Functional Characterization of a Venom Protein Calreticulin in the Ectoparasitoid Pachycrepoideus vindemiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Sequence and Phylogenetic Analysis
2.3. Venom Apparatus Collection and Isolation of Total RNA
2.4. cDNA Synthesis and Quantitative Real-Time PCR
2.5. Gene Cloning
2.6. Gal4-Driven Expression of PvCRT
2.7. Genomic DNA Extraction and PCR Detection
2.8. Western Blotting
2.9. Immunofluorescence Staining
2.10. Bacterial Infection and Survival Analysis of D. melanogaster Adults
2.11. Data Analysis
3. Results
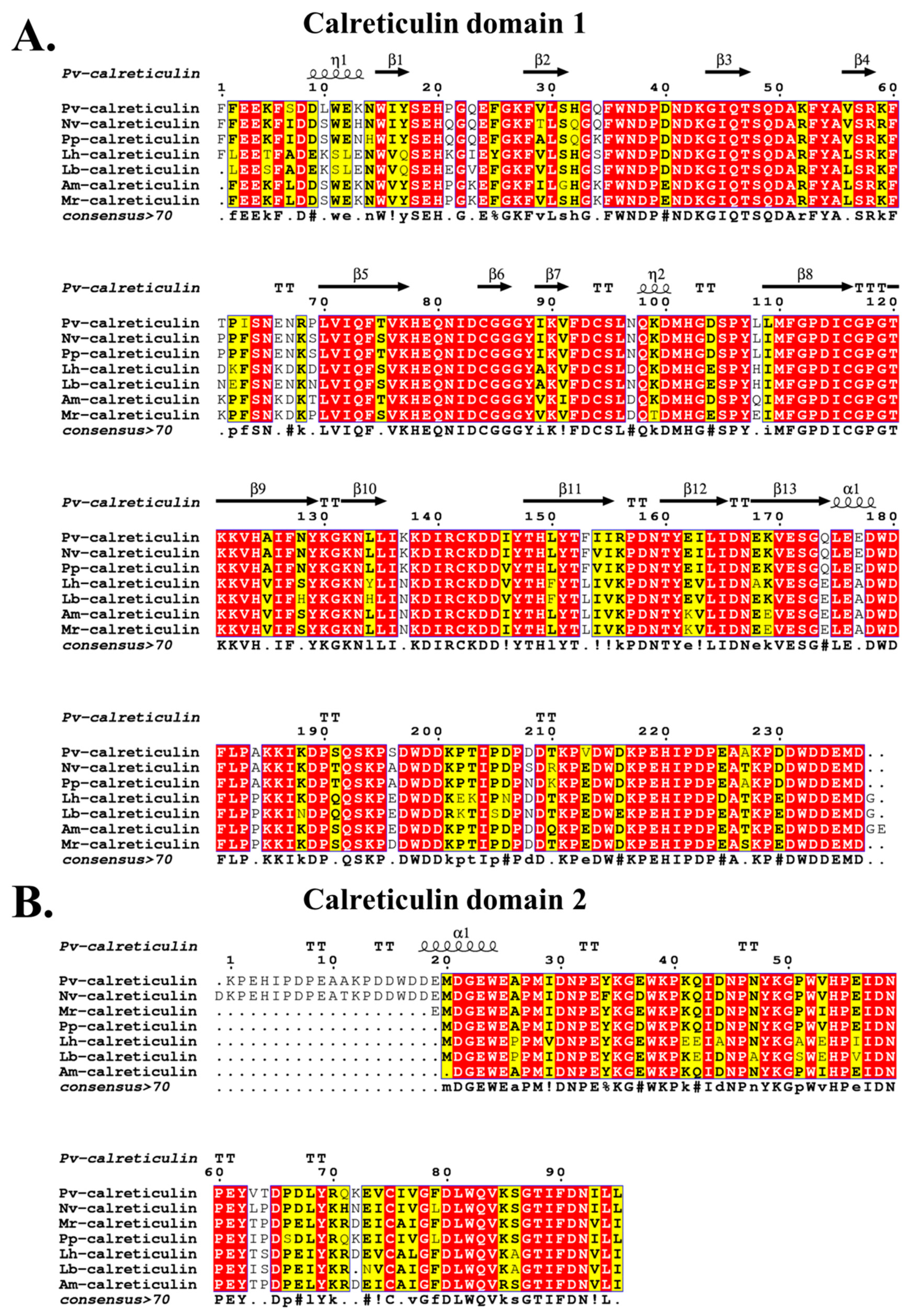
3.1. Sequence Analysis of PvCRT
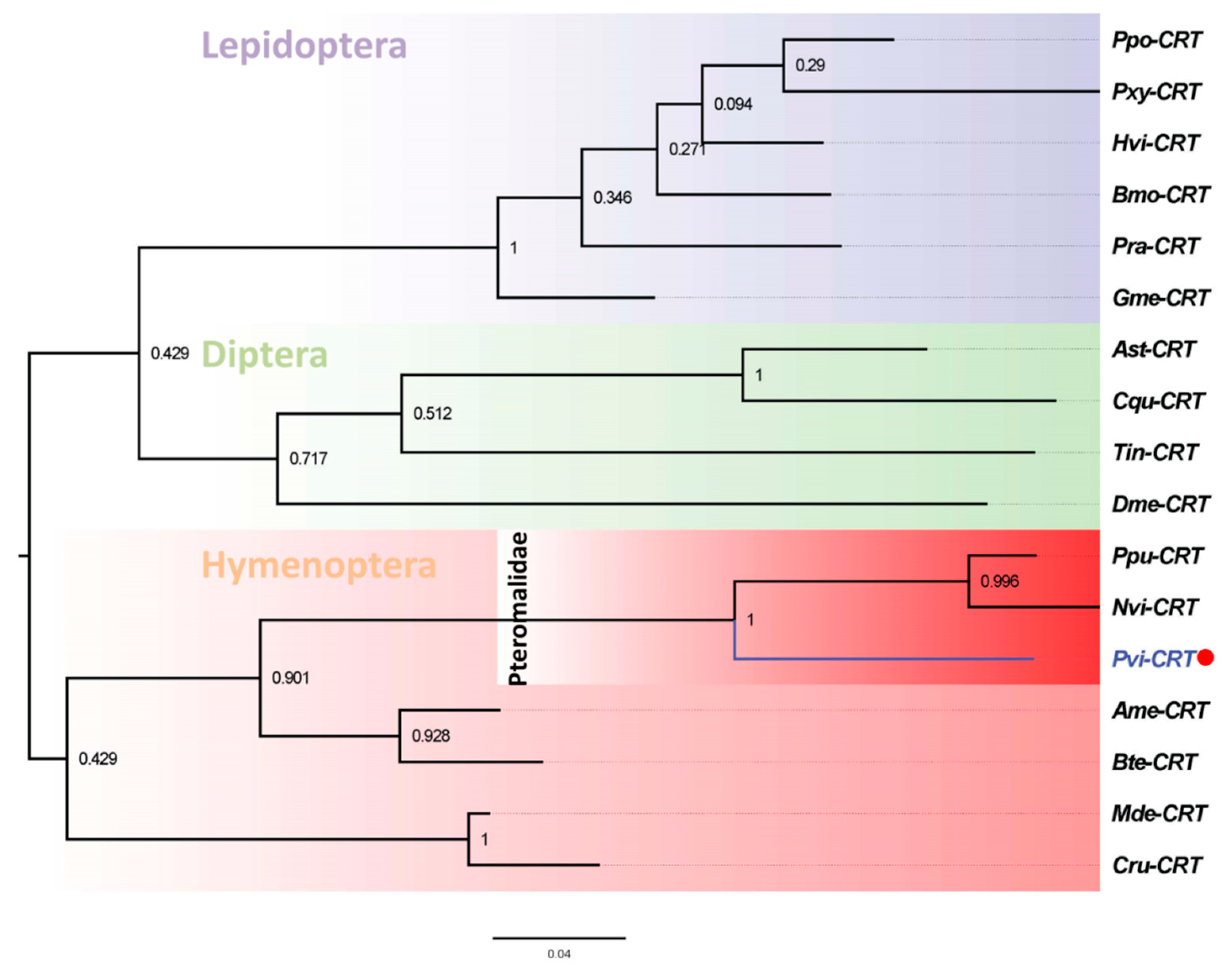
3.2. Phylogenetic Analysis
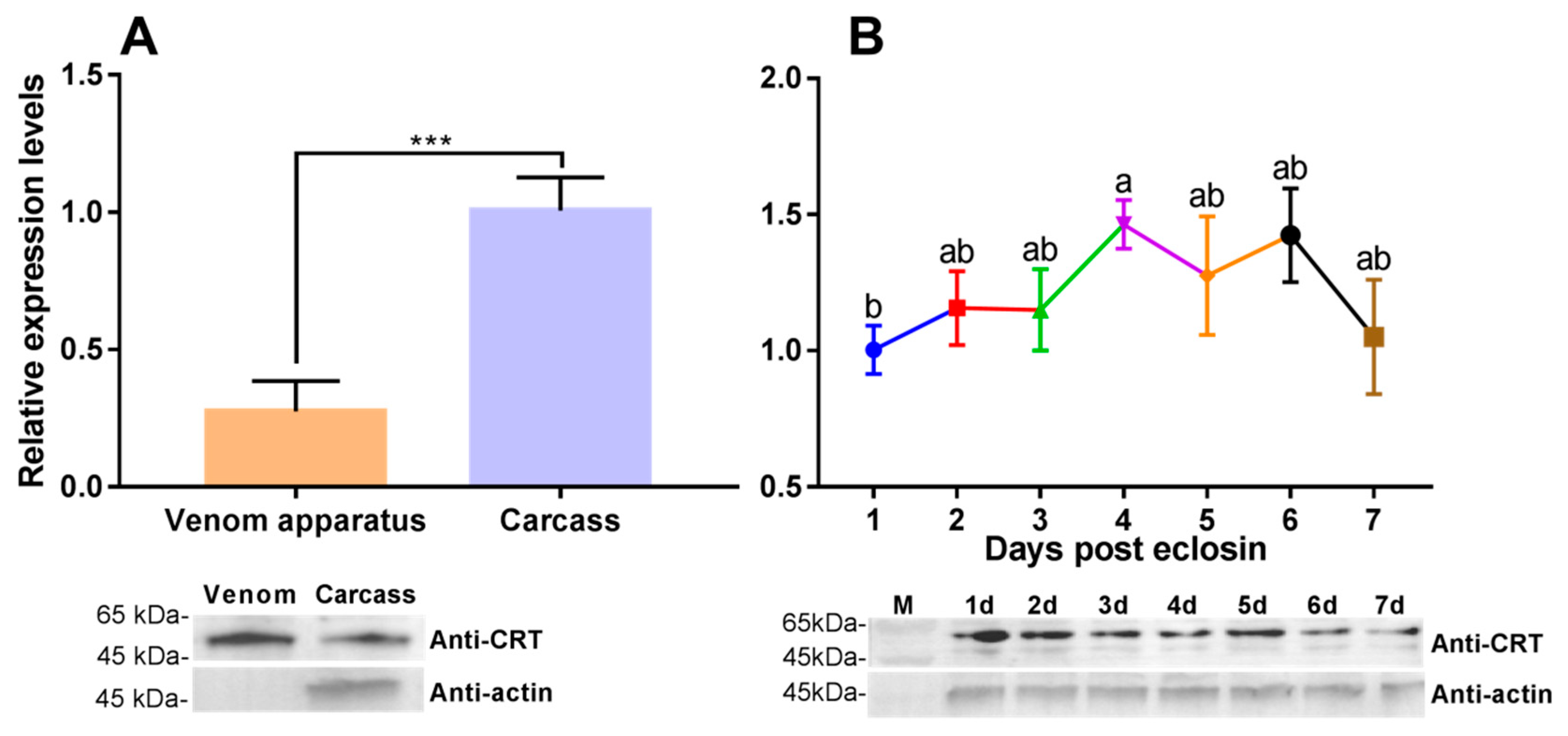
3.3. Transcriptional Profiles of PvCRT
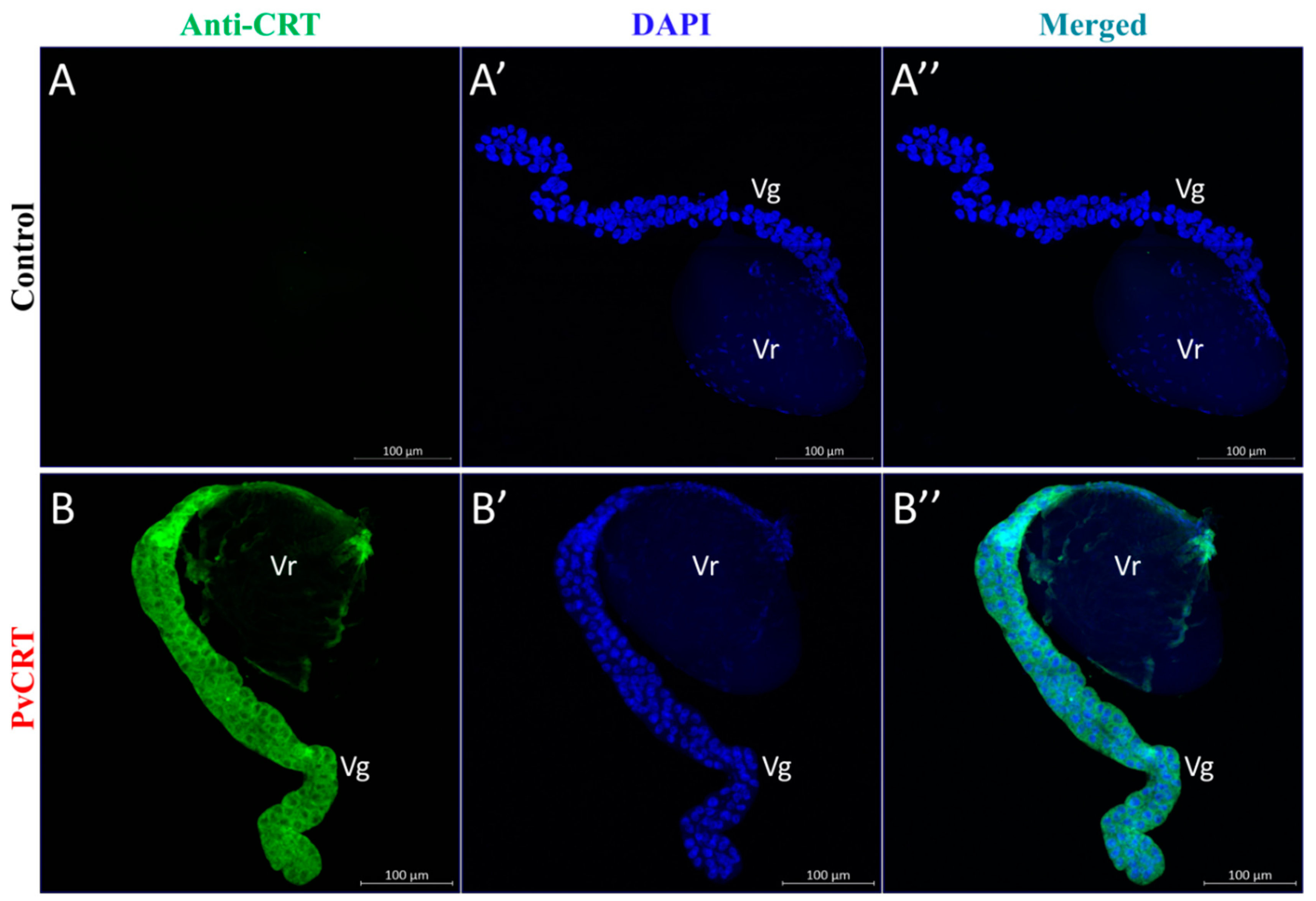
3.4. Immunohistochemical Visualization of PvCRT in Venom Apparatus
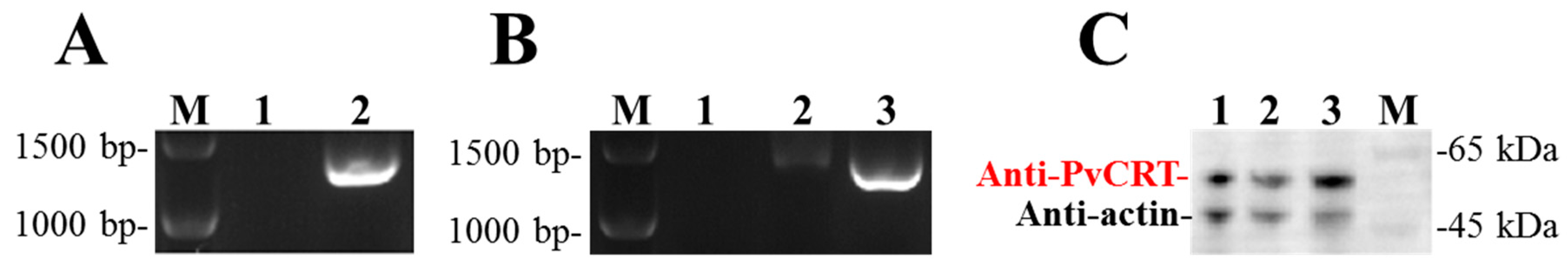
3.5. Construction of PvCRT Transgenic Drosophila
3.6. PvCRT Inhibits Encapsulation of the Host
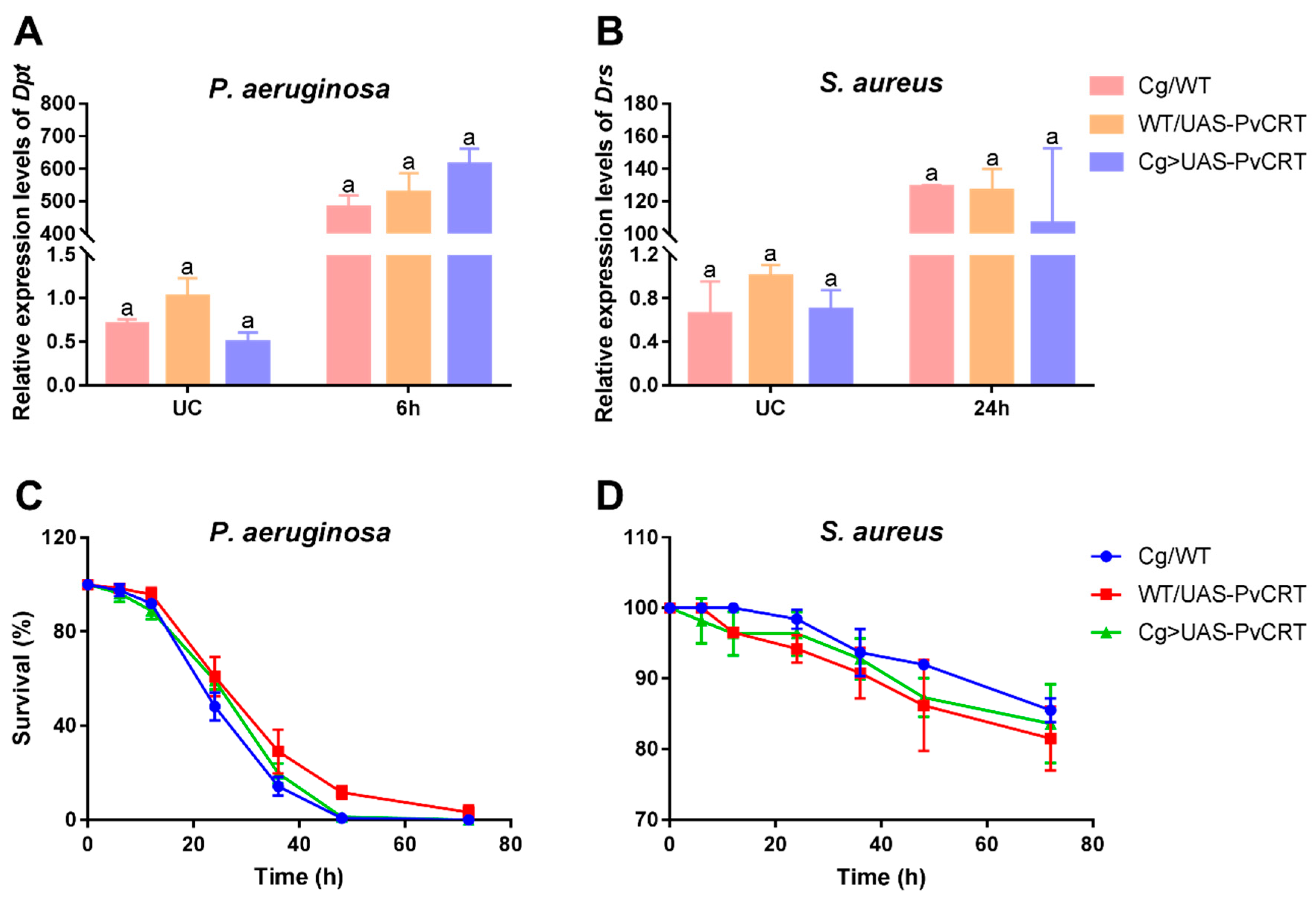
3.7. The Roles of PvCRT in Antimicrobial Immunity of the Host
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreau, S.J.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins (Basel) 2015, 7, 2385–2412. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Glupov, V.V.; Kryukova, N.A. Physiological and biochemical aspects of interactions between insect parasitoids and their hosts. Entomol. Rev. 2016, 96, 513–524. [Google Scholar] [CrossRef]
- Gundersen-Rindal, D.; Dupuy, C.; Huguet, E.; Drezen, J.-M. Parasitoid polydnaviruses: Evolution, pathology and applications. Biocontrol Sci. Technol. 2013, 23, 1–61. [Google Scholar] [CrossRef]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Mabiala-Moundoungou, A.D.; Doury, G.; Eslin, P.; Cherqui, A.; Prevost, G. Deadly venom of Asobara japonica parasitoid needs ovarian antidote to regulate host physiology. J. Insect Physiol. 2010, 56, 35–41. [Google Scholar] [CrossRef]
- Teng, Z.; Wu, H.; Ye, X.; Xiong, S.; Xu, G.; Wang, F.; Fang, Q.; Ye, G. An ovarian protein involved in passive avoidance of an endoparasitoid to evade its host immune response. J. Proteome Res. 2019, 18, 2695–2705. [Google Scholar] [CrossRef]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One protein, one gene, many functions. Biochem. J. 1999, 344, 281–292. [Google Scholar] [CrossRef]
- Siebert, A.L.; Wheeler, D.; Werren, J.H. A new approach for investigating venom function applied to venom calreticulin in a parasitoid wasp. Toxicon 2015, 107, 304–316. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Q.; Qian, C.; Wang, F.; Yu, X.Q.; Ye, G.Y. Inhibition of host cell encapsulation through inhibiting immune gene expression by the parasitic wasp venom calreticulin. Insect Biochem. Mol. Biol. 2013, 43, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Schmidt, O.; Asgari, S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 2006, 30, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S. Polydnavirus particle proteins with similarities to molecular chaperones, heat-shock protein 70 and calreticulin. J. Gen. Virol. 2003, 84, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.M.; Brauning, R.; Smolenski, G.; Ferguson, C.; Barton, D.; Wheeler, T.T.; Mcculloch, A. The constituents of Microctonus sp. parasitoid venoms. Insect Mol. Biol. 2008, 17, 313–324. [Google Scholar] [CrossRef]
- Cha, W.H.; Kim, Y.; Lee, D.-W. Calreticulin in Cotesia plutellae suppresses immune response of Plutella xylostella (L.). J. Asia Pac. Entomol. 2015, 18, 27–31. [Google Scholar] [CrossRef]
- Hubert, H.M.; Colinet, D.; Deleury, E.; Belghazi, M.; Ravallec, M.; Poulain, J.; Dossat, C.; Poirie, M.; Gatti, J.L. Comparative venomics of Psyttalia lounsburyi and P. concolor, two olive fruit fly parasitoids: A hypothetical role for a GH1 β-glucosidase. Sci. Rep. 2016, 6, 35873. [Google Scholar] [CrossRef]
- Tang, B.Z.; Meng, E.; Zhang, H.J.; Zhang, X.M.; Asgari, S.; Lin, Y.P.; Lin, Y.Y.; Peng, Z.Q.; Qiao, T.; Zhang, X.F.; et al. Combination of label-free quantitative proteomics and transcriptomics reveals intraspecific venom variation between the two strains of Tetrastichus brontispae, a parasitoid of two invasive beetles. J. Proteom. 2018, 192, 37–53. [Google Scholar] [CrossRef]
- Marchiori, C.H. Parasitóides de estágios imaturos de dípteros sinantrópicos coletados em vários ambientes em Itumbiara-GO. Acta Sci. 2000, 22, 655–661. [Google Scholar]
- Marchiori, C.H.; Barbaresco, L.F. Occurrence of Pachycrepoideus vindemmiae (Rondani, 1875) (Hymenoptera: Pteromalidae) as a parasitoid of Megaselia scalaris (Loew, 1866) (Diptera: Phoridae) in Brazil. Braz. J. Biol. 2007, 67, 577–578. [Google Scholar] [CrossRef]
- Marchiori, C.H.; Borges, L.M.F.; Ferreira, L.L. Hosts of the parasitoid Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae) of medical-veterinary and economic importance collected in the state of Goiás, Brazil. Am. J. Life Med. 2013, 1, 228–231. [Google Scholar] [CrossRef][Green Version]
- Marchiori, C.H.; Borges, L.M.F. First report of the parasitoid Pachycrepoideus vindemmiae (Rondani, 1875) (Hymenoptera: Pteromalidae) parasitizing Synthesiomyia nudiseta (Van der Wulp, 1883) (Diptera: Muscidae). Braz. J. Biol. 2017, 77, 1519–6984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchiori, C.H.; Pereira, L.A.; Filho, O.M.S. Primeiro relato do parasitóide Pachycrepoideus vindemmiae (Rondani) (Hymenoptera Pteromalidae) parasitando pupas de Sarcodexia lambens Wiedemann (Diptera: Sarcophagidae). Ciênc. Rural. 2003, 33, 173–175. [Google Scholar] [CrossRef]
- Yang, L.; Wan, B.; Wang, B.-B.; Liu, M.-M.; Fang, Q.; Song, Q.-S.; Ye, G.-Y. The pupal ectoparasitoid Pachycrepoideus vindemmiae regulates cellular and humoral immunity of host Drosophila melanogaster. Front. Physiol. 2019, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Rizki, T.M.; Rizki, R.M. Developmental analysis of a temperature-sensitive melanotic tumor mutant in Drosophila melanogaster. Wilehm Roux’s Arch. Dev. Biol. 1980, 189, 197–206. [Google Scholar] [CrossRef]
- Hennig, K.M.; Colombani, J.; Neufeld, T.P. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J. Cell Biol. 2006, 173, 963–974. [Google Scholar] [CrossRef]
- Chen, W.; He, Z.; Ji, X.L.; Tang, S.T.; Hu, H.Y. Hyperparasitism in a generalist ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), on its own conspecifics: When the lack of resource lead to cannibalism. PLoS ONE 2015, 10, e0124305. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2017, 46, D493–D496. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Ma, J.; Luo, X.; Nie, P.; Zuo, Z.; Lahrmann, U.; Zhao, Q.; Zheng, Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef]
- SWISS-MODEL. Available online: http://www.swissmodel.expasy.org/ (accessed on 2 July 2018).
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Clustal Omega. Available online: http://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 9 May 2018).
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, M.J.; Perlman, S.J. Generality of toxins in defensive symbiosis: Ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLoS Pathog. 2017, 13, e1006431. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta (Delta CT)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.B. GAL4 system in Drosophila: A fly geneticist’s swiss army knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef]
- Mortimer, N.T.; Goecks, J.; Kacsoh, B.Z.; Mobley, J.A.; Bowersock, G.J.; Taylor, J.; Schlenke, T.A. Parasitoid wasp venom SERCA regulates Drosophila calcium levels and inhibits cellular immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 9427–9432. [Google Scholar] [CrossRef]
- Yan, Z.C.; Fang, Q.; Wang, L.; Liu, J.D.; Zhu, Y.; Wang, F.; Li, F.; Werren, J.H.; Ye, G.Y. Insights into the venom composition and evolution of an endoparasitoid wasp by combining proteomic and transcriptomic analyses. Sci. Rep. 2016, 6, 19604. [Google Scholar] [CrossRef]
- Wu, J.S.; Luo, L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protoc. 2006, 1, 2110–2115. [Google Scholar] [CrossRef]
- Neyen, C.; Bretscher, A.J.; Binggeli, O.; Lemaitre, B. Methods to study Drosophila immunity. Methods 2014, 68, 116–128. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Goecks, J.; Mortimer, N.T.; Mobley, J.A.; Bowersock, G.J.; Taylor, J.; Schlenke, T.A. Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS ONE 2013, 8, e64125. [Google Scholar] [CrossRef] [PubMed]
- ESPript 3.0 Server. Available online: http://espript.ibcp.fr/ (accessed on 1 July 2014).
- Weinberger, K.; Collazo, N.; Aguillon, J.C.; Molina, M.C.; Rosas, C.; Pena, J.; Pizarro, J.; Maldonado, I.; Cattan, P.E.; Apt, W.; et al. Triatoma infestans calreticulin: Gene cloning and expression of a main domain that interacts with the host complement system. Am. J. Trop. Med. Hyg. 2017, 96, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Messing, R.H. The ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), attacks other primary tephritid fruit fly parasitoids: Host expansion and potential non-target impact. Biol. Control. 2004, 31, 227–236. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Buffington, M.; Daane, K.M.; Dalton, D.T.; Grassi, A.; Kaçar, G.; Miller, B.; Miller, J.C.; Baser, N.; Ioriatti, C.; et al. Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biol. Control. 2015, 84, 28–35. [Google Scholar] [CrossRef]
- Lee, J.C.; Wang, X.; Daane, K.M.; Hoelmer, K.A.; Isaacs, R.; Sial, A.A.; Walton, V.M. Biological control of spotted-wing Drosophila (Diptera: Drosophilidae)—Current and pending tactics. J. Integr. Pest Manag. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Wan, B.; Goguet, E.; Ravallec, M.; Pierre, O.; Lemauf, S.; Volkoff, A.-N.; Gatti, J.-L.; Poirié, M. Venom atypical extracellular vesicles as interspecies vehicles of virulence factors involved in host specificity: The case of a Drosophila parasitoid wasp. Front. Immunol. 2019, 10, 1688. [Google Scholar] [CrossRef]
- Asgari, S.; Zhang, G.; Zareie, R.; Schmidt, O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003, 33, 1017–1024. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, Z.Q.; Jiang, H.; Asgari, S. Negative regulation of prophenoloxidase (proPO) activation by a clip-domain serine proteinase homolog (SPH) from endoparasitoid venom. Insect Biochem. Mol. Biol. 2004, 34, 477–483. [Google Scholar] [CrossRef]
- Zhang, G.; Schmidt, O.; Asgari, S. A novel venom peptide from an endoparasitoid wasp is required for expression of polydnavirus genes in host hemocytes. J. Biol. Chem. 2004, 279, 41580–41585. [Google Scholar] [CrossRef]
- Colinet, D.; Schmitz, A.; Depoix, D.; Crochard, D.; Poirie, M. Convergent use of RhoGAP toxins by eukaryotic parasites and bacterial pathogens. PLoS Pathog. 2007, 3, e203. [Google Scholar] [CrossRef]
- Colinet, D.; Dubuffet, A.; Cazes, D.; Moreau, S.; Drezen, J.M.; Poirie, M. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev. Comp. Immunol. 2009, 33, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.C.; Fang, Q.; Liu, Y.; Xiao, S.; Yang, L.; Wang, F.; An, C.J.; Werren, J.H.; Ye, G.Y. A venom serpin splicing isoform of the endoparasitoid wasp Pteromalus puparum suppresses host prophenoloxidase cascade by forming complexes with host hemolymph proteinases. J. Biol. Chem. 2017, 292, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, Y.; Liu, M.; Yang, L.; Stanley, D.W.; Fang, Q.; Ye, G.Y. A digestive tract expressing alpha-amylase influences the adult lifespan of Pteromalus puparum revealed through RNAi and rescue analyses. Pest Manag. Sci. 2019, 75, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, D.C.; Aerts, M.; Brunain, M.; Desjardins, C.A.; Jacobs, F.J.; Werren, J.H.; Devreese, B. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Mol. Biol. 2010, 19, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Martinson, E.O.; Kelkar, Y.D.; Chang, C.H.; Werren, J.H. The evolution of venom by co-option of single-copy genes. Curr. Biol. 2017, 27, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Grbic, M.; Strand, M.R. Shifts in the life history of parasitic wasps correlate with pronounced alterations in early development. Proc. Natl. Acad. Sci. USA 1998, 95, 1097–1101. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wang, B.; Qiu, L.; Wan, B.; Yang, Y.; Liu, M.; Wang, F.; Fang, Q.; Stanley, D.W.; Ye, G. Functional Characterization of a Venom Protein Calreticulin in the Ectoparasitoid Pachycrepoideus vindemiae. Insects 2020, 11, 29. https://doi.org/10.3390/insects11010029
Yang L, Wang B, Qiu L, Wan B, Yang Y, Liu M, Wang F, Fang Q, Stanley DW, Ye G. Functional Characterization of a Venom Protein Calreticulin in the Ectoparasitoid Pachycrepoideus vindemiae. Insects. 2020; 11(1):29. https://doi.org/10.3390/insects11010029
Chicago/Turabian StyleYang, Lei, Beibei Wang, Liming Qiu, Bin Wan, Yi Yang, Mingming Liu, Fang Wang, Qi Fang, David W. Stanley, and Gongyin Ye. 2020. "Functional Characterization of a Venom Protein Calreticulin in the Ectoparasitoid Pachycrepoideus vindemiae" Insects 11, no. 1: 29. https://doi.org/10.3390/insects11010029
APA StyleYang, L., Wang, B., Qiu, L., Wan, B., Yang, Y., Liu, M., Wang, F., Fang, Q., Stanley, D. W., & Ye, G. (2020). Functional Characterization of a Venom Protein Calreticulin in the Ectoparasitoid Pachycrepoideus vindemiae. Insects, 11(1), 29. https://doi.org/10.3390/insects11010029





