasions in Europe. PLoS ONE 2012, 7, e47689. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.A. Survival and development of Lymantria monacha (Lepidoptera: Lymantriidae) on North American and introduced Eurasian tree species. J. Econ. Entomol. 2003, 96, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.A.; Shi, J. Effects of temperature on first instar Lymantria (Lepidoptera: Erebidae) survival and development with and without food. Environ. Entomol. 2019, 48, 655–666. [Google Scholar] [CrossRef]
- Dempster, J.P.; Pollard, E. Fluctuations in resource availability and insect populations. Oecologia 1981, 50, 412–416. [Google Scholar] [CrossRef]
- Scharf, I. The multifaceted effects of starvation on arthropod behaviour. Anim. Behav. 2016, 119, 37–48. [Google Scholar] [CrossRef]
- Hunter, A.F. Gypsy moth population sizes and the window of opportunity in spring. Oikos 1993, 68, 531. [Google Scholar] [CrossRef]
- McCue, M.D. Comparative Physiology of Fasting, Starvation, and Food Limitation; McCue, M.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-29055-8. [Google Scholar]
- Peters, T.M.; Barbosa, P. Influence of population density on size, fecundity, and developmental rate of insects in culture. Annu. Rev. Entomol. 1977, 22, 431–450. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M. Summary of Concepts of Dynamic Energy Budget Theory; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780521131919. [Google Scholar]
- Oku, K.; Vermeer, K.M.C.A.; Verbaarschot, P.; de Jong, P.W. Effects of starvation and mating status on the activity of the flea beetle, Phyllotreta nemorum (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2010, 107, 549–551. [Google Scholar] [CrossRef]
- Gergs, A.; Jager, T. Body size-mediated starvation resistance in an insect predator. J. Anim. Ecol. 2014, 83, 758–768. [Google Scholar] [CrossRef]
- Kolk, A.; Starzyk, J.R. Atlas of Forest Insect Pests; MULTICO Publishing House Ltd.: Warsaw, Poland, 1996. [Google Scholar]
- Sierpińska, A. Towards an integrated management of Dendrolimus pini L. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating, Insects, Banska hiavnica, Slovak Republic, 18‒23 August 1996; pp. 129–142. [Google Scholar]
- Skrzecz, I.; Ślusarski, S.; Tkaczyk, M. Integration of science and practice for Dendrolimus pini (L.) management—A review with special reference to Central Europe. For. Ecol. Manag. 2020, 455, 117697. [Google Scholar] [CrossRef]
- Sukovata, L. A comparison of three approaches for larval instar separation in insects—A case study of Dendrolimus pini. Insects 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Sukovata, L.; Kolk, A.; Jaroszynska, J.; Krajewska, U.; Purzynska, A.; Isidorov, V. Host-tree preferences of the pine moth (Lepidoptera: Lasiocampidae) and pine beauty moth (Lepidopera: Noctuidae) larvae in relation to needle quality. Ecol. Surv. Manag. For. Insects Proc. 2003, 98–106. [Google Scholar]
- Le Mellec, A.; Michalzik, B. Impact of a pine lappet (Dendrolimus pini) mass outbreak on C and N fluxes to the forest floor and soil microbial properties in a Scots pine forest in Germany. Can. J. For. Res. 2008, 38, 1829–1841. [Google Scholar] [CrossRef]
- Björkman, C.; Lindelöw, Å.; Eklund, K.; Kyrk, S.; Klapwijk, J.; Fedderwitz, F.; Nordlander, G. A rare event—An isolated outbreak of the pine-tree lappet moth (Dendrolimus pini) in the Stockholm archipelago. Entomol. Tidskr. 2013, 134, 1–9. [Google Scholar]
- Grodner, J.; Zander, R. Sex propheromone of the pine tree lappet moth Dendrolimus pini and its use in attractant-based monitoring system. Pesticides 2010, 1–4, 43–49. [Google Scholar]
- Diaz, J.H. The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming. Am. J. Trop. Med. Hyg. 2005, 72, 347–357. [Google Scholar] [CrossRef]
- Reim, C.; Teuschl, Y.; Blanckenhorn, W.U. Size-dependent effects of temperature and food stress on energy reserves and starvation resistance in yellow dung flies. Evol. Ecol. Res. 2006, 8, 1215–1234. [Google Scholar]
- Stockhoff, B.A. Starvation resistance of gypsy moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae): Tradeoffs among growth, body size, and survival. Oecologia 1991, 88, 422–429. [Google Scholar] [CrossRef]
- Awmack, C.; Leather, S. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Karolewski, P.; Jagodziński, A.M.; Giertych, M.J.; Łukowski, A.; Baraniak, E.; Oleksyn, J. Invasive Prunus serotina-a new host for Yponomeuta evonymellus (Lepidoptera: Yponomeutidae)? Eur. J. Entomol. 2014, 111, 227–236. [Google Scholar] [CrossRef]
- Friend, W.G. Nutritional requirements of phytophagous insects. Annu. Rev. Entomol. 1958, 3, 57–74. [Google Scholar] [CrossRef]
- Montezano, D.G.; Hunt, T.E.; Specht, A.; Luz, P.M.C.; Peterson, J.A. Survival and development of Striacosta albicosta (Smith) (Lepidoptera: Noctuidae) immature stages on dry beans, non-Bt, Cry1F, and Vip3A maize. Insects 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E.; Austarå, Ø. Development of Neodiprion sertifer (Geoff.) (Hym., Diprionidae) on drought-stressed pines: A laboratory experiment. J. Appl. Entomol. 1996, 120, 221–223. [Google Scholar] [CrossRef]
- Galway, K.E.; Duncan, R.P.; Syrett, P.; Emberson, R.M.; Sheppard, A. Insect performance and host-plant stress: A review from a biological control perspective. In Proceedings of the XI International Biological Control of Weeds Symposium, Canberra, Australia, 27 April–2 May 2003; pp. 394–399. [Google Scholar]
- Weiss, M.J.; Seevers, K.P.; Mayo, Z.B. Influence of western corn rootworm (Diabrotica virgifera virgifera) larval densities and damage on corn rootworm survival, developmental time, size and sex ratio (Coleoptera: Chrysomelidae). J. Kansas Entomol. Soc. 1985, 58, 397–402. [Google Scholar]
- Daglish, G.J. Survival and reproduction of Tribolium castaneum (Herbst), Rhyzopertha dominica (F.) and Sitophilus oryzae (L.) following periods of starvation. J. Stored Prod. Res. 2006, 42, 328–338. [Google Scholar] [CrossRef]
- Miller, W.E. Extrinsic effects on fecundity-maternal weight relations in capital-breeding Lepidoptera. J. Lepid. Soc. 2005, 59, 143–160. [Google Scholar]
- Kanturski, M.; Bugaj-Nawrocka, A.; Wieczorek, K. Pine pest aphids of the genus Eulachnus (Hemiptera: Aphididae: Lachninae): How far can their range extend? Agric. For. Entomol. 2016, 18, 398–408. [Google Scholar] [CrossRef]
- Mercader, R.J.; Siegert, N.W.; Liebhold, A.M.; McCullough, D.G. Dispersal of the emerald ash borer, Agrilus planipennis, in newly-colonized sites. Agric. For. Entomol. 2009, 11, 421–424. [Google Scholar] [CrossRef]
- Short, M.; Chase, K.; Feeley, T.; Kees, A.; Wittman, J.; Aukema, B. Rail transport as a vector of emerald ash borer. Agric. For. Entomol. 2020, 22, 92–97. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Poland, T.M.; Mccullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- Venette, R.C. Exotic Pine Pests: Survey Reference; USDA Forest Service: Washington, DC, USA, 2008.
- Williams, T.; Virto, C.; Murillo, R.; Caballero, P. Covert infection of insects by baculoviruses. Front. Microbiol. 2017, 8, 1337. [Google Scholar] [CrossRef] [PubMed]
- Kasianov, N.S.; Belousova, I.A.; Pavlushin, S.V.; Dubovskiy, I.M.; Podgwaite, J.D.; Martemyanov, V.V.; Bakhvalov, S.A. The activity of phenoloxidase in haemolymph plasma is not a predictor of Lymantria dispar resistance to its baculovirus. PLoS ONE 2017, 12, e0183940. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Baz, F.; Romo, H.; González, J.M.; de Hernández, M.J.M.; Pastrana, R.G. Maximum entropy niche-based modeling (Maxent) of potential geographical distribution of Coreura albicosta (Lepidoptera: Erebidae: Ctenuchina) in Mexico. Florida Entomol. 2016, 99, 376–380. [Google Scholar] [CrossRef]
- Bauchinger, U.; McWilliams, S.R. Tissue-specific mass changes during fasting: The protein turnover hypothesis. In Comparative Physiology of Fasting, Starvation, and Food Limitation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 193–206. [Google Scholar]
- Renault, D.; Hance, T.; Vannier, G.; Vernon, P. Is body size an influential parameter in determining the duration of survival at low temperatures in Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae)? J. Zool. 2003, 259, 381–388. [Google Scholar] [CrossRef]
- Lehmann, T.; Dalton, R.; Kim, E.H.; Dahl, E.; Diabate, A.; Dabire, R.; Dujardin, J.P. Genetic contribution to variation in larval development time, adult size, and longevity of starved adults of Anopheles gambiae. Infect. Genet. Evol. 2006, 6, 410–416. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Dornhaus, A. Small worker bumble bees (Bombus impatiens) are hardier against starvation than their larger sisters. Insectes Soc. 2010, 57, 193–197. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Khokhlova, I.S.; Fielden, L.J.; Burdelova, N.I. Time of survival under starvation in two flea species (Siphonaptera: Pulicidae) at different air temperatures and relative humidities. J. Vector Ecol. 2002, 27, 70–81. [Google Scholar]
- Barahona-Segovia, R.M.; Grez, A.A.; Bozinovic, F. Testing the hypothesis of greater eurythermality in invasive than in native ladybird species: From physiological performance to life-history strategies. Ecol. Entomol. 2016, 41, 182–191. [Google Scholar] [CrossRef]
- Karolewski, P.; Grzebyta, J.; Oleksyn, J.; Giertych, M.J. Effects of temperature on larval survival rate and duration of development in Lymantria monacha (L.) on needles of Pinus sylvestris (L.) and in L. dispar (L.) on leaves of Quercus robur (L.). Polish J. Ecol. 2007, 55, 595–600. [Google Scholar]
- Sandi, C.; Pinelo-Nava, M.T. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007, 2007, 78970. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukowski, A.; Adamczyk, D.; Karolewski, P. Survival and Recovery of the Pine-Tree Lappet Dendrolimus pini When Subjected to Simulated Starvation. Insects 2020, 11, 67. https://doi.org/10.3390/insects11010067
Łukowski A, Adamczyk D, Karolewski P. Survival and Recovery of the Pine-Tree Lappet Dendrolimus pini When Subjected to Simulated Starvation. Insects. 2020; 11(1):67. https://doi.org/10.3390/insects11010067
Chicago/Turabian StyleŁukowski, Adrian, Dawid Adamczyk, and Piotr Karolewski. 2020. "Survival and Recovery of the Pine-Tree Lappet Dendrolimus pini When Subjected to Simulated Starvation" Insects 11, no. 1: 67. https://doi.org/10.3390/insects11010067
APA StyleŁukowski, A., Adamczyk, D., & Karolewski, P. (2020). Survival and Recovery of the Pine-Tree Lappet Dendrolimus pini When Subjected to Simulated Starvation. Insects, 11(1), 67. https://doi.org/10.3390/insects11010067





