Rectal Gland Chemistry, Volatile Emissions, and Antennal Responses of Male and Female Banana Fruit Fly, Bactrocera musae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Banana Fruit Fly Rearing
2.2. Rectal Gland Extractions
2.3. Headspace Collections
2.4. GC-MS Analysis
2.5. GC-EAD Experiments
3. Results
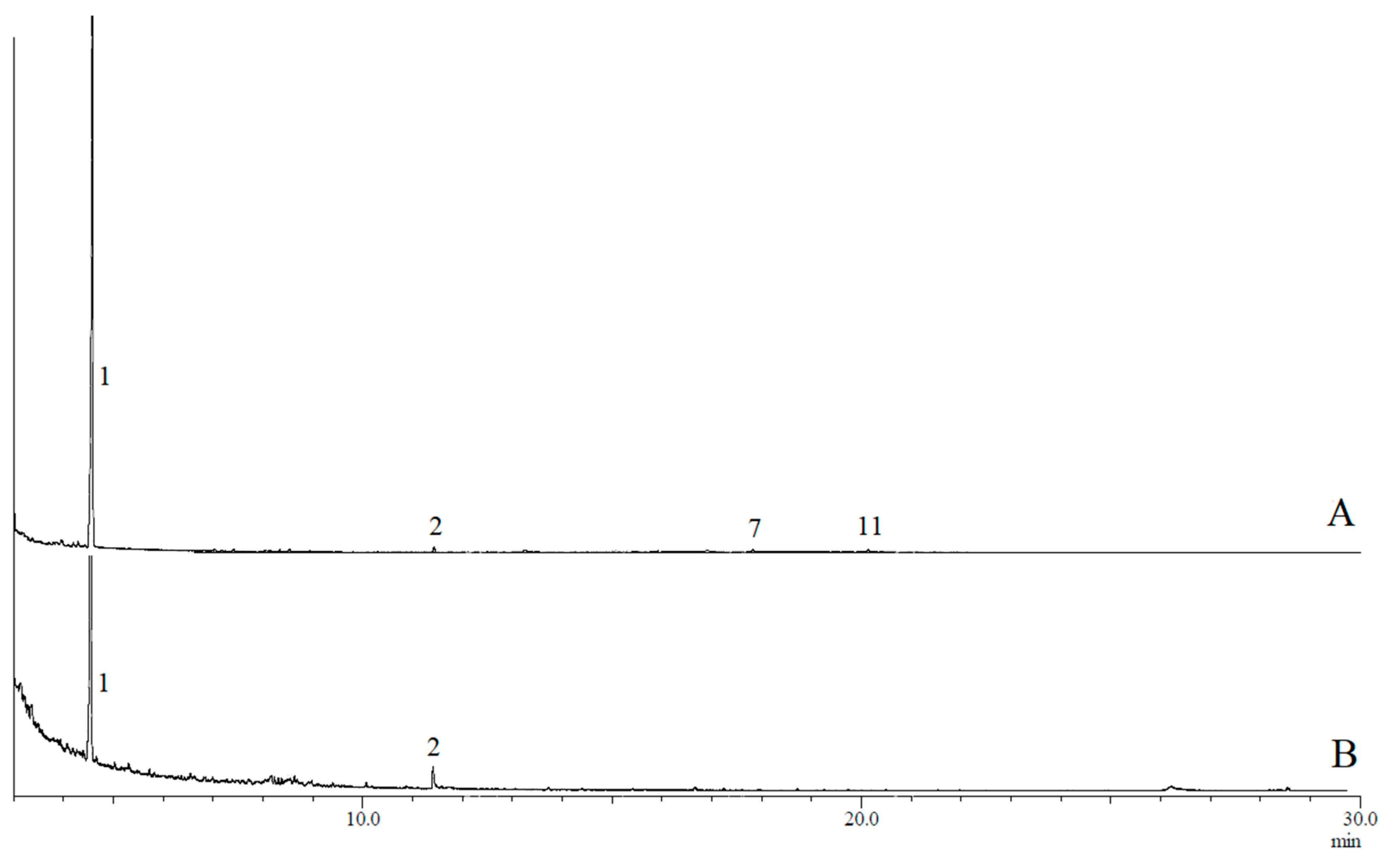
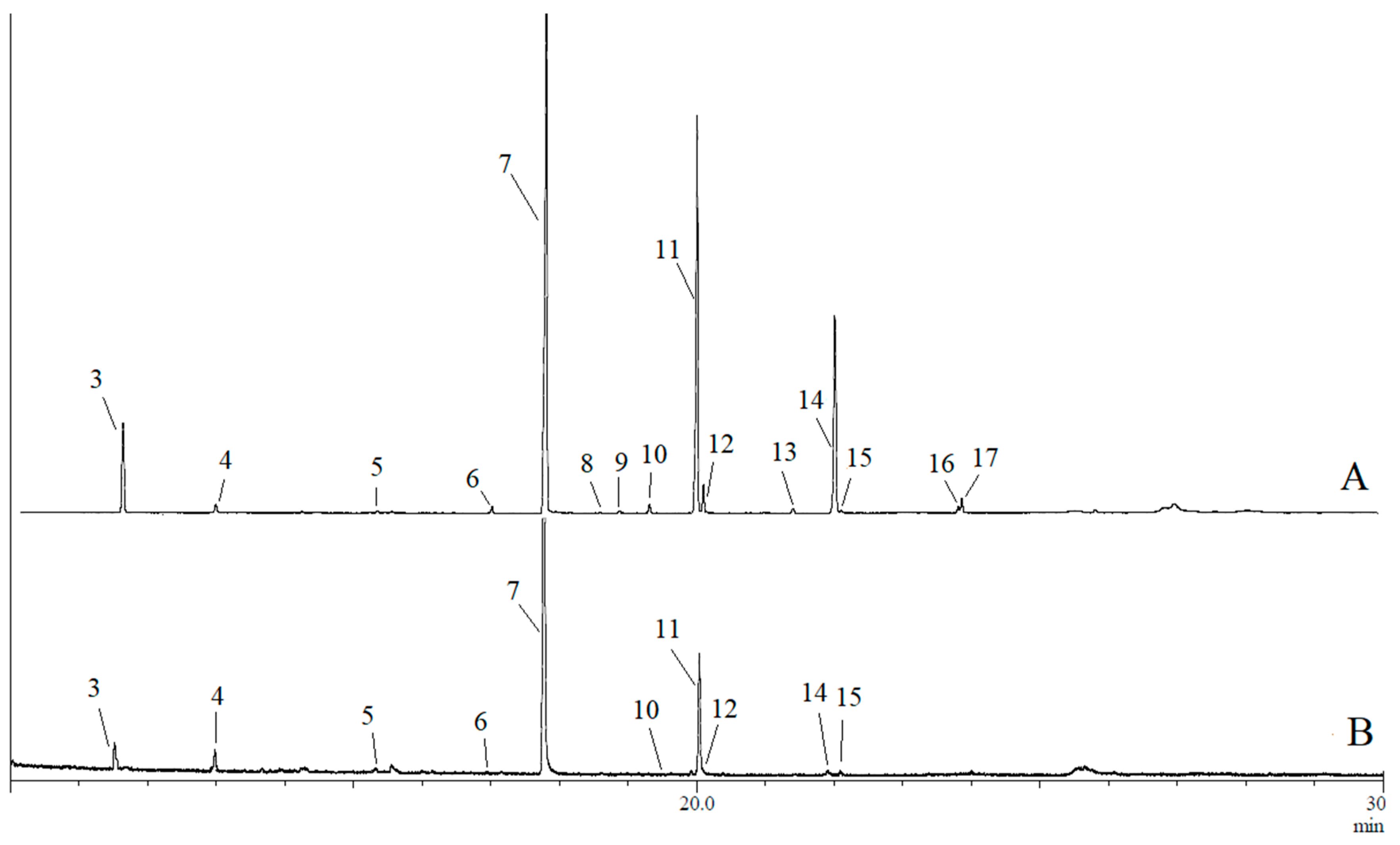
3.1. Analysis of Volatile Compounds
3.2. Assignment of Compounds
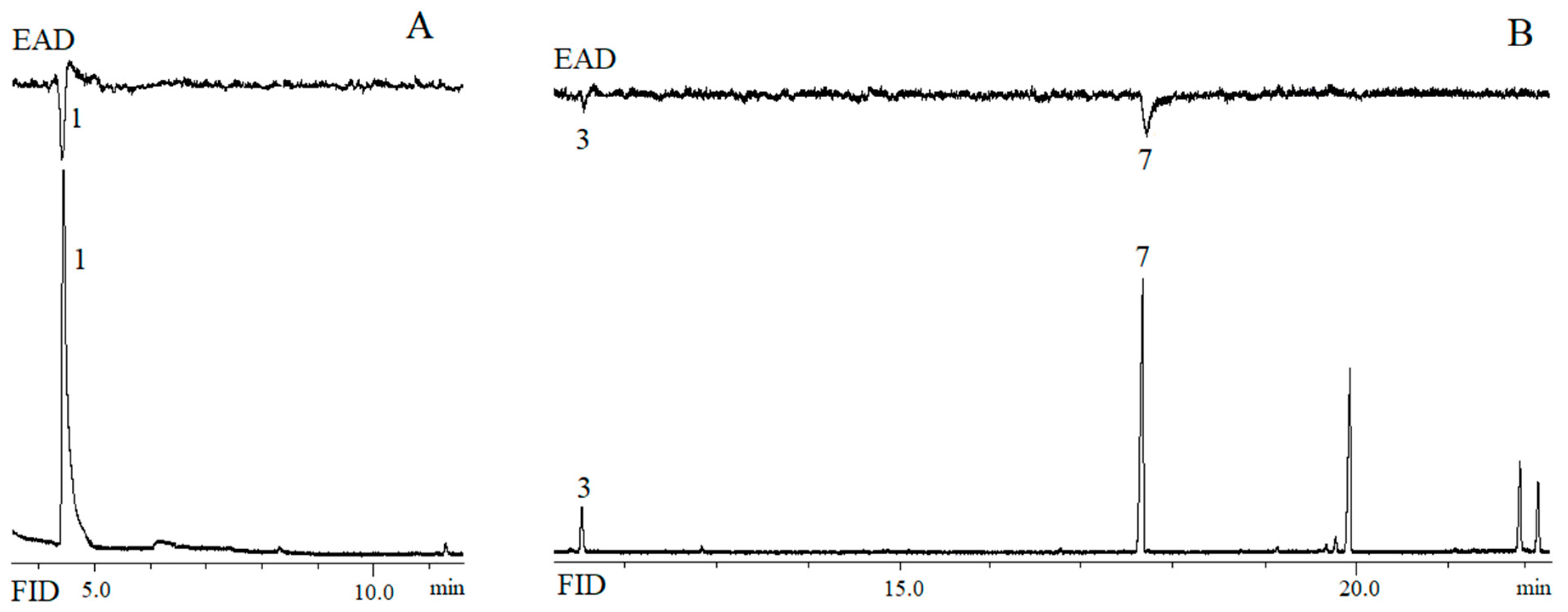
3.3. GC-EAD Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Daane, K.M.; Canale, A.; Niu, C.-Y.; Messing, R.H.; Vargas, R.I. Sexual communication and related behaviours in Tephritidae: Current knowledge and potential applications for integrated pest management. J. Pest Sci. 2014, 87, 385–405. [Google Scholar] [CrossRef]
- Perkins, M.V.; Fletcher, M.T.; Kitching, W.; Drew, R.A.I.; Moore, C.J. Chemical studies of rectal gland secretions of some species of Bactrocera dorsalis complex of fruit flies (diptera: Tephritidae). J. Chem. Ecol. 1990, 16, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, P. Insect sex-communication and prospects for pheromones in pest management. Bolletino di Zool. 1980, 47, 397–408. [Google Scholar] [CrossRef]
- Tokushima, I.; Orankanok, W.; Tan, K.H.; Ono, H.; Nishida, R. Accumulation of phenylpropanoid and sesquiterpenoid volatiles in male rectal pheromonal glands of the guava fruit fly, Bactrocera correcta. J. Chem. Ecol. 2010, 36, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L.; Tan, K.H. Female sexual response to male rectal volatile constituents in the fruit fly, Bactrocera carambolae (Diptera: Tephritidae). Appl. Entomol. Zool. 2005, 40, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Nation, J.L. Courtship behavior and evidence for a sex attractant in the male Caribbean fruit fly, Anastrepha suspensa. Ann. Entomol. Soc. Am. 1972, 65, 1364–1367. [Google Scholar] [CrossRef]
- Perkins, M.V. Characterisation and Synthesis of Bactrocera Fruit Fly Pheromones. Ph.D. Thesis, Department of Chemistry, The University of Queensland, Brisbane, Australia, 1990. [Google Scholar]
- Sivinski, J.; Aluja, M.; Dodson, G.N.; Freidberg, A.; Headrick, D.H.; Kaneshiro, K.Y.; Landolt, P.J. Topics in the evolution of sexual behavior in the Tephritidae. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 751–792. ISBN 9781420074468. [Google Scholar]
- Cruz-López, L.; Malo, E.A.; Rojas, J.C. Sex pheromone of Anastrepha striata. J. Chem. Ecol. 2015, 41, 458–464. [Google Scholar] [CrossRef]
- Sivinski, J.M.; Calkins, C. Use of pheromones in tropical crops: Pheromones and parapheromones in the control of tephritids. Fla. Entomol. 1986, 69, 157–168. [Google Scholar] [CrossRef]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Fla. Entomol. 2002, 85, 1–14. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com/ (accessed on 17 March 2019).
- Heath, R.R.; Landolt, P.J.; Robacker, D.C.; Dueben, B.D.; Epsky, N.D. Sexual pheromones of tephritid flies: Clues to unravel phylogeny and behavior. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 793–809. [Google Scholar]
- Haniotakis, G.E. Sexual attraction in the olive fruit fly, Dacus oleae (Gmelin). Environ. Entomol. 1974, 3, 82–86. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. Male olive fruit fly attraction to synthetic sex pheromone components in laboratory and field tests. J. Chem. Ecol. 1985, 11, 397–405. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.H.; Lomer, R.A. Chemical components of the rectal gland secretions of male Dacus cucurbitae, the melon fly. Experientia 1982, 38, 232–233. [Google Scholar] [CrossRef]
- Baker, R.; Bacon, A.J. The identification of spiroacetals in the volatile secretions of two species of fruit fly (Dacus dorsalis, Dacus curcurbitae). Experientia 1985, 41, 1484–1485. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Serit, M.; Lajis, N.H.; Sukari, A.M.; Takahashi, S.; Fukami, H. Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis. Experientia 1988, 44, 534–536. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Fukami, H. Cis-3,4-dimethoxycinnamyl alcohol from the rectal glands of male oriental fruit fly, Dacus dorsalis. Chem. Express 1988, 3, 207–210. [Google Scholar]
- Drew, R.A.I.; Hooper, G.H.S.; Bateman, M.A. Economic fruit flies of the South Pacific Region, 2nd ed.; Queensland Department of Primary Industries: Brisbane, Australia, 1982.
- Hancock, D.L.; Hamacek, E.L.; Lloyd, A.C.; Elson-Harris, M.M. The Distribution and Host Plants of Fruit Flies (Diptera: Tephritidae) in Australia; Queensland Department of Primary Industries: Brisbane, Australia, 2000.
- Schutze, M.; McMahon, J.; Krosch, M.; Strutt, F.; Royer, J.; Bottrill, M.; Woods, N.; Cameron, S.; Woods, B.; Blacket, M. The Australian Handbook for the Identification of Fruit Flies; Version 3.1; Plant Health Australia: Canberra, Australia, 2018; ISBN 9780648245605. [Google Scholar]
- Hamacek, E. Host records of fruit flies in the South Pacific. In Management of Fruit Flies in the Pacific; Allwood, A.J., Drew, R.A.I., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1997; p. 102. [Google Scholar]
- Leblanc, L.; Tora, E.; Drew, R.A.I.; Allwood, A.J. Host plant records for fruit flies (Diptera: Tephritidae: Dacini) in the Pacific Islands. Proc. Hawaiian Entomol. Soc. 2013, 44, 11–53. [Google Scholar]
- Baker, R.; Herbert, R.H. Isolation and synthesis of 1,7-dioxaspiro[5.5]undecane and 1,7-dioxaspiro[5.5]undecan-3-and -4-ols from the olive fly (Dacus oleae). J. Chem. Soc. Perkin Trans. 1 1987, 1123. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.; Howse, P.E.; Jones, O.T.; Francke, W.; Reith, W. Identification and synthesis of the major sex pheromone of the olive fly (Dacus oleae). J. Chem. Soc. Chem. Commun. 1980, 52–53. [Google Scholar] [CrossRef]
- Fletcher, B.S. Storage and release of sex pheromone by the Queensland fruit fly, Dacus tryoni (Diptera:Trypetidae). Nature 1968, 219, 631–632. [Google Scholar] [CrossRef]
- Bellas, T.E.; Fletcher, B.S. Identification of the major components in the secretion from the rectal pheromone glands of the Queensland fruit flies Dacus tryoni and Dacus neohumeralis (Diptera: Tephritidae). J. Chem. Ecol. 1979, 5, 795–803. [Google Scholar] [CrossRef]
- Nation, J.L. Biology of pheromone release by male Caribbean fruit flies, Anastrepha suspensa (Diptera: Tephritidae). J. Chem. Ecol. 1990, 16, 553–572. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992; ISBN 0851987907.
- Steiner, L.F.; Mitchell, S. Tephritid fruit flies. In Insect Colonization and Mass Production; Smith, C.N., Ed.; Academic Press: Cambridge, MA, USA, 1966; pp. 555–583. ISBN 978-0-12-395601-9. [Google Scholar]
- Pérez, J.; Park, S.J.; Taylor, P.W. Domestication modifies the volatile emissions produced by male Queensland fruit flies during sexual advertisement. Sci. Rep. 2018, 8, 16503. [Google Scholar] [CrossRef] [PubMed]
- Kitching, W.; Lewis, J.A.; Perkins, M.V.; Drew, R.; Moore, C.J.; Schurig, V.; Koenig, W.A.; Francke, W. Chemistry of fruit flies. Composition of the rectal gland secretion of (male) Dacus cucumis (cucumber fly) and Dacus halfordiae. Characterization of (Z,Z)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane. J. Org. Chem. 1989, 54, 3893–3902. [Google Scholar] [CrossRef]
- Aluja, M.; Norrbom, A.L. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 0849312752. [Google Scholar]
- El-Sayed, A.M.; Byers, J.A.; Manning, L.M.; Jürgens, A.; Mitchell, V.J.; Suckling, D.M. Floral scent of Canada thistle and its potential as a generic insect attractant. J. Econ. Entomol. 2008, 101, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Booth, Y.K.; Hayes, P.Y.; Moore, C.J.; Lambert, L.K.; Kitching, W.; De Voss, J.J. Synthesis and absolute configuration of a constitutionally-new [5.6] spiroacetal from B. tryoni (Queensland fruit fly). Org. Biomol. Chem. 2007, 5, 1111–1117. [Google Scholar] [CrossRef]
- Gilpin, J.A. Mass spectrometric analysis of aliphatic amides. Anal. Chem. 1959, 31, 935–939. [Google Scholar] [CrossRef]
- Sharkey, A.G.; Shultz, J.L.; Friedel, R.A. Mass spectra of esters. Formation of rearrangement ions. Anal. Chem. 1959, 31, 87–94. [Google Scholar] [CrossRef]
- Francke, W.; Kitching, W. Spiroacetals in insects. Curr. Org. Chem. 2001, 5, 233–251. [Google Scholar] [CrossRef]
- Schwartz, B.D.; Booth, Y.K.; Fletcher, M.T.; Kitching, W.; Voss, J.J. De Spiroacetal biosynthesis in fruit flies is complex: Distinguishable origins of the same major spiroacetal released by different Bactrocera spp. Chem. Commun. 2010, 46, 1526–1528. [Google Scholar] [CrossRef]
- López-Guillén, G.; Cruz-López, L.; Malo, E.A.; González-Hernández, H.; Cazares, C.L.; López-Collado, J.; Toledo, J.; Rojas, J.C. Factors influencing the release of volatiles in Anastrepha obliqua males (Diptera: Tephritidae). Environ. Entomol. 2008, 37, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Bosa, C.F.; Cruz-López, L.; Zepeda-Cisneros, C.S.; Valle-Mora, J.; Guillén-Navarro, K.; Liedo, P. Sexual behavior and male volatile compounds in wild and mass-reared strains of the Mexican fruit fly Anastrepha ludens (Diptera: Tephritidae) held under different colony management regimes. Insect Sci. 2016, 23, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Vaníčková, L.; Do Nascimento, R.R.; Hoskovec, M.; Ježková, Z.; Břízová, R.; Tomčala, A.; Kalinová, B. Are the wild and laboratory insect populations different in semiochemical emission? the case of the medfly sex pheromone. J. Agric. Food Chem. 2012, 60, 7168–7176. [Google Scholar] [CrossRef] [PubMed]
- Kamala Jayanthi, P.D.; Woodcock, C.M.; Caulfield, J.; Birkett, M.A.; Bruce, T.J.A. Isolation and identification of host cues from mango, Mangifera indica, that attract gravid female Oriental fruit fly, Bactrocera dorsalis. J. Chem. Ecol. 2012, 38, 361–369. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M.; Venkatesham, U.; Unelius, C.R.; Sporle, A.; Pérez, J.; Taylor, P.W.; Suckling, D.M. Chemical composition of the rectal gland and volatiles released by female Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Environ. Entomol. 2019, 48, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.T.; Kitching, W. Chemistry of fruit flies. Chem. Rev. 1995, 95, 789–828. [Google Scholar] [CrossRef]
- Booth, Y.K.; Schwartz, B.D.; Fletcher, M.T.; Lambert, L.K.; Kitching, W.; De Voss, J.J. A diverse suite of spiroacetals, including a novel branched representative, is released by female Bactrocera tryoni (Queensland fruit fly). Chem. Commun. 2006, 3975–3977. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. A multicomponent female sex pheromone of Dacus oleae Gmelin: Isolation and bioassay. J. Chem. Ecol. 1981, 7, 437–444. [Google Scholar] [CrossRef]
- Shen, J.; Hu, L.; Zhou, X.; Dai, J.; Chen, B.; Li, S. Allyl-2,6-dimethoxyphenol, a female-biased compound, is robustly attractive to conspecific males of Bactrocera dorsalis at close range. Entomol. Exp. Appl. 2019, 167, 811–819. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Jacobs, M.F.; Kitching, W.; Krohn, S.; Drew, R.A.I.; Haniotakis, G.E.; Francke, W. Absolute stereochemistry of the 1,7-dioxaspiro[5.5]undecanols in fruit-fly species, including the olive-fly. J. Chem. Soc. Chem. Commun. 1992, 1457–1459. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.H.; Grant, G.G. Isolation and identification of the sex pheromone of the Mediterranean fruit fly, Ceratitis capitata (Wied). J. Chem. Soc. Chem. Commun. 1985, 824–825. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Moussalli, A.; Elgar, M.A. The evolution of sex pheromones in an ecologically diverse genus of flies. Biol. J. Linn. Soc. 2009, 97, 594–603. [Google Scholar] [CrossRef]
- Perkins, M.V.; Kitching, W.; Drew, R.A.I.; Moore, C.J.; Konig, W.A. Chemistry of fruit flies: Composition of the male rectal gland secretions of some species of South-East Asian Dacinae. Re-examination of Dacus cucurbitae (melon fly). J. Chem. Soc. Perkin Trans. 1 1990, 1111–1117. [Google Scholar] [CrossRef]
- Perkins, M.V.; Kitching, W.; König, W.A.; Drew, R.A.I. An (S)-(+)-lactic acid route to (2S,6R,8S)-2,8 dimethyl-1,7-dioxaspiro[5,5]undecane and (2S,6R,8S)-2-ethyl-8-methyl-1,7-dioxaspiro[5,5]undecane and demonstration of their presence in the rectal glandular se. J. Chem. Soc. Perkin Trans. 1 1990, 2501–2506. [Google Scholar] [CrossRef]
| KI | Compound | Female | Male | ||||
|---|---|---|---|---|---|---|---|
| Rectal Glands | Headspace | Rectal Glands | Headspace | ||||
| 2014 | 2017 | 2017 | 2014 | 2017 | 2017 | ||
| 828 | Ethyl butanoate (1) | ND | ND | ND | 99.0 | 99.2 | 98.3 |
| 1162 | N-(3-Methylbutyl)acetamide (2) | ND | ND | ND | <1 | <1 | 1.7 |
| 1179 | (E,E)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane (3) | 5.0 | 5.7 | 2.1 | ND | ND | ND |
| 1266 | (E,E)-2-Ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane (4) | <1 | <1 | 2.0 | ND | ND | ND |
| 1437 | Ethyl caprate (5) | <1 | <1 | <1 | ND | ND | ND |
| 1570 | Methyl laurate (6) | <1 | <1 | <1 | ND | ND | ND |
| 1637 | Ethyl laurate (7) | 40.0 | 47.3 | 72.5 | <1 | ND | ND |
| 1705 | Ethyl tridecanaote (8) | <1 | <1 | ND | ND | ND | ND |
| 1731 | Propyl laurate (9) | <1 | <1 | ND | ND | ND | ND |
| 1771 | Methyl myristate (10) | <1 | <1 | <1 | ND | ND | ND |
| 1837 | Ethyl myristate (11) | 21.8 | 25.2 | 19.2 | <1 | ND | ND |
| 1845 | Ethyl myristoleate (12) | 1.9 | 1.7 | <1 | ND | ND | ND |
| 1974 | Methyl palmitate (13) | <1 | <1 | ND | ND | ND | ND |
| 2037 | Ethyl palmitate (14) | 21.7 | 16.4 | 1.6 | ND | ND | ND |
| 2047 | Ethyl palmitoleate (15) | <1 | <1 | <1 | ND | ND | ND |
| 2233 | Ethyl oleate (16) | <1 | <1 | ND | ND | ND | ND |
| 2239 | Ethyl elaidate (17) | <1 | <1 | ND | ND | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noushini, S.; Perez, J.; Park, S.J.; Holgate, D.; Jamie, I.; Jamie, J.; Taylor, P. Rectal Gland Chemistry, Volatile Emissions, and Antennal Responses of Male and Female Banana Fruit Fly, Bactrocera musae. Insects 2020, 11, 32. https://doi.org/10.3390/insects11010032
Noushini S, Perez J, Park SJ, Holgate D, Jamie I, Jamie J, Taylor P. Rectal Gland Chemistry, Volatile Emissions, and Antennal Responses of Male and Female Banana Fruit Fly, Bactrocera musae. Insects. 2020; 11(1):32. https://doi.org/10.3390/insects11010032
Chicago/Turabian StyleNoushini, Saeedeh, Jeanneth Perez, Soo Jean Park, Danielle Holgate, Ian Jamie, Joanne Jamie, and Phillip Taylor. 2020. "Rectal Gland Chemistry, Volatile Emissions, and Antennal Responses of Male and Female Banana Fruit Fly, Bactrocera musae" Insects 11, no. 1: 32. https://doi.org/10.3390/insects11010032
APA StyleNoushini, S., Perez, J., Park, S. J., Holgate, D., Jamie, I., Jamie, J., & Taylor, P. (2020). Rectal Gland Chemistry, Volatile Emissions, and Antennal Responses of Male and Female Banana Fruit Fly, Bactrocera musae. Insects, 11(1), 32. https://doi.org/10.3390/insects11010032







