Influence of Forest Disturbance on La Crosse Virus Risk in Southwestern Virginia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Disturbance Treatments
2.3. Contiguous Control Sites
2.4. Mosquito Sampling
2.5. Quantitative LACV Real-Time RT-PCR of 2008 Mosquito Pools
2.6. Quantitative LACV Real-Time RT-PCR of 2009–2010 Mosquito Pools
2.7. Quantitative LACV Real-Time RT-PCR with Novel Primers
2.8. Chipmunk Mark–Recapture Study
2.9. Chipmunk Plaque-Reduction Neutralization Test (PRNT) for La Crosse Virus Antibodies
2.10. Statistical Analysis
3. Results
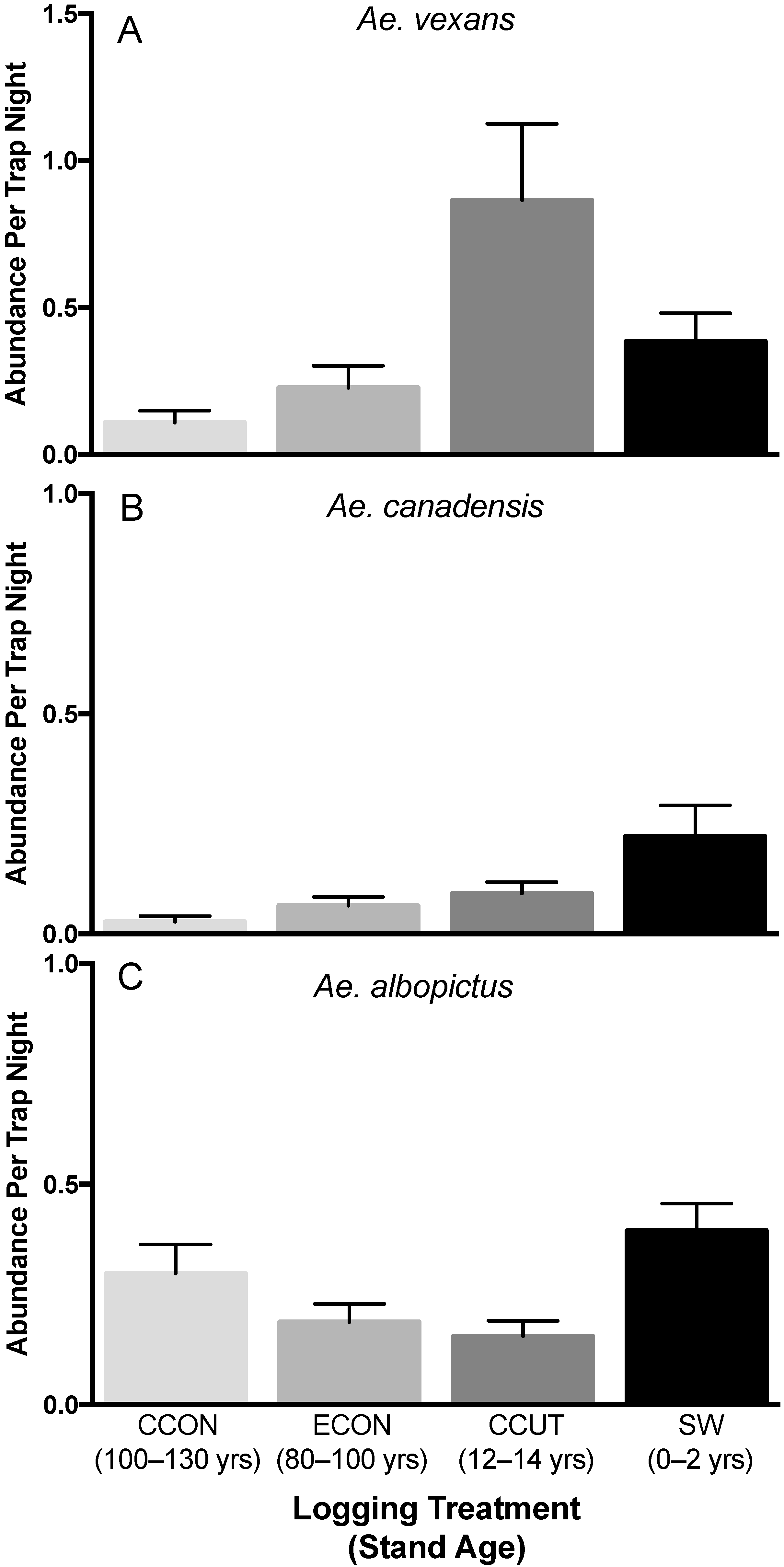
3.1. Mosquito Accessory Vector Abundance
3.1.1. Aedes vexans
3.1.2. Aedes canadensis
3.1.3. Aedes albopictus
3.1.4. LACV Mosquito Surveillance
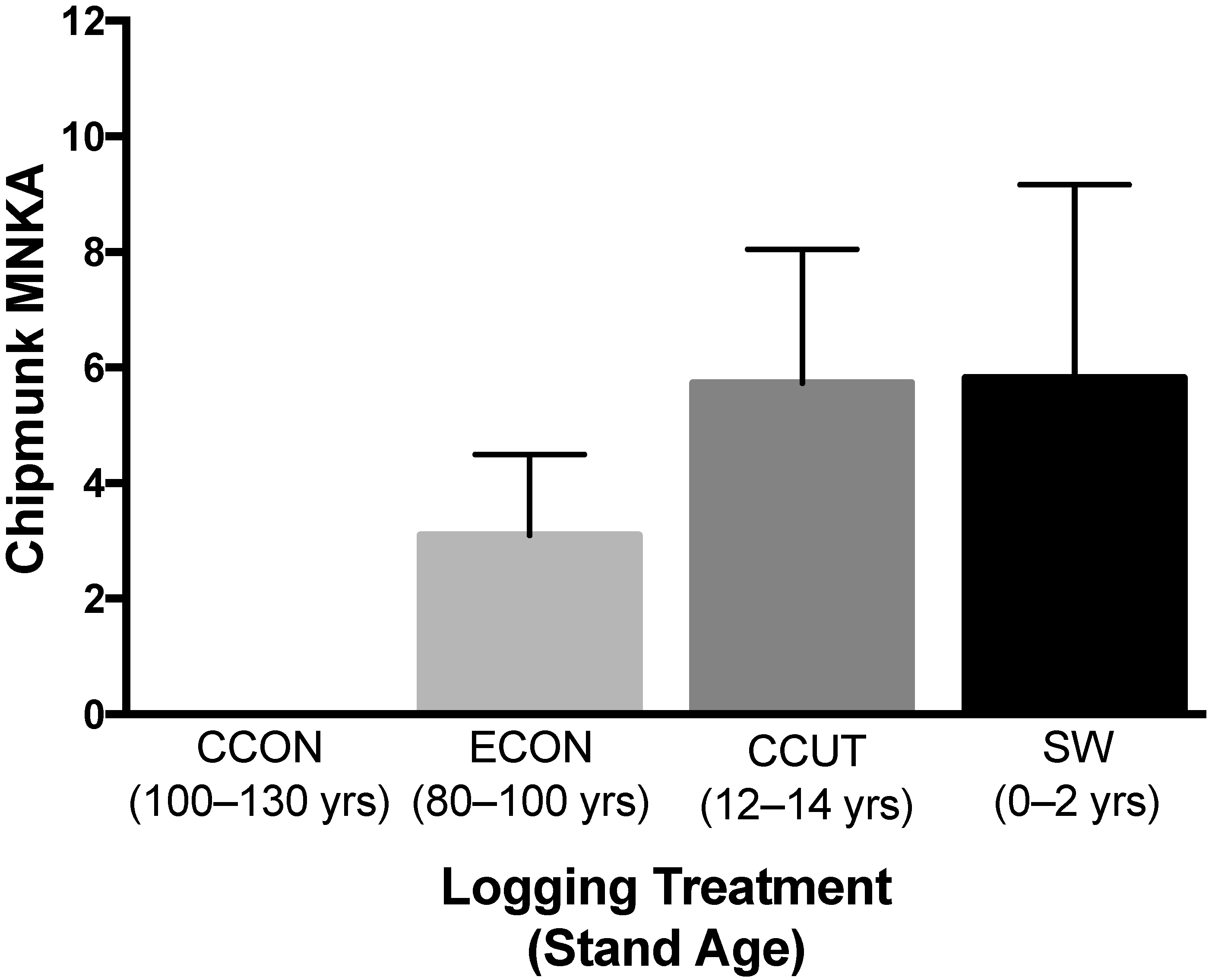
3.1.5. Chipmunk Mark–Recapture Study
3.1.6. Chipmunk Plaque-Reduction Neutralization Test (PRNT) for La Crosse Virus Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vora, N. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape J. Med. 2008, 10, 238. [Google Scholar] [PubMed]
- Walsh, J.F.; Molyneux, D.H.; Birley, M.H. Deforestation: Effects on vector-borne disease. Parasitology 1993, 106, S55–S75. [Google Scholar] [CrossRef] [PubMed]
- Allan, B.F.; Keesing, F.; Ostfeld, R.S. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 2003, 17, 267–272. [Google Scholar] [CrossRef]
- Nupp, T.E.; Swihart, R.K. Effects of forest fragmentation on population attributes of white-footed mice and eastern chipmunks. J. Mammal. 1998, 79, 1234–1243. [Google Scholar] [CrossRef]
- Krohne, D.T.; Hoch, G.A. Demography of Peromyscus leucopus populations on habitat patches: The role of dispersal. Can. J. Zool. 1999, 77, 1247–1253. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Little, T.J.; Lawson, B.W.; Githeko, A.K.; Yan, G.Y. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008, 14, 1533–1538. [Google Scholar] [CrossRef]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Githeko, A.K.; Yan, G.Y. The ecology of Anopheles mosquitoes under climate change: Case studies from the effects of deforestation in East African highlands. Ann. N. Y. Acad. Sci. 2012, 1249, 204–210. [Google Scholar] [CrossRef]
- Bonneaud, C.; Sepil, I.; Milá, B.; Buermann, W.; Pollinger, J.; Sehgal, R.N.; Valkiūnas, G.; Iezhova, T.A.; Saatchi, S.; Smith, T.B. The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. J. Trop. Ecol. 2009, 25, 439–447. [Google Scholar] [CrossRef]
- Chasar, A.; Loiseau, C.; Valkiunas, G.; Iezhova, T.; Smith, T.B.; Sehgal, R.N.M. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol. Ecol. 2009, 18, 121–4133. [Google Scholar] [CrossRef]
- Laurance, S.G.; Jones, D.; Westcott, D.; Mckeown, A.; Harrington, G.; Hilbert, D.W. Habitat fragmentation and ecological traits influence the prevalence of avian blood parasites in a tropical rainforest landscape. PLoS ONE 2013, 8, e76227. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.M.; Paulson, S.L.; Cantrell, S.; Davis, B.S. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. J. Med. Entomol. 2003, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Bixler, D.; Schuh, A.J. The Demographic and Socioeconomic Factors Predictive for Populations at High-Risk for La Crosse Virus Infection in West Virginia. PLoS ONE 2011, 6, e25739. [Google Scholar] [CrossRef] [PubMed]
- McJunkin, J.E.; Khan, R.R.; Tsai, T.F. California La Crosse encephalitis. Infect. Dis. Clin. N. Am. 1998, 12, 83–93. [Google Scholar] [CrossRef]
- Miller, B.R.; Defoliart, G.R.; Yuill, T.M. Vertical transmission of La Crosse virus (California encephalitis group)—Transovarial and filial infection rates in Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 1977, 14, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H.; Beaty, B.J. Venereal transmission of La Crosse (California encephalitis) arbovirus in Aedes triseriatus mosquitoes. Science 1977, 196, 530–531. [Google Scholar] [CrossRef]
- Moulton, D.W.; Thompson, W.H. California group virus infections in small, forest-dwelling mammals of Wisconsin: Some ecological consideration. Am. J. Trop. Med. Hyg. 1971, 20, 474–482. [Google Scholar] [CrossRef]
- Gauld, L.; Hanson, R.; Thompson, W.; Sinha, S. Observations on a natural cycle of La Crosse virus (California group) in Southwestern Wisconsin. Am. J. Trop. Med. Hyg. 1974, 23, 983–992. [Google Scholar] [CrossRef]
- Watts, D.M.; Thompson, W.H.; Yuill, T.M.; DeFoliart, G.R.; Hanson, R.P. Overwintering of La Crosse virus in Aedes triseriatus. Am. J. Trop. Med. Hyg. 1974, 23, 694–700. [Google Scholar] [CrossRef]
- Lambert, A.J.; Blair, C.D.; D’Anton, M.; Ewing, W.; Harborth, M.M.; Seiferth, R.; Xiang, J.; Lanciotti, R.S. La Crosse virus in Aedes albopictus mosquitoes, Texas, USA, 2009. Emerg. Infect. Dis. 2010, 16, 856–858. [Google Scholar] [CrossRef]
- Westby, K.; Fritzen, C.; Huang, J.; Jaske, E.; Paulsen, D.; Jones, C.; Moncayo, A. La Crosse encephalitis in eastern Tennessee: Evidence of invasive mosquito (Aedes albopictus and Ochlerotatus japonicus) involvement in the transmission of an indigenous disease. Am. J. Trop. Med. Hyg. 2011, 85, 1476–1645. [Google Scholar]
- Harris, M.C.; Dotseth, E.J.; Jackson, B.T.; Zink, S.D.; Marek, P.E.; Kramer, L.D.; Paulson, S.L.; Hawley, D.M. La Crosse virus in Aedes japonicus japonicus Mosquitoes in the Appalachian Region, USA. Emerg. Infect. Dis. 2015, 21, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.L.; Parsons, M.A.; Lalondeweigert, B.J.; Lebio, J.; Stegmiller, H.; Bear, G.T. Aedes canadensis, a vector of La Crosse virus (California serogroup) in Ohio. J. Am. Mosq. Control Assoc. 1986, 2, 73–78. [Google Scholar]
- Berry, R.L.; Parsons, M.A.; Restifo, R.A.; Peterson, E.D.; Gordon, S.W. Reed, Kin Ohio: An 18-year retrospective summary. Prog. Clin. Biol. Res. 1983, 123, 215–223. [Google Scholar] [PubMed]
- Thompson, W.H.; Anslow, R.O.; Hanson, R.P.; Defoliart, G.R. La Crosse virus isolations from mosquitoes in Wisconsin 1964–1968. Am. J. Trop. Med. Hyg. 1972, 21, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.C.; Jackson, D.M.; Yang, F.; Dotseth, E.J.; Paulson, S.L.; Hawley, D.M. La Crosse virus field detection and vector competence of Culex mosquitoes. Am. J. Trop. Med. Hyg. 2015, 93, 461–467. [Google Scholar] [CrossRef]
- Hopkins, M.C.; Thomason, C.A.; Brown, B.L.; Kirkpatrick, L.T.; Paulson, S.L.; Hawley, D.M. Experimental logging alters the abundance and community composition of ovipositing mosquitoes in the southern Appalachians. Ecol. Entomol. 2018, 43, 463–472. [Google Scholar] [CrossRef]
- Antonovics, J.; Iwasa, Y.; Hassell, M.P. A generalized-model of parasitoid, venereal, and vector-based transmission processes. Am. Nat. 1995, 145, 661–675. [Google Scholar] [CrossRef]
- Mather, T.N.; Nicholson, M.C.; Donnelly, E.F.; Matyas, B.T. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 1996, 144, 1066–1069. [Google Scholar] [CrossRef]
- Kellner, K.F.; Urban, N.A.; Swihart, R.K. Short-Term Responses of Small Mammals to Timber Harvest in the United States Central Hardwood Forest Region. J. Wildl. Manag. 2013, 77, 1650–1663. [Google Scholar] [CrossRef]
- Kirkland, G.L. Responses of small mammals to clearcutting of northern Appalachian forests. J. Mammal. 1977, 58, 600–609. [Google Scholar] [CrossRef]
- Krull, J.N. Response of chipmunks and red squirrels to commercial clear-cut logging. N. Y. Fish Game J. 1970, 17, 58–59. [Google Scholar]
- Yahner, R.H. Dynamics of a small mammal community in a fragmented forest. Am. Midl. Nat. 1992, 127, 381–391. [Google Scholar] [CrossRef]
- Slajchert, T.; Kitron, U.D.; Jones, C.J.; Mannelli, A. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern Illinois, USA. J. Wildl. Dis. 1997, 33, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hersh, M.H.; Tibbetts, M.; Strauss, M.; Ostfeld, R.S.; Keesing, F. Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 2012, 18, 1951–1957. [Google Scholar] [CrossRef]
- Johnson, R.C.; Kodner, C.; Jarnefeld, J.; Eck, D.K.; Xu, Y.N. Agents of Human Anaplasmosis and Lyme Disease at Camp Ripley, Minnesota. Vector Borne Zoonotic Dis. 2011, 11, 1529–1534. [Google Scholar] [CrossRef]
- Platt, K.B.; Tucker, B.J.; Halbur, P.G.; Tiawsirisup, S.; Blitvich, B.J.; Fabiosa, F.G.; Bartholomay, L.C.; Rowley, W.A. West Nile virus viremia in eastern chipmunks (Tamias striatus) sufficient for infecting different mosquitoes. Emerg. Infect. Dis. 2007, 13, 831–837. [Google Scholar] [CrossRef]
- Gauld, L.W.; Yuill, T.M.; Hanson, R.P.; Sinha, S.K. Isolation of La Crosse virus (California encephalitis group) from the chipmunk (Tamias striatus), an amplifier host. Am. J. Trop. Med. Hyg. 1975, 24, 999–1005. [Google Scholar] [CrossRef]
- Patrican, L.; DeFoliart, G.; Yuill, T. La Crosse viremias in juvenile, subadult and adult chipmunks (Tamias striatus) following feeding by transovarially-infected Aedes triseriatus. Am. J. Trop. Med. Hyg. 1985, 34, 596–602. [Google Scholar] [CrossRef]
- Belote, R.T.; Jones, R.H.; Hood, S.M.; Wender, B.W. Diversity-invasibility across an experimental disturbance gradient in Appalachian forests. Ecology 2008, 89, 183–192. [Google Scholar] [CrossRef]
- Atwood, C.J.; Fox, T.R.; Loftis, D.L. Effects of alternative silviculture on stump sprouting in the southern Appalachians. For. Ecol. Manag. 2009, 257, 1305–1313. [Google Scholar] [CrossRef]
- Homyack, J.A.; Haas, C.A. Effects of repeated-stand entries on terrestrial salamanders and their habitat. Southeast. Nat. 2013, 12, 353–366. [Google Scholar] [CrossRef]
- Atwood, C.J. Effects of Alternative Silvicultural Treatments on Regeneration in the Southern Appalachians. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2008. [Google Scholar]
- Jackson, B.T.; Paulson, S.L.; Youngman, R.R.; Scheffel, S.L.; Hawkins, B. Oviposition preferences of Culex restuans and Culex pipiens (Diptera: Culicidae) for selected infusions in oviposition traps and gravid traps. J. Am. Mosq. Control Assoc. 2005, 21, 360–365. [Google Scholar] [CrossRef]
- Stone, C.M.; Foster, W.A. Plant-sugar feeding and vectorial capacity. In Ecology of Parasite-Vector Interactions; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; Volume 3, pp. 35–79. [Google Scholar]
- Saul, S.H.; Grimstad, P.R.; Craig, G.B. Identification of Culex species by electrophoresis. Am. J. Trop. Med. Hyg. 1977, 26, 1009–1012. [Google Scholar] [CrossRef]
- Harrington, L.C.; Poulson, R.L. Considerations for accurate identification of adult Culex restuans (Diptera: Culicidae) in field studies. J. Med. Entomol. 2008, 45, 1–8. [Google Scholar] [CrossRef]
- Nasci, R.S.; Gottfried, K.L.; Burkhalter, K.L.; Kulasekera, V.L.; Lambert, A.J.; Lanciotti, R.S.; Hunt, A.R.; Ryan, J.R. Comparison of Vero cell plaque assay, TaqMan (R) reverse transcriptase polymerase chain reaction RNA assay, and VecTest (TM) antigen assay for detection of West Nile virus in field-collected mosquitoes. J. Am. Mosq. Control Assoc. 2002, 18, 294–300. [Google Scholar]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001, 7, 807–811. [Google Scholar] [CrossRef]
- Lambert, A.J.; Nasci, R.S.; Cropp, B.C.; Martin, D.A.; Rose, B.C.; Russell, B.J.; Lanciotti, R.S. Nucleic acid amplification assays for detection of La Crosse virus RNA. J. Clin. Microbiol. 2005, 43, 1885–1889. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Beaty, B.; Calisher, C.; Shope, R. Arboviruses. In Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections, 7th ed.; Lennette, D.L.E., Lennette, E., Eds.; American Public Health Association: Washington, DC, USA, 1995; pp. 189–212. [Google Scholar]
- Johnson, B.W.; Kosoy, O.; Hunsperger, E.; Beltran, M.; Delorey, M.; Guirakhoo, F.; Monath, T. Evaluation of chimeric Japanese encephalitis and dengue viruses for use in diagnostic plaque reduction neutralization tests. Clin. Vaccine Immunol. 2009, 16, 1052–1059. [Google Scholar] [CrossRef]
- Slade, N.A.; Blair, S.M. An empirical test of using counts of individuals captured as indices of population size. J. Mammol. 2000, 81, 1035–1045. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J.; Andre, A.R.G. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Nasci, R.S.; Moore, C.G.; Biggerstaff, B.J.; Panella, N.A.; Liu, H.Q.; Karabatsos, N.; Davis, B.S.; Brannon, E.S. La Crosse encephalitis virus habitat associations in Nicholas County, West Virginia. J. Med. Entomol. 2000, 37, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Pantuwatana, S.; Thompson, W.H.; Watts, D.M.; Yuill, T.M.; Hanson, R.P. Isolation of La Crosse virus from field collected Aedes triseriatus larvae. Am. J. Trop. Med. Hyg. 1974, 23, 246–250. [Google Scholar] [CrossRef]
- Haramis, L.D. Aedes triseriatus—A comparison of density in tree holes vs discarded tires. Mosq. News 1984, 44, 485–489. [Google Scholar]
- Nasci, R.S. Biology of Aedes triseriatus (Diptera, Culicidae) developing in tires in Louisiana. J. Med. Entomol. 1988, 25, 402–405. [Google Scholar] [CrossRef]
- Hawley, W.A.; Reiter, P.; Copeland, R.S.; Pumpuni, C.B.; Craig, G.B., Jr. Aedes albopictus in North America—Probable introduction in used tires from northern Asia. Science 1987, 236, 1114–1116. [Google Scholar] [CrossRef]
- Moore, C.G. Aedes albopictus in the United States: Current status and prospects for further spread. J. Am. Mosq. Control Assoc. 1999, 15, 221–227. [Google Scholar]
- Peyton, E.L.; Campbell, S.R.; Candeletti, T.M.; Romanowski, M.; Crans, W.J. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J. Am. Mosq. Control Assoc. 1999, 15, 238–241. [Google Scholar]
- Kaufman, M.G.; Fonseca, D.M. Invasion Biology of Aedes japonicus japonicus (Diptera: Culicidae). In Annual Review of Entomology; Berenbaum, M.R., Ed.; Annual Reviews: Palo Alto, CA, USA, 2014; Volume 59, pp. 31–49. [Google Scholar]
- Grim, D.C.; Jackson, B.T.; Paulson, S.L. Abundance and bionomics of Ochlerotatus j. japonicus in two counties in southwestern Virginia. J. Am. Mosq. Control Assoc. 2007, 23, 259–263. [Google Scholar] [CrossRef]
- Tesh, R.B.; Gubler, D.J. Laboratory studies of transovarial transmission of La Crosse and other arboviruses by Aedes albopictus and Culex fatigans. Am. J. Trop. Med. Hyg. 1975, 24, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.T.; Gonzalez, J.A.; Reagan, K.L.; Blair, C.D.; Beaty, B.J. Comparative potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to transovarially transmit La Crosse virus. J. Med. Entomol. 2006, 43, 757–761. [Google Scholar] [CrossRef]
- Troyano, N.M. Transmission of La Crosse Virus in Southwest Virginia: Role of Accessory Vectors, Microfilarial Coinfection, and Canine Seroprevalence. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2009. [Google Scholar]
- Bewick, S.; Agusto, F.; Calabrese, J.M.; Muturi, E.J.; Fagan, W.F. Epidemiology of La Crosse virus emergence, Appalachia region, United States. Emerg. Infect. Dis. 2016, 22, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Masterson, R.A.; Stegmiller, H.W.; Parsons, M.A.; Spencer, C.B.; Croft, C.C. California encephalitis—An endemic puzzle in Ohio. Health Lab. Sci. 1971, 8, 89–96. [Google Scholar]
- Sudia, W.; Newhouse, V.; Calisher, C.; Chamberlain, R. California group arboviruses: Isolations from mosquitoes in North America. Mosq. News 1971, 73, 576–600. [Google Scholar]
- Watts, D.M.; Grimstad, P.R.; DeFoliart, G.R.; Yuill, T.M.; Hanson, R.P. Laboratory transmission of La Crosse encephalitis virus by several species of mosquitoes. J. Med. Entomol. 1973, 10, 583–586. [Google Scholar] [CrossRef]
- Crans, W.J. A classification system for mosquito life cycles: Life cycle types for mosquitoes of the northeastern United States. J. Vector Ecol. 2004, 29, 1–10. [Google Scholar]
- Jackson, B.T. La Crosse Virus in Southwestern Virginia: Role of Exotic Mosquito Species and Effect of Virus Infection on Feeding. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2009. [Google Scholar]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host Selection by Culex pipiens Mosquitoes and West Nile Virus Amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef]
- Nupp, T.E.; Swihart, R.K. Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. J. Mammal. 2000, 81, 512–526. [Google Scholar] [CrossRef]
- Pantuwatana, S.; Thompson, W.H.; Watts, D.M.; Hanson, R.P. Experimental infection of chipmunks and squirrels with La Crosse and Trivittatus viruses and biological transmission of La Crosse virus by Aedes triseriatus. Am. J. Trop. Med. Hyg. 1972, 21, 476–481. [Google Scholar] [CrossRef]
- Wright, R.E.; DeFoliart, G.R. Associations of Wisconsin mosquitoes and woodland vertebrate hosts. Ann. Entomol. Soc. Am. 1970, 63, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Nasci, R.S. Local variation in blood feeding by Aedes triseriatus and Aedes hendersoni (Diptera: Culicidae). J. Med. Entomol. 1985, 22, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.; Greig, J.; Mascarenhas, M.; Young, I.; Waddell, L.A. La Crosse virus: A scoping review of the global evidence. Epidemiol. Infect. 2019, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]

| Year | Laboratory | Primer/Probe Name | LACV M Segment Primer/Probe Sequence (5’→3’) | Source |
|---|---|---|---|---|
| 2008 | VA DCLS | LAC836 LP1 LAC812 LF1 LAC881 LR1 LAC2387 LP2 LAC2364 LF2 LAC2448 LR2 | CATCCATTCACAGAGTGTGGCACGC TGCAAGCTATGCGGCCTAGT AGCGAGCACCACAGACACAA AATGGGCCAAGTGTGTATAGGAAACCATCA CAATAATTCCGTGTGGTGAACC GACCGATCAGTGCTAGATTGGAA | R. Lanciotti, CDC, pers. comm. |
| 2009 | CDC | AGTAGTGTACTACC TTRAARCADGCATGGAA | [50] |
| Species | Year | LACV + Pools/Total Pools | Mean CT Value | Pool Size | Treatment | County | Month |
|---|---|---|---|---|---|---|---|
| Ae. triseriatus | 2008 2009 2010 | 0/59 0/12 0/11 | |||||
| Ae. japonicus | 2008 2009 2010 | 1/53 2/27 0/16 | 38 a 14, 23 a n/a | 22 3, 50 n/a | ECON SW, ECON n/a | M M, C n/a | July July n/a |
| Ae. albopictus | 2008 2009 | 0/10 0/1 | |||||
| Cx. pipiens/restuans | 2008 2009 2010 | 2/64 0/1 0/3 | 42, 42 b n/a n/a | 3, 7 n/a n/a | ECON n/a n/a | M n/a n/a | July, August n/a n/a |
| Ae. vexans | 2008 | 0/18 * |
| Primer/Probe | Location in M Segment (GU206142) | LACV M Segment Primer/Probe Sequence (5’→3’) | Primer Size (bp) |
|---|---|---|---|
| F Primer | 817 | CTATGCGGCCTAGTGTATC | 19 |
| R Primer | 872 | GGAAGTATCATAGCGAGCACC | 21 |
| Probe | 844 | CY5-CACAGAGTGTGGCACGCATTGTGTC-3BHQ_2 | 25 |
| Forest Disturbance Treatment | No. Seropositive/Total Tested | Seroprevalence |
|---|---|---|
| Contiguous Control (CCON) | N/A | N/A |
| Embedded Control (ECON) | 0/7 | 0% |
| Clearcut (CCUT) | 2/14 | 14.3% |
| Shelterwood (SW) | 3/17 | 17.6% |
| Overall Total | 5/38 | 13.2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopkins, M.C.; Zink, S.D.; Paulson, S.L.; Hawley, D.M. Influence of Forest Disturbance on La Crosse Virus Risk in Southwestern Virginia. Insects 2020, 11, 28. https://doi.org/10.3390/insects11010028
Hopkins MC, Zink SD, Paulson SL, Hawley DM. Influence of Forest Disturbance on La Crosse Virus Risk in Southwestern Virginia. Insects. 2020; 11(1):28. https://doi.org/10.3390/insects11010028
Chicago/Turabian StyleHopkins, M. Camille, Steven D. Zink, Sally L. Paulson, and Dana M. Hawley. 2020. "Influence of Forest Disturbance on La Crosse Virus Risk in Southwestern Virginia" Insects 11, no. 1: 28. https://doi.org/10.3390/insects11010028
APA StyleHopkins, M. C., Zink, S. D., Paulson, S. L., & Hawley, D. M. (2020). Influence of Forest Disturbance on La Crosse Virus Risk in Southwestern Virginia. Insects, 11(1), 28. https://doi.org/10.3390/insects11010028





