A Survey of Tick-Borne Bacterial Pathogens in Florida
Abstract
1. Introduction
2. Materials and Methods
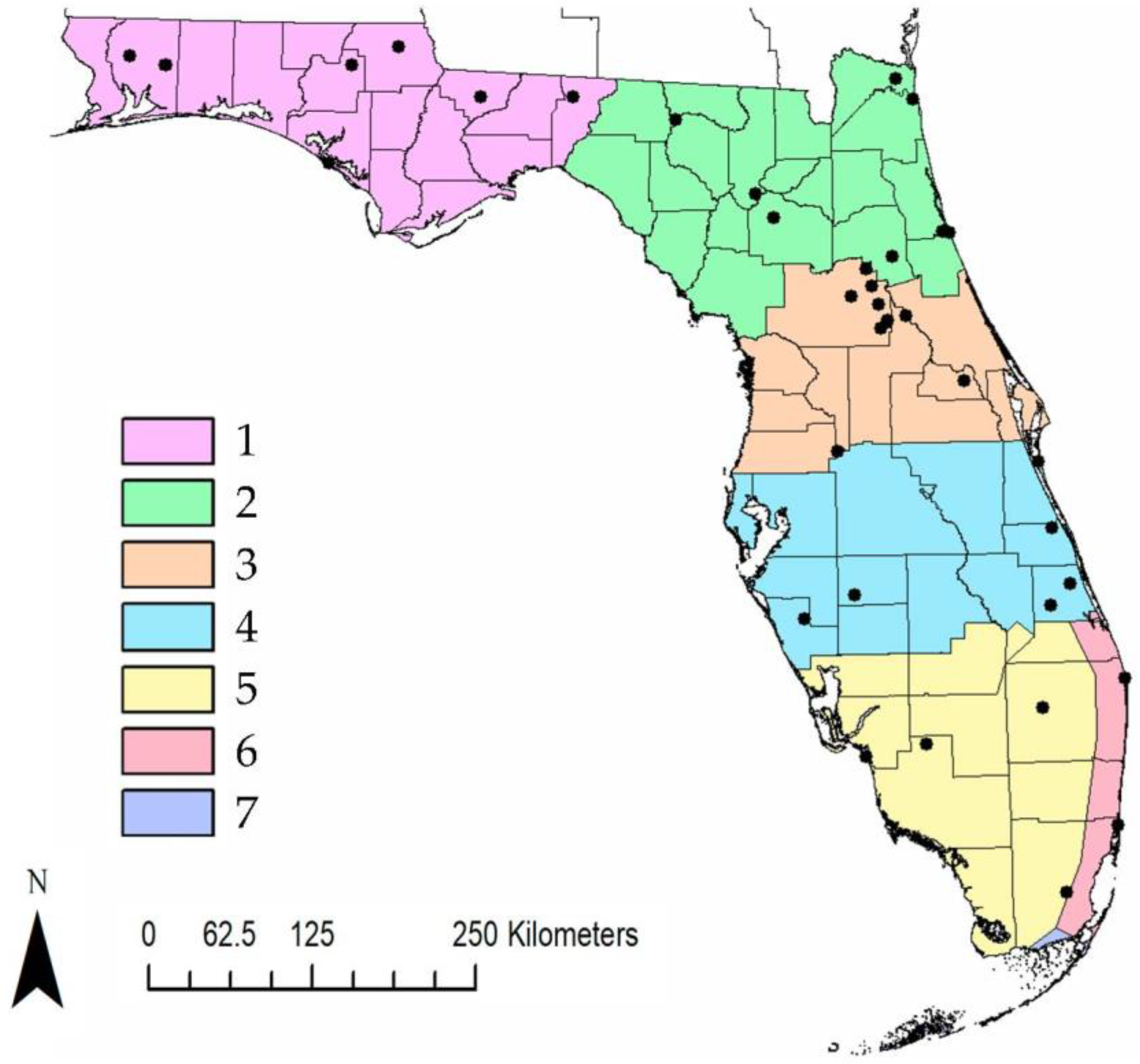
2.1. Tick Collections
2.2. DNA Extractions
2.3. Pathogen Screening
3. Results
3.1. Tick Collections
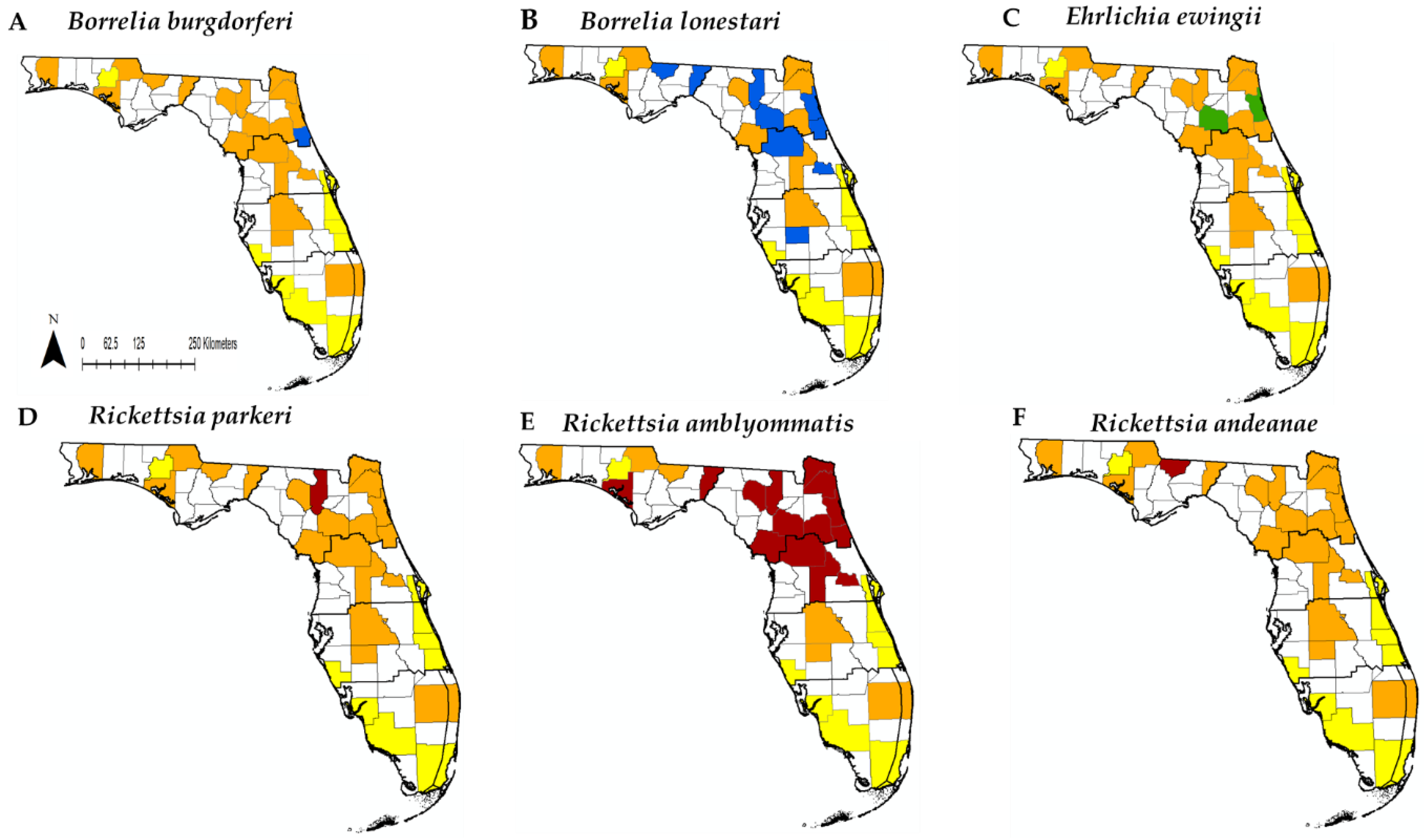
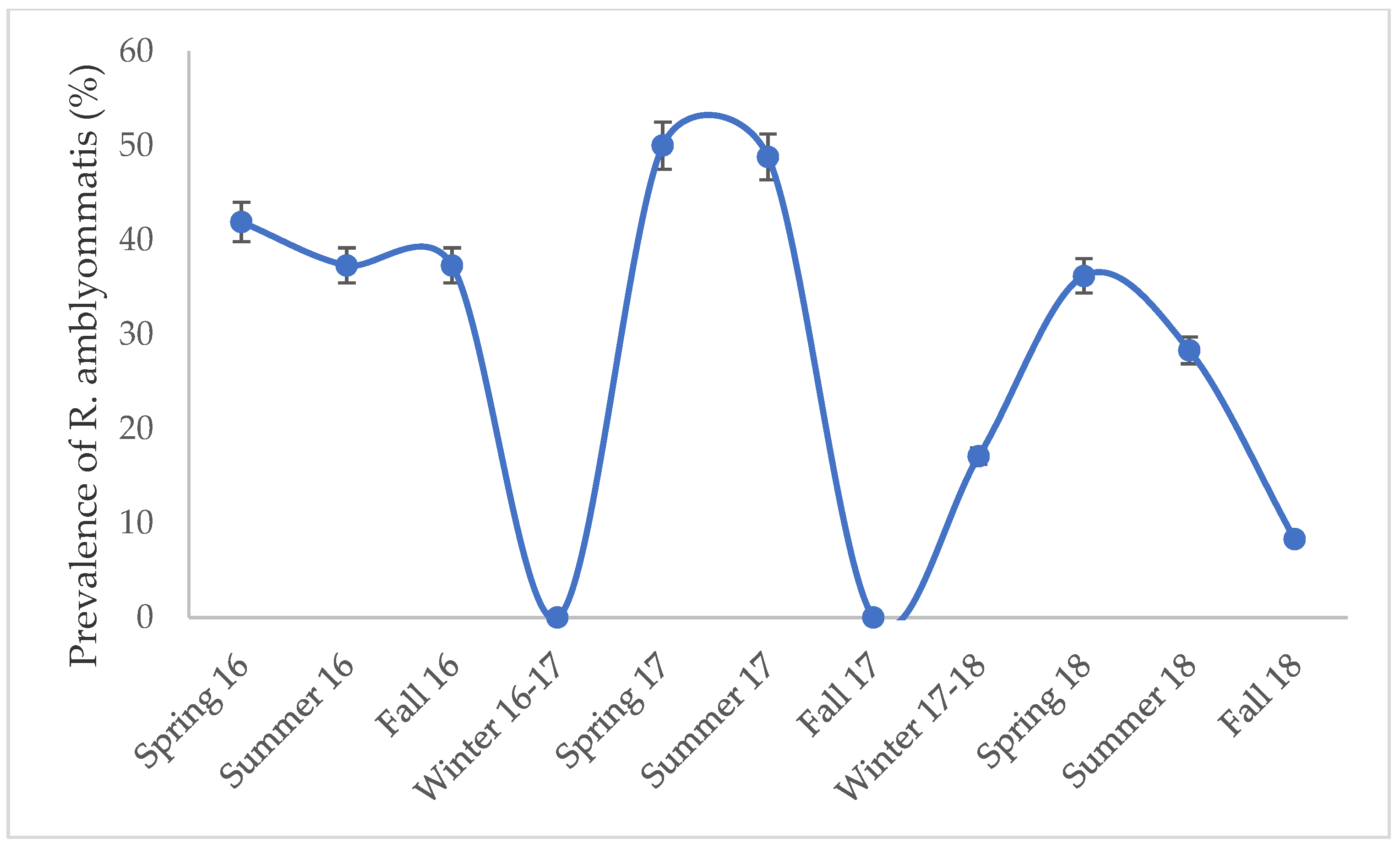
3.2. Pathogen Screening
3.3. Comparison of DNA Extraction Protocols
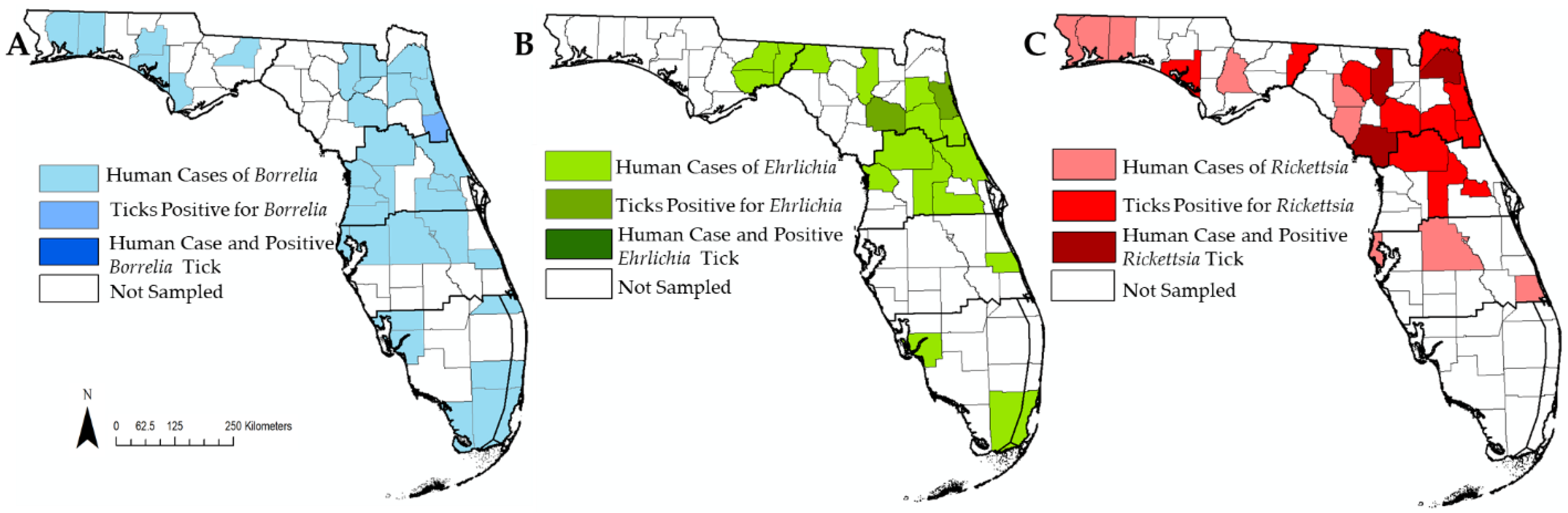
3.4. Pathogen Detection and Human Cases in Florida
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J. Vital signs: Trends in reported vector-borne disease cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 496. [Google Scholar] [CrossRef] [PubMed]
- Biggs, H.M.; Barton Behravesh, C.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.L.; Ryan, R.; Levin, M.L. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States: A practical guide for health care and public health professionals. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Trout Fryxell, R.T.; Hendricks, B.M.; Pompo, K.; Mays, S.E.; Paulsen, D.J.; Operario, D.J.; Houston, A.E. Investigating the adult ixodid tick populations and their associated Anaplasma, Ehrlichia, and Rickettsia bacteria at a Rocky Mountain spotted fever hotspot in Western Tennessee. Vector-Borne Zoonotic Dis. 2017, 17, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Trout Fryxell, R.; Steelman, C.; Szalanski, A.; Billingsley, P.; Williamson, P. Molecular detection of Rickettsia species within ticks (Acari: Ixodidae) collected from Arkansas United States. J. Med. Entomol. 2015, 52, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, C.M.; Huang, J.; Westby, K.; Freye, J.D.; Dunlap, B.; Yabsley, M.J.; Schardein, M.; Dunn, J.R.; Jones, T.F.; Moncayo, A.C. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am. J. Trop. Med. Hyg. 2011, 85, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Mixson, T.R.; Campbell, S.R.; Gill, J.S.; Ginsberg, H.S.; Reichard, M.V.; Schulze, T.L.; Dasch, G.A. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 2006, 43, 1261–1268. [Google Scholar] [CrossRef]
- Roellig, D.M.; Fang, Q.Q. Detection of Anaplasma phagocytophilum in ixodid ticks from equine-inhabited sites in the Southeastern United States. Vector-Borne Zoonotic Dis. 2012, 12, 330–332. [Google Scholar] [CrossRef]
- Loftis, A.D.; Mixson, T.R.; Stromdahl, E.Y.; Yabsley, M.J.; Garrison, L.E.; Williamson, P.C.; Fitak, R.R.; Fuerst, P.A.; Kelly, D.J.; Blount, K.W. Geographic distribution and genetic diversity of the Ehrlichia sp. from Panola Mountain in Amblyomma americanum. BMC Infect. Dis. 2008, 8, 54. [Google Scholar] [CrossRef]
- Anderson, B.; Dawson, J.; Jones, D.; Wilson, K. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 1991, 29, 2838–2842. [Google Scholar]
- Buller, R.S.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikihisa, Y.; Unver, A.; Gaudreault-Keener, M.; Manian, F.A.; Liddell, A.M. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/ticks/surveillance/index.html (accessed on 29 July 2019).
- Eisen, R.J.; Eisen, L. Spatial modeling of human risk of exposure to vector-borne pathogens based on epidemiological versus arthropod vector data. J. Med. Entomol. 2014, 45, 181–192. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/monitoring-references/maps/us-climate-divisions.php (accessed on 20 June 2019).
- Allan, S.A.; Simmons, L.; Burridge, M.J. Ixodid ticks on white-tailed deer and feral swine in Florida. J. Vector Ecol. 2001, 26, 93–102. [Google Scholar] [PubMed]
- Rogers, A.J. A Study of the Ixodid Ticks of Northern Florida, Including the Biology and Life History of Ixodes Scapularis Say (Ixodidae: Acarina). Ph.D. Thesis, University of Maryland, College Park, MD, USA, 1953. [Google Scholar]
- Sayler, K.; Rowland, J.; Boyce, C.; Weeks, E. Borrelia burgdorferi DNA absent, multiple Rickettsia spp. DNA present in ticks collected from a teaching forest in North Central Florida. Ticks Tick-Borne Dis. 2017, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sayler, K.A.; Loftis, A.D.; Beatty, S.K.; Boyce, C.L.; Garrison, E.; Clemons, B.; Cunningham, M.; Alleman, A.R.; Barbet, A.F. Prevalence of tick-borne pathogens in host-seeking Amblyomma Am. (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida. J. Med. Entomol. 2016, 53, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Florida Department of Health. Annual Morbidity Statistics Report; Technical Report for Florida Department of Health; Florida Department of Health: Tallahassee, FL, USA, 2011–2017. [Google Scholar]
- Clark, K. Borrelia species in host-seeking ticks and small mammals in northern Florida. J. Clin. Microbiol. 2004, 42, 5076–5086. [Google Scholar] [CrossRef]
- Burkot, T.R.; Mullen, G.R.; Anderson, R.; Schneider, B.S.; Happ, C.M.; Zeidner, N.S. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg. Infect. Dis. 2001, 7, 471–473. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Dugan, V.G.; Stallknecht, D.E.; Little, S.E.; Lockhart, J.M.; Dawson, J.E.; Davidson, W.R. Evaluation of a prototype Ehrlichia chaffeensis surveillance system using white-tailed deer (Odocoileus virginianus) as natural sentinels. Vector-Borne Zoonotic Dis. 2003, 3, 195–207. [Google Scholar] [CrossRef]
- Comer, J.A.; Nicholson, W.L.; Paddock, C.D.; Sumner, J.W.; Childs, J.E. Detection of antibodies reactive with Ehrlichia chaffeensis in the raccoon. J. Wildl. Dis. 2000, 36, 705–712. [Google Scholar] [CrossRef][Green Version]
- Castellaw, A.H.; Chenney, E.F.; Varela-Stokes, A.S. Tick-borne disease agents in various wildlife from Mississippi. Vector-Borne Zoonotic Dis. 2011, 11, 439–442. [Google Scholar] [CrossRef]
- Demma, L.J.; Traeger, M.S.; Nicholson, W.L.; Paddock, C.D.; Blau, D.M.; Eremeeva, M.E.; Dasch, G.A.; Levin, M.L.; Singleton, J., Jr.; Zaki, S.R. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 2005, 353, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Berrada, Z.L.; Goethert, H.K.; Cunningham, J.; Telford III, S.R. Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from Kansas. J. Med. Entomol. 2011, 48, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, J.E.; Thomasson, J.A.; Marsella, R.; Greiner, E.C.; Allan, S.A. Ticks associated with domestic dogs and cats in Florida, USA. Exp. Appl. Acarol. 2016, 69, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Yabsley, M.J.; Garrison, L.E.; Freye, J.D.; Dunlap, B.G.; Dunn, J.R.; Mead, D.G.; Jones, T.F.; Moncayo, A.C. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg. Infect. Dis. 2009, 15, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Apperson, C.S.; Engber, B.; Nicholson, W.L.; Mead, D.G.; Engel, J.; Yabsley, M.J.; Dail, K.; Johnson, J.; Watson, D.W. Tick-borne diseases in North Carolina: Is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector-Borne Zoonotic Dis. 2008, 8, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, F.S.; Paddock, C.D.; Springer, Y.P.; Eisen, R.J.; Behravesh, C.B. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am. J. Trop. Med. Hyg. 2016, 94, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E.; Ganser, C.; Kessler, W.H. Standardized Ixodid Tick Surveys in Mainland Florida. Insects 2019, 10, 235. [Google Scholar] [CrossRef]
- Kessler, W.H.; Blackburn, J.K.; Sayler, K.A.; Glass, G.E. Estimating the geographic distribution of host-seeking adult Amblyomma americanum (Acari: Ixodidae) in Florida. J. Med. Entomol. 2019, 56, 55–64. [Google Scholar] [CrossRef]
- Clifford, C.M.; Anastos, G.; Van der Borght-Elbl, A. The Larval Ixodid Ticks of the Eastern United States (Acarina-Ixodidae); Entomological Society of America: Annapolis, MD, USA, 1961; Volume 26, pp. 435–448. [Google Scholar]
- Keirans, J.E.; Litwak, T.R. Pictorial Key to the Adults of Hard Ticks, Family Ixodidae (Ixodida, Ixodoidea), East of the Mississippi River. J. Med. Entomol. 1989, 26, 435–448. [Google Scholar] [CrossRef]
- Durden, L.A.; Keirans, J.E. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance; Entomological Society of America: Lanham, MD, USA, 1996; p. 95. [Google Scholar]
- Keirans, J.E.; Durden, L.A. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998, 35, 489–495. [Google Scholar] [CrossRef]
- Qiagen DNeasy Blood and Tissue Handbook. Available online: https://www.qiagen.com/us/resources/resourcedetail?id=6b09dfb8-6319-464d-996c-79e8c7045a50&lang=en (accessed on 28 July 2019).
- Thermo Fisher Trizol Reagent Hanbook. Available online: http://tools.thermofisher.com/content/sfs/manuals/trizol_reagent.pdf (accessed on 28 July 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Tabara, K.; Arai, S.; Kawabuchi, T.; Itagaki, A.; Ishihara, C.; Satoh, H.; Okabe, N.; Tsuji, M. Molecular survey of Babesia microti, Ehrlichia species and Candidatus Neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 2007, 51, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Maupin, G.O.; Teltow, G.J.; Carter, C.J.; Piesman, J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996, 173, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic Identification of Rickettsiae and Estimation of Intraspecies Sequence Divergence for Portions of 2 Rickettsial Genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.; Sumner, J.; Nicholson, W.; Paddock, C.; Shen, J.; Piesman, J. Survey of ticks collected in Mississippi for Rickettsia, Ehrlichia, and Borrelia species. J. Vector Ecol. 2003, 28, 184–189. [Google Scholar] [PubMed]
- Eremeeva, M.; Yu, X.J.; Raoult, D. Differentiation among Spotted-Fever Group Rickettsiae Species by Analysis of Restriction-Fragment-Length-Polymorphism of PCR-Amplified Dna. J. Clin. Microbiol. 1994, 32, 803–810. [Google Scholar] [PubMed]
- Raoult, D.; Parola, P. Rocky Mountain spotted fever in the USA: A benign disease or a common diagnostic error? Lancet Infect. Dis. 2008, 8, 587–589. [Google Scholar] [CrossRef]
- Paddock, C.D.; Fournier, P.; Sumner, J.W.; Goddard, J.; Elshenawy, Y.; Metcalfe, M.G.; Loftis, A.D.; Varela-Stokes, A. Isolation of Rickettsia parkeri and Identification of a Novel Spotted Fever Group Rickettsia sp. from Gulf Coast Ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 2010, 76, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Denison, A.M.; Dryden, M.W.; Noden, B.H.; Lash, R.R.; Abdelghani, S.S.; Evans, A.E.; Kelly, A.R.; Hecht, J.A.; Karpathy, S.E.; et al. High prevalence of “Candidatus Rickettsia andeanae” and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculatum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks Tick-Borne Dis. 2015, 6, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Stromdahl, E.Y.; Richards, A.L. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast Ticks Collected from Humans in the United States. Vector-Borne Zoonotic Dis. 2012, 12, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W.; Hayes, S.F.; Mavros, A.J. Nonpathogenic rickettsiae in Dermacentor andersoni: A limiting factor for the distribution of Rickettsia rickettsii. Rickettsiae and rickettsial diseases. In Rickettsiae and Rickettsial Diseases; Burgdorfer, W., Anacker, R.L., Eds.; Academic Press: New York, NY, USA, 1980; pp. 585–594. [Google Scholar]
- Stromdahl, E.Y.; Jiang, J.; Vince, M.; Richards, A.L. Infrequency of Rickettsia rickettsii in Dermacentor variabilis Removed from Humans, with Comments on the Role of Other Human-Biting Ticks Associated with Spotted Fever Group Rickettsiae in the United States. Vector-Borne Zoonotic Dis. 2011, 11, 969–977. [Google Scholar] [CrossRef]
- Lin, T.; Gao, L.H.; Seyfang, A.; Oliver, J.H. ‘Candidatus Borrelia texasensis’, from the American dog tick Dermacentor variabilis. Int. J. Syst. Evol. Microbiol. 2005, 55, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.C.; Billingsley, P.M.; Teltow, G.J.; Seals, J.P.; Turnbough, M.A.; Atkinson, S.F. Borrelia, Ehrlichia, and Rickettsia spp. in Ticks Removed from Persons, Texas, USA. Emerg. Infect. Dis. 2010, 16, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Arsnoe, I.M.; Hickling, G.J.; Ginsberg, H.S.; McElreath, R.; Tsao, J.I. Different Populations of Blacklegged Tick Nymphs Exhibit Differences in Questing Behavior That Have Implications for Human Lyme Disease Risk. PLoS ONE 2015, 10, e0127450. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.S.; Ewing, C.P. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol. 1989, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Merrill, M.M.; Boughton, R.K.; Lord, C.C.; Sayler, K.A.; Wight, B.; Anderson, W.M.; Wisely, S.M. Wild pigs as sentinels for hard ticks: A case study from south-central Florida. Int. J. Parasitol. Parasites Wildl. 2018, 7, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, ESG. Epitools Epidemiological Calculators. Ausvet Pty Ltd. Available online: http://epitools.ausvet.com.au (accessed on 18 December 2018).
| Disease | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|
| Anaplasmosis | 6 | 1 | 0 | 0 | 0 | 1 | 0 |
| Ehrlichiosis | 13 | 20 | 17 | 23 | 16 | 23 | 12 |
| Lyme disease | 22 | 39 | 21 | 35 | 35 | 36 | 27 |
| Spotted fever rickettsiosis | 9 | 20 | 15 | 21 | 12 | 7 | 15 |
| Pathogen | Target Gene | Primer Sequences 5’-3’ | Positive Control | Reference |
|---|---|---|---|---|
| Anaplasma/Ehrlichia | groESL | gro607F GAAGATGC(A/T)GT(A/T)GG(A/T)TGTAC(G/T)GC gro677F ATTACTCAGAGTGCTTCTCA(A/G)TG gro1294R AG(A/C)GCTTC(A/T)CCTTC(A/T)AC(A/G)TC(C/T)TC gro1121R TGCATACC(A/G)TCAGT(C/T)TTTTCAAC | E. chaffensis | [40] |
| Borrelia | Flagellin b | FlaLL ACATATTCAGATGCAAGACAGA FlaRL GCAATCATAGCCATTGCAGATTGT FlaLS AACAGCTGAAGAGCTTGGAATG FlaRS CTTTGATCACTTATCATTCTAATAGC | B. lonestari | [41] |
| Rickettsia | ompA | Rr 190.70p ATGGCGAATATTTCTCCAAAA 190.701 GTTCCGTTAATGGCAGCATCT | R.sp endosymbiont | [42] |
| Species | Spring 16 | Summer 16 | Fall 16 | Winter 16–17 | Spring 17 | Summer 17 | Fall 17 | Winter 17–18 | Spring 18 | Summer 18 | Fall 18 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. americanum | ||||||||||||
| Adult | 60 | 45 | 0 | 0 | 52 | 0 | 5 | 34 | 33 | 173 | 0 | 402 |
| Nymph | 133 | 89 | 0 | 8 | 75 | 0 | 75 | 30 | 238 | 106 | 156 | 910 |
| Larvae | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 6 | 2 | 0 | 0 | 13 |
| A. maculatum | ||||||||||||
| Adult | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| Nymph | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| D. variabilis | ||||||||||||
| Adult | 6 | 8 | 0 | 0 | 5 | 0 | 2 | 1 | 10 | 8 | 6 | 46 |
| I. scapularis | ||||||||||||
| Adult | 10 | 1 | 0 | 94 | 62 | 0 | 7 | 18 | 36 | 2 | 0 | 230 |
| Nymph | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Tick Species | Pathogen Species | No. Positive/No. Tested (%) [95% CI] | % Identical (Accession #) * |
|---|---|---|---|
| I. scapularis | B. burgdorferi | 1/233 (0.43%) [0–0.02] | 98% (AF264895.1) |
| I. scapularis | R. endosymbiont | 80/233 (34.3%) [0.3–0.42] | 98% (AB002268) |
| D. variabilis | R. endosymbiont | 1/46 (2.2%) [0–0.11] | 97% (AB002268) |
| D. variabilis | B. lonestari | 1/46(2.2%) [0–0.11] | 99% (AF273670) |
| A. americanum | B. lonestari | 17/1312 (1.29%) [0.01–0.02] | 100% (AF273670) |
| A. americanum | E. ewingii | 2/1312 (0.16%) [0–0.001] | 98% (AF195273) |
| A. americanum | R. amblyommatis | 391/1312 (29%) [0.27–0.32] | 100% (CP003334) |
| A. americanum | R. parkeri | 2/1312 (0.16%) [0–0.01] | 99.7% (MH247927) |
| A. maculatum | R. andeanae | 1/4 (25%) [0.05–0.7] | 100% (KY628370) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesus, C.E.; Ganser, C.; Kessler, W.H.; White, Z.S.; Bhosale, C.R.; Glass, G.E.; Wisely, S.M. A Survey of Tick-Borne Bacterial Pathogens in Florida. Insects 2019, 10, 297. https://doi.org/10.3390/insects10090297
De Jesus CE, Ganser C, Kessler WH, White ZS, Bhosale CR, Glass GE, Wisely SM. A Survey of Tick-Borne Bacterial Pathogens in Florida. Insects. 2019; 10(9):297. https://doi.org/10.3390/insects10090297
Chicago/Turabian StyleDe Jesus, Carrie E., Claudia Ganser, William H. Kessler, Zoe S. White, Chanakya R. Bhosale, Gregory E. Glass, and Samantha M. Wisely. 2019. "A Survey of Tick-Borne Bacterial Pathogens in Florida" Insects 10, no. 9: 297. https://doi.org/10.3390/insects10090297
APA StyleDe Jesus, C. E., Ganser, C., Kessler, W. H., White, Z. S., Bhosale, C. R., Glass, G. E., & Wisely, S. M. (2019). A Survey of Tick-Borne Bacterial Pathogens in Florida. Insects, 10(9), 297. https://doi.org/10.3390/insects10090297






