Encapsulated Entomopathogenic Nematodes Can Protect Maize Plants from Diabrotica balteata Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Culture and Plant Growth
2.2. EPN Beads Production
2.3. Ability of Encapsulated Nematodes to Kill D. balteata Larvae
2.4. Ability of Encapsulated Nematodes to Protect Maize Plants
3. Results
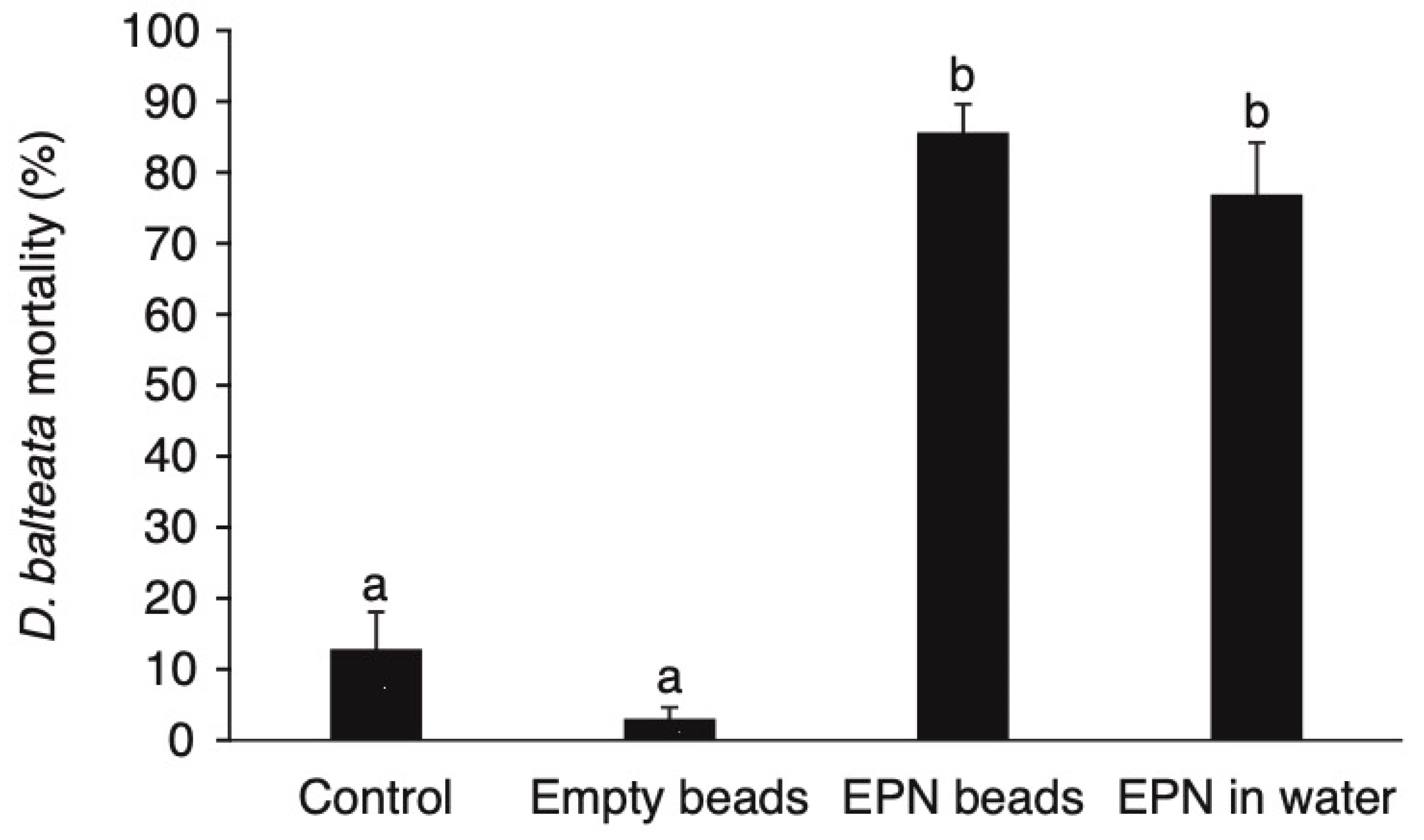
3.1. The Ability of Encapsulated Nematodes to Kill D. balteata larvae
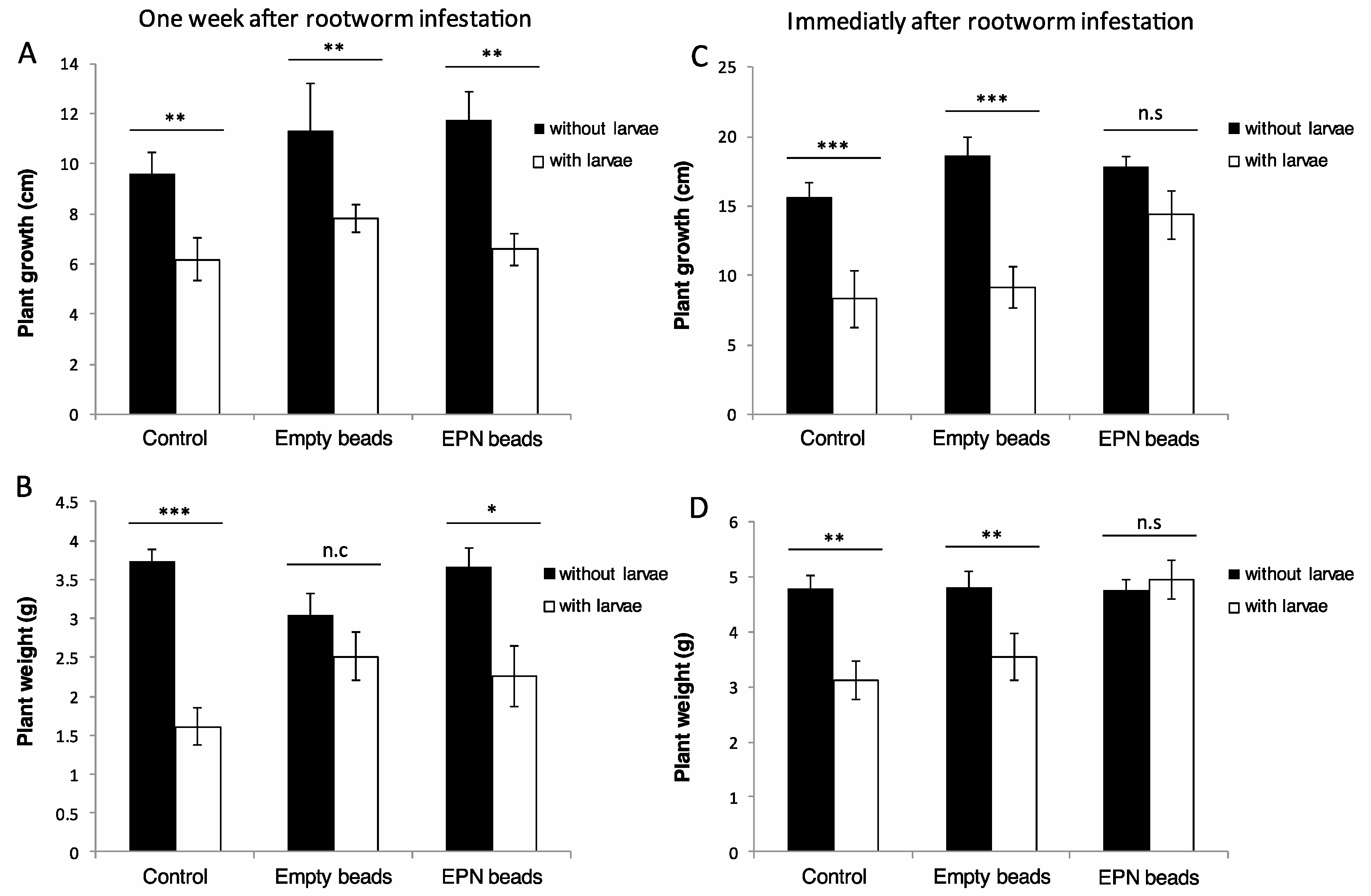
3.2. The Ability of Encapsulated Nematodes to Protect Infested Maize Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McEvoy, P.B. Host specificity and biological pest control. BioScience 1996, 46, 401–405. [Google Scholar] [CrossRef][Green Version]
- Shapiro-Ilan, D.I.; Han, R.; Dolinksi, C. Entomopathogenic nematode production and application technology. J. Nematol. 2012, 44, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.J.; Fodor, A.; Koppenhöfer, H.S.; Stackebrandt, E.; Patricia Stock, S.; Klein, M.G. Biodiversity and systematics of nematode–bacterium entomopathogens. Biol. Control. 2006, 38, 4–21. [Google Scholar] [CrossRef]
- Miles, C.; Blethen, C.; Gaugler, R.; Shapiro-Ilan, D.; Murray, T. Using entomopathogenic nematodes for crop insect pest control. Pacific NorthWest Extension. 2012, 544, 1–9. [Google Scholar]
- Koppenhöfer, A.M. Nematodes. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 249–264. [Google Scholar] [CrossRef]
- Burnell, A.M.; Stock, S.P. Heterorhabditis, Steinernema and their bacterial symbionts—Lethal pathogens of insects. Nematology 2000, 2, 31–42. [Google Scholar] [CrossRef]
- Dillman, A.R.; Chaston, J.M.; Adams, B.J.; Ciche, T.A.; Goodrich-Blair, H.; Patricia, S.; Sternberg, P.W. An entomopathogenic nematode by any other name. PLoS Pathog. 2012, 8, e1002527. [Google Scholar] [CrossRef]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Lewis, E.E.; Campbell, J.; Griffin, C.; Kaya, H.; Peters, A. Behavioral ecology of entomopathogenic nematodes. Biol. Control 2006, 38, 66–79. [Google Scholar] [CrossRef]
- Gaugler, R.E. Ntomopathogenic Nematology; CABI Publishing: Wallingford, UK, 2002. [Google Scholar] [CrossRef]
- Georgis, R.; Koppenhöfer, A.M.; Lacey, L.A.; Bélair, G.; Duncan, L.W.; Grewal, P.S.; Samish, M.; Tan, L.; Torr, P.; van Tol, R.W.H.M. Successes and failures in the use of parasitic nematodes for pest control. Biol. Control 2006, 38, 103–123. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Yabur, R.; Bashan, Y.; Hernández-Carmona, G. Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J. Appl. Phycol. 2007, 19, 43–53. [Google Scholar] [CrossRef]
- Kaya, H.K.; Nelsen, C.E. Encapsulation of steinernematid and heterorhabditid nematodes with calcium alginate: A new approach for insect control and other applications. Environ. Entomol. 1985, 14, 572–574. [Google Scholar] [CrossRef]
- Hiltpold, I.; Hibbard, B.E.; French, B.W.; Turlings, T.C.J. Capsules containing entomopathogenic nematodes as a Trojan horse approach to control the western corn rootworm. Plant Soil 2012, 358, 11–25. [Google Scholar] [CrossRef]
- Kim, J.; Jaffuel, G.; Turlings, T.C.J. Enhanced alginate capsule properties as a formulation of entomopathogenic nematodes. BioControl 2014, 60, 527–535. [Google Scholar] [CrossRef]
- Kim, J.; Hiltpold, I.; Jaffuel, G.; Sbaiti, I.; Hibbard, B.E.; Turlings, T.C.J. Calcium-alginate beads as a formulation for the application of entomopathogenic nematodes to control the Western corn rootworm. Pest Manag. Sci. 2019. under review. [Google Scholar]
- Saba, F. Host plant spectrum and temperature limitations of Diabrotica balteata. Can. Entomol. 1970, 102, 684–691. [Google Scholar] [CrossRef]
- Chittenden, F.H. Notes on the cucumber beetles. USDA Bur. Entomol. Bull. 1912, 82, 67–75. [Google Scholar]
- Creighton, C.S.; Fassuliotis, F. Heterorhabditis sp. (Nematoda: Heterorhabditidae): A nematode isolated from the banded cucumber beetle Diabrotica balteata. J. Nematol. 1985, 17, 150–153. [Google Scholar]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 1997; pp. 281–324. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 21 December 2019).
- Kuhlmann, U.; Van der Burgt, A.C.M. Possibilities for biological control of the western corn rootworm, Diabrotica virgifera virgifera LeConte, in Central Europe. Biocontrol News Inf. 1998, 19, 59–68. [Google Scholar]
- Jackson, J.J. Field performance of entomopathogenic nematodes for suppression of western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1996, 89, 366–372. [Google Scholar] [CrossRef]
- Toepfer, S.; Hatala-Zseller, I.; Ehlers, R.U.; Peters, A.; Kuhlmann, U. The effect of application techniques on field–scale efficacy: Can the use of entomopathogenic nematodes reduce damage by western corn rootworm larvae? Agric. For. Entomol. 2010, 12, 389–402. [Google Scholar] [CrossRef]
- Chen, S.; Glazer, I. A novel method for long-term storage of the entomopathogenic nematode Steinernema feltiae at room temperature. Biol. Control 2005, 32, 104–110. [Google Scholar] [CrossRef]
- Kaya, H.K.; Mannion, C.M.; Burlando, T.M.; Nelsen, C.E. Escape of Steinernema feltiae from alginate capsules containing tomato seeds. J. Nematol. 1987, 19, 287–291. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffuel, G.; Sbaiti, I.; Turlings, T.C.J. Encapsulated Entomopathogenic Nematodes Can Protect Maize Plants from Diabrotica balteata Larvae. Insects 2020, 11, 27. https://doi.org/10.3390/insects11010027
Jaffuel G, Sbaiti I, Turlings TCJ. Encapsulated Entomopathogenic Nematodes Can Protect Maize Plants from Diabrotica balteata Larvae. Insects. 2020; 11(1):27. https://doi.org/10.3390/insects11010027
Chicago/Turabian StyleJaffuel, Geoffrey, Ilham Sbaiti, and Ted C. J. Turlings. 2020. "Encapsulated Entomopathogenic Nematodes Can Protect Maize Plants from Diabrotica balteata Larvae" Insects 11, no. 1: 27. https://doi.org/10.3390/insects11010027
APA StyleJaffuel, G., Sbaiti, I., & Turlings, T. C. J. (2020). Encapsulated Entomopathogenic Nematodes Can Protect Maize Plants from Diabrotica balteata Larvae. Insects, 11(1), 27. https://doi.org/10.3390/insects11010027




