Neuronal Innervation of the Subgenual Organ Complex and the Tibial Campaniform Sensilla in the Stick Insect Midleg
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Leg Morphology
2.3. Scanning Electron Microscopy
2.4. Staining of Nerves and Sensory Structures by Axonal Tracing
2.5. Terminology of Nerves and Nerve Branches
2.6. Microscopy and Documentation
3. Results
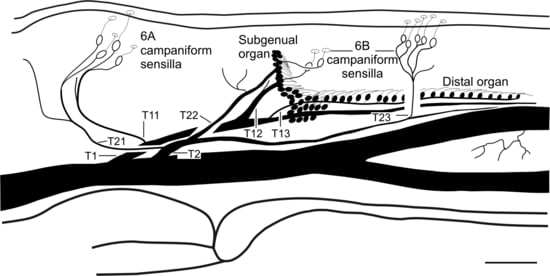
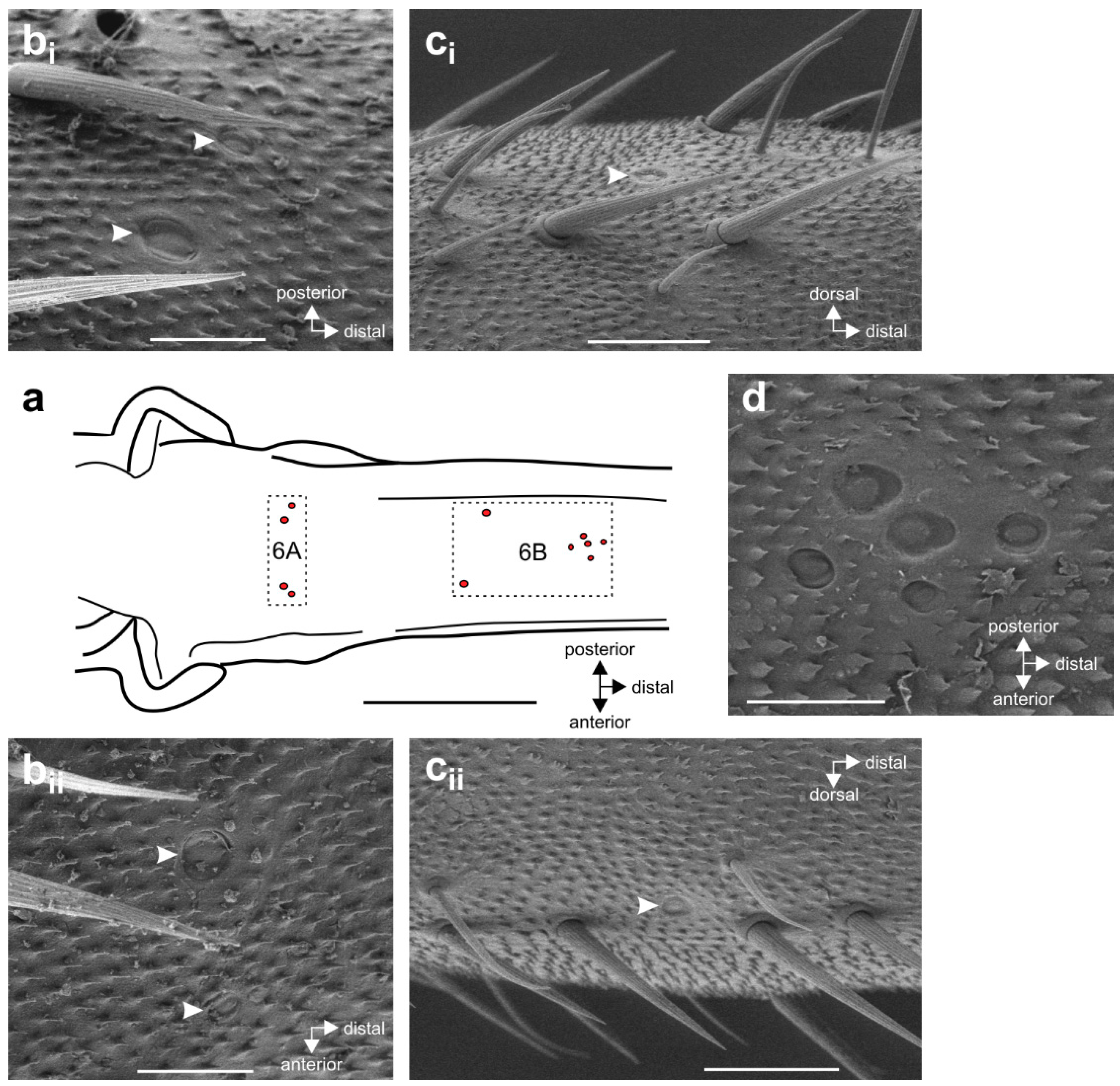
3.1. External Morphology of the Midleg and Location of Campaniform Sensilla
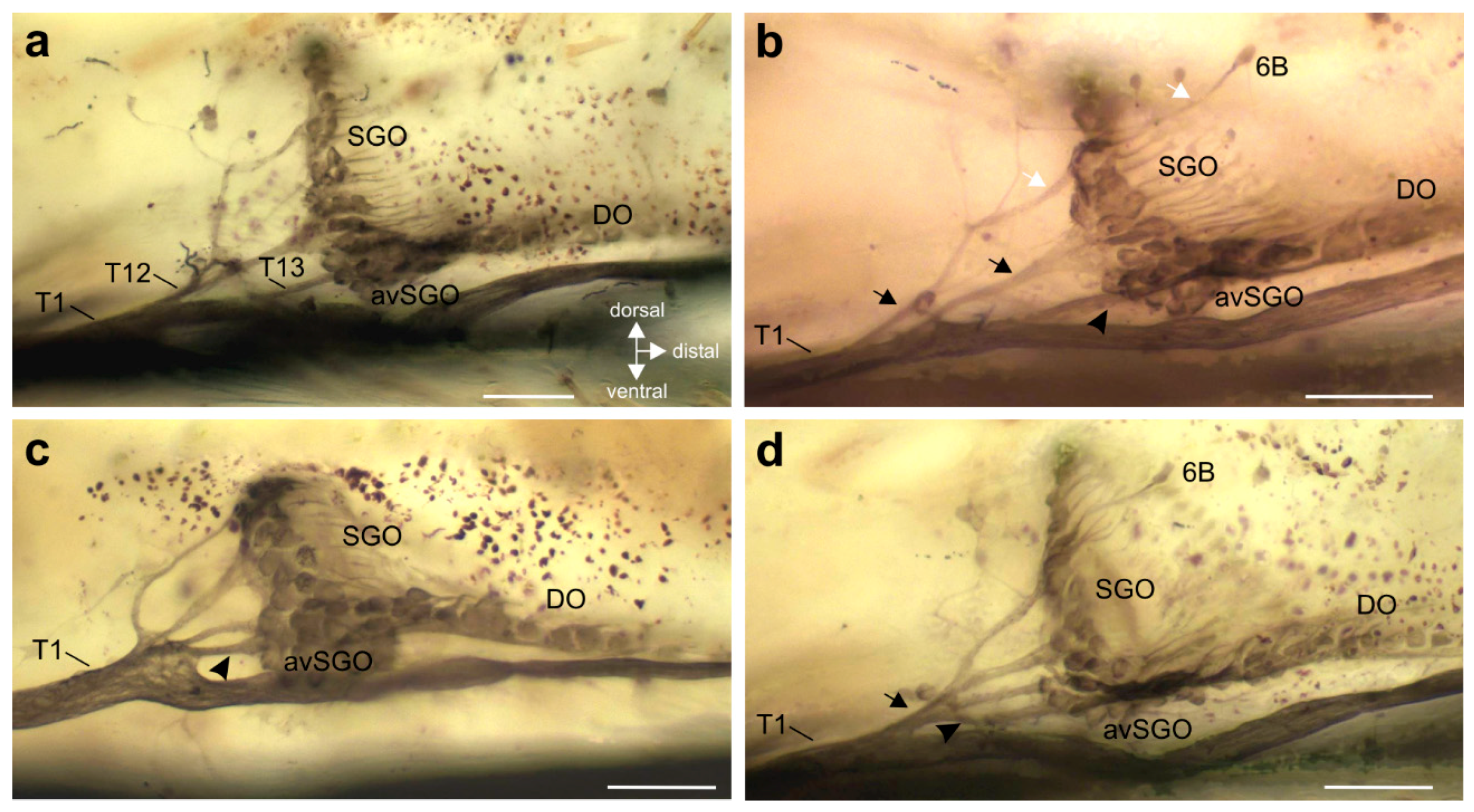
3.2. Innervation Pattern of Sensory Elements in the Proximal Tibia
3.3. Variation in the Innervation of Sensory Structures
4. Discussion
4.1. Innervation of the Subgenual Organ Complex and the Campaniform Sensilla in the Proximal Tibia
4.2. Variation in the Neuronal Innervation
4.3. Functional Aspects of Innervations Considering the Central Projections
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Iwasaki, M.; Itoh, T.; Tominaga, Y. Mechano- and phonoreceptors. In Atlas of Arthropod Sensory Receptors. Dynamic Morphology in Relation to Function; Eguchi, E., Tominaga, Y., Eds.; Springer: Tokyo, Japan, 1999; pp. 177–196. [Google Scholar]
- Devetak, D.; Pabst, M.A.; Delakorda, S.L. Leg chordotonal organs and campaniform sensilla in Chrysoperla Steinmann 1964 (Neuroptera): Structure and function. Denisia 2004, 13, 163–171. [Google Scholar]
- Čokl, A.; Virant-Doberlet, M.; Zorović, M. Sense organs involved in the vibratory communication of bugs. In Insect Sounds and Communication: Physiology, Behaviour, Ecology and Evolution; Drosopoulos, S., Claridge, M., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 71–80. [Google Scholar]
- Joel, A.-C.; Adamova, H.; Bräunig, P. Mechanoreceptive sensillum fields at the tarsal tip of insect legs. J. Morphol. 2018, 279, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Nowińska, A.; Brożek, J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology 2019, 138, 307–319. [Google Scholar] [CrossRef]
- Wang, X.-S.; Shaukat, A.; Han, Y.; Yang, B.; Tang, L.-D.; Wu, J.-H. Morphology and distribution of the antennal sensilla of two species, Megalurothrips usitatus and Thrips palmi (Thysanoptera: Thripidae). Insects 2019, 10, 251. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, N.; Brożek, J.; Dai, W. Antennal morphology and sexual dimorphism of antennal sensilla in Callitettix versicolor (Fabricius) (Hemiptera: Cercopidae). Insects 2019, 10, 56. [Google Scholar] [CrossRef]
- Godden, D.H. The motor innervation of the leg musculature and motor output during thanatosis in the stick insect, Carausius morosus Br. J. Comp. Physiol. 1972, 80, 201–225. [Google Scholar] [CrossRef]
- Bässler, U. Neural Basis of Elementary Behaviour on Stick Insects; Springer: Berlin, Germany, 1983. [Google Scholar]
- Schumacher, R. Morphologische Untersuchungen der tibialen Tympanalorgane von neun einheimischen Laubheuschrecken-Arten (Orthoptera, Tettigonioidea). Zoomorphology 1973, 75, 267–282. [Google Scholar] [CrossRef]
- Hustert, R.; Pflüger, J.H.; Bräunig, P. Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. III. The external mechanoreceptors: The campaniform sensilla. Cell Tissue Res. 1981, 216, 97–111. [Google Scholar] [CrossRef]
- Mücke, A. Innervation pattern and sensory supply of the midleg of Schistocerca gregaria (Insecta, Orthopteroidea). Zoomorphology 1991, 110, 175–187. [Google Scholar] [CrossRef]
- Nishino, H.; Field, L.H. Somatotopic mapping of chordotonal organ neurons in a primitive ensiferan, the New Zealand tree weta Hemideina femorata: II. Complex tibial organ. J. Comp. Neurol. 2003, 464, 327–342. [Google Scholar] [CrossRef]
- Keil, T. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 1997, 39, 506–531. [Google Scholar] [CrossRef]
- Field, L.H.; Matheson, T. Chordotonal organs of insects. Adv. Insect Physiol. 1998, 27, 1–228. [Google Scholar]
- Strauß, J.; Lakes-Harlan, R. Vibrational sensitivity of the subgenual organ complex in female Sipyloidea sipylus stick insects in different experimental paradigms of stimulus direction, leg attachment, and ablation of a connective tibial sense organ. Comp. Biochem. Physiol. A 2017, 203, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J.; Stritih, N.; Lakes-Harlan, R. The subgenual organ complex in the cave cricket Troglophilus neglectus (Orthoptera: Rhaphidophoridae): Comparative innervation and sensory evolution. R. Soc. Open Sci. 2014, 1, 140240. [Google Scholar] [CrossRef]
- Strauß, J.; Lakes-Harlan, R. Sensory neuroanatomy of stick insects highlights the evolutionary diversity of the orthopteroid subgenual organ complex. J. Comp. Neurol. 2013, 521, 3791–3803. [Google Scholar] [CrossRef]
- Kalmring, K.; Rössler, W.; Unrast, C. Complex tibial organs in the forelegs, midlegs, and hindlegs of the bushcricket Gampsocleis gratiosa (Tettigoniidae): Comparison of the physiology of the organs. J. Exp. Zool. 1994, 270, 155–161. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Ann. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef]
- Tuthill, J.C.; Wilson, R.A. Mechanosensation and adaptive motor control in insects. Curr. Biol. 2016, 26, R1022–R1038. [Google Scholar] [CrossRef]
- Delcomyn, F. Activity and directional sensitivity of leg campaniform sensilla in a stick insect. J. Comp. Physiol. A 1991, 168, 113–119. [Google Scholar] [CrossRef]
- Noah, J.A.; Quimby, L.; Frazier, S.F.; Zill, S.N. Force detection in cockroach walking reconsidered: Discharges of proximal tibial campaniform sensilla when body load is altered. J. Comp. Physiol. A 2001, 187, 769–784. [Google Scholar] [CrossRef]
- Ritzmann, R.E.; Zill, S.N. Walking and jumping. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 1044–1048. [Google Scholar]
- Zill, S.N.; Büschges, A.; Schmitz, J. Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J. Comp. Physiol. A 2011, 197, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Zill, S.N.; Schmitz, J.; Chaudhry, S.; Büschges, A. Force encoding in stick insect legs delineates a reference frame for motor control. J. Neurophysiol. 2012, 108, 1453–1472. [Google Scholar] [CrossRef] [PubMed]
- Zill, S.N.; Chaudhry, S.; Büschges, A.; Schmitz, J. Directional specificity and encoding of muscle forces and loads by stick insect campaniform sensilla, including receptors with round cuticular caps. Arthropod Struct. Dev. 2013, 42, 455–467. [Google Scholar] [CrossRef]
- Zill, S.N.; Chaudhry, S.; Büschges, A.; Schmitz, J. Force feedback reinforces muscle synergies in insect legs. Arthropod Struct. Dev. 2015, 44, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, A.; Gruhn, M.; Zill, S.N.; Büschges, A. Identification of the origin of force feedback signals influencing motor neurons of the thoraco-coxal joint in an insect. J. Comp. Physiol. A 2019, 205, 253–270. [Google Scholar] [CrossRef]
- Schnorbus, H. Die subgenualen Sinnesorgane von Periplaneta americana: Histologie und Vibrationsschwellen. Zeitschrift für vergleichende Physiologie 1971, 71, 14–48. [Google Scholar]
- Kühne, R. Neurophysiology of the vibration sense in locusts and bushcrickets: Response characteristics of single receptor units. J. Insect Physiol. 1982, 28, 155–163. [Google Scholar] [CrossRef]
- Stritih-Peljhan, N.; Rühr, P.T.; Buh, B.; Strauß, J. Low-frequency vibration transmission and mechanosensory detection in the legs of cave crickets. Comp. Biochem. Physiol. A 2019, 233, 89–96. [Google Scholar] [CrossRef]
- Zill, S.N.; Schmitz, J.; Büschges, A. Load sensing and control of posture and locomotion. Arthropod Struct. Dev. 2004, 22, 273–286. [Google Scholar] [CrossRef]
- Büschges, A.; Gruhn, M. Mechanosensory feedback in walking: From joint control to locomotor patterns. Adv. Insect Physiol. 2008, 34, 193–230. [Google Scholar]
- Bidaye, S.S.; Bockemühle, T.; Büschges, A. Six-legged walking in insects: How CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J. Neurophysiol. 2018, 119, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Lakes, R.; Mücke, A. Regeneration of the foreleg tibia and tarsi of Ephippiger ephippiger (Orthoptera: Tettigoniidae). J. Exp. Zool. 1989, 250, 176–187. [Google Scholar] [CrossRef]
- Strauß, J.; Lomas, K.; Field, L.H. The complex tibial organ of the New Zealand ground weta: Sensory adaptations for vibrational signal detection. Sci. Rep. 2017, 7, 2031. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, U. Postembryonic ontogeny in Sipyloidea sipylus. Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere 1987, 115, 273–279. [Google Scholar]
- Stein, W.; Sauer, A.E. Physiology of vibration-sensitive afferents in the femoral chordotonal organ of the stick insect. J. Comp. Physiol. A 1999, 184, 253–263. [Google Scholar] [CrossRef]
- Akay, T.; Bässler, U.; Gerharz, P.; Büschges, A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J. Neurophysiol. 2001, 85, 594–604. [Google Scholar] [CrossRef]
- Von Twickel, A.; Guschlbauer, C.; Hooper, S.L.; Büschges, A. Swing velocity profiles of small limbs can arise from transient passive torques of the antagonist muscle alone. Curr. Biol. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Mulisch, M.; Welsch, U. (Eds.) Romeis—Mikroskopische Technik, 19th ed.; Springer: Berlin, Germany, 2015. [Google Scholar]
- Pitman, R.M.; Tweedle, C.D.; Cohen, M.J. The form of nerve cells: Determination by cobalt impregnation. In Intracellular Staining in Neurobiology; Nicholson, C., Kater, S.B., Eds.; Springer: Berlin, Germany, 1973; pp. 83–97. [Google Scholar]
- Bässler, U. Sense organs in the femur of the stick insect and their relevance to the control of position of the femur-tibia-joint. J. Comp. Physiol. 1977, 121, 99–113. [Google Scholar] [CrossRef]
- Goldammer, J.; Büschges, A.; Schmidt, J. Motoneurons, DUM Cells, and sensory neurons in an insect thoracic ganglion: A tracing study in the stick insect Carausius morosus. J. Comp. Neurol. 2012, 520, 230–257. [Google Scholar] [CrossRef]
- Büschges, A. The physiology of sensory cells in the ventral scoloparium of the stick insect femoral chordotonal organ. J. Exp. Biol. 1994, 189, 285–292. [Google Scholar]
- Strauß, J. The scolopidial accessory organs and Nebenorgans in orthopteroid insects: Comparative neuroanatomy, mechanosensory function and evolutionary origin. Arthropod Struct. Dev. 2017, 46, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J. Are the scolopidial accessory organs and Nebenorgans in orthopteroid insects homologous? A comparative neuroanatomical analysis. Mitteilungen der Deutschen Gesellschaft für Allgemeine und Angewandte Entomologie 2018, 21, 165–169. [Google Scholar]
- Strauß, J.; Stritih, N. Neuronal regression of internal leg vibroreceptor organs in a cave-dwelling insect (Orthoptera: Rhaphidophoridae: Dolichopoda araneiformis). Brain Behav. Evol. 2017, 89, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J. Scolopidial accessory organ in the Jerusalem cricket (Orthoptera: Stenopelmatidae). Arthropod Struct. Dev. 2017, 46, 171–177. [Google Scholar] [CrossRef]
- Keshishian, H.; Bentley, D. Embryogenesis of peripheral nerve pathways in grasshopper leg. II. The major nerve routes. Dev. Biol. 1983, 96, 103–115. [Google Scholar] [CrossRef]
- Hartenstein, V. Development of insect sensilla. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Reproduction and development; Elsevier: Amsterdam, The Netherlands, 2005; Volume 1, pp. 379–419. [Google Scholar]
- Bonner, J.; O’Connor, T.P. Semaphorin function in the developing invertebrate peripheral nervous system. Biochem. Cell Biol. 2000, 78, 603–611. [Google Scholar] [CrossRef]
- Isbister, C.M.; Mackenzie, P.J.; To, K.C.; O’Connor, T.P. Gradient steepness influences the pathfinding decisions of neuronal growth cones in vivo. J. Neurosci. 2003, 23, 193–202. [Google Scholar] [CrossRef]
- Pflüger, H.-J.; Bräunig, P.; Hustert, R. The organization of mechanosensory neuropiles in locust thoracic ganglia. Philos. Trans. R. Soc. Lond. B 1988, 321, 1–26. [Google Scholar] [CrossRef]
- Blagburn, J.M.; Bacon, J.P. Control of central synaptic specificity in insect sensory neurons. Ann. Rev. Neurosci. 2004, 27, 29–51. [Google Scholar] [CrossRef]
- Kent, K.S.; Levine, R.B. Neural control of leg movements in a metamorphic insect: Sensory and motor elemenst of the larval thoracic legs in Manduca sexta. J. Comp. Neurol. 1988, 271, 559–576. [Google Scholar] [CrossRef]
- Thompson, K.S.; Blagburn, J.M.; Gibbon, C.R.; Bacon, J.P. Correlation of filiform hair position with sensory afferent morphology and synaptic connections in the second instar cockroach. J. Comp. Neurol. 1992, 320, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Mücke, A.; Lakes-Harlan, R. Central projections of sensory cells of the midleg of the locust, Schistocerca gregaria. Cell Tissue Res. 1995, 280, 391–400. [Google Scholar] [CrossRef]
- Nishino, H. Topographic mapping of the axons of the femoral chordotonal organ neurons in the cricket Gryllus bimaculatus. Cell Tissue Res. 2000, 299, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H. Somatotopic mapping of chordotonal organ neurons in a primitive ensiferan, the New Zealand tree weta Hemideina femorata: I. Femoral chordotonal organ. J. Comp. Neurol. 2003, 464, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Bräunig, P.; Pflüger, H.-J.; Hustert, R. The specificity of central nervous projections of locust mechanoreceptors. J. Comp. Neurol. 1983, 218, 197–207. [Google Scholar] [CrossRef]
- Schmitz, J.; Dean, J.; Kittmann, R. Central projections of leg sensory organs in Carausius morosus (Insecta, Phasmida). Zoomorphology 1991, 111, 19–33. [Google Scholar] [CrossRef]




© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauß, J. Neuronal Innervation of the Subgenual Organ Complex and the Tibial Campaniform Sensilla in the Stick Insect Midleg. Insects 2020, 11, 40. https://doi.org/10.3390/insects11010040
Strauß J. Neuronal Innervation of the Subgenual Organ Complex and the Tibial Campaniform Sensilla in the Stick Insect Midleg. Insects. 2020; 11(1):40. https://doi.org/10.3390/insects11010040
Chicago/Turabian StyleStrauß, Johannes. 2020. "Neuronal Innervation of the Subgenual Organ Complex and the Tibial Campaniform Sensilla in the Stick Insect Midleg" Insects 11, no. 1: 40. https://doi.org/10.3390/insects11010040
APA StyleStrauß, J. (2020). Neuronal Innervation of the Subgenual Organ Complex and the Tibial Campaniform Sensilla in the Stick Insect Midleg. Insects, 11(1), 40. https://doi.org/10.3390/insects11010040