Effects of Propoxur Exposure on Insecticidal Susceptibility and Developmental Traits in Culex pipiens quinquefasciatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Insecticides
2.3. Bioassay
2.4. Resistance Selection and Sublethal Effects of Short-Term Exposure to Propoxur
2.5. Assessment of the Transgenerational Effects of Propoxur Exposure
2.6. Detection of Mutation in AChE Encoding Gene Ace-1
2.7. Enzyme Activity Assays
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
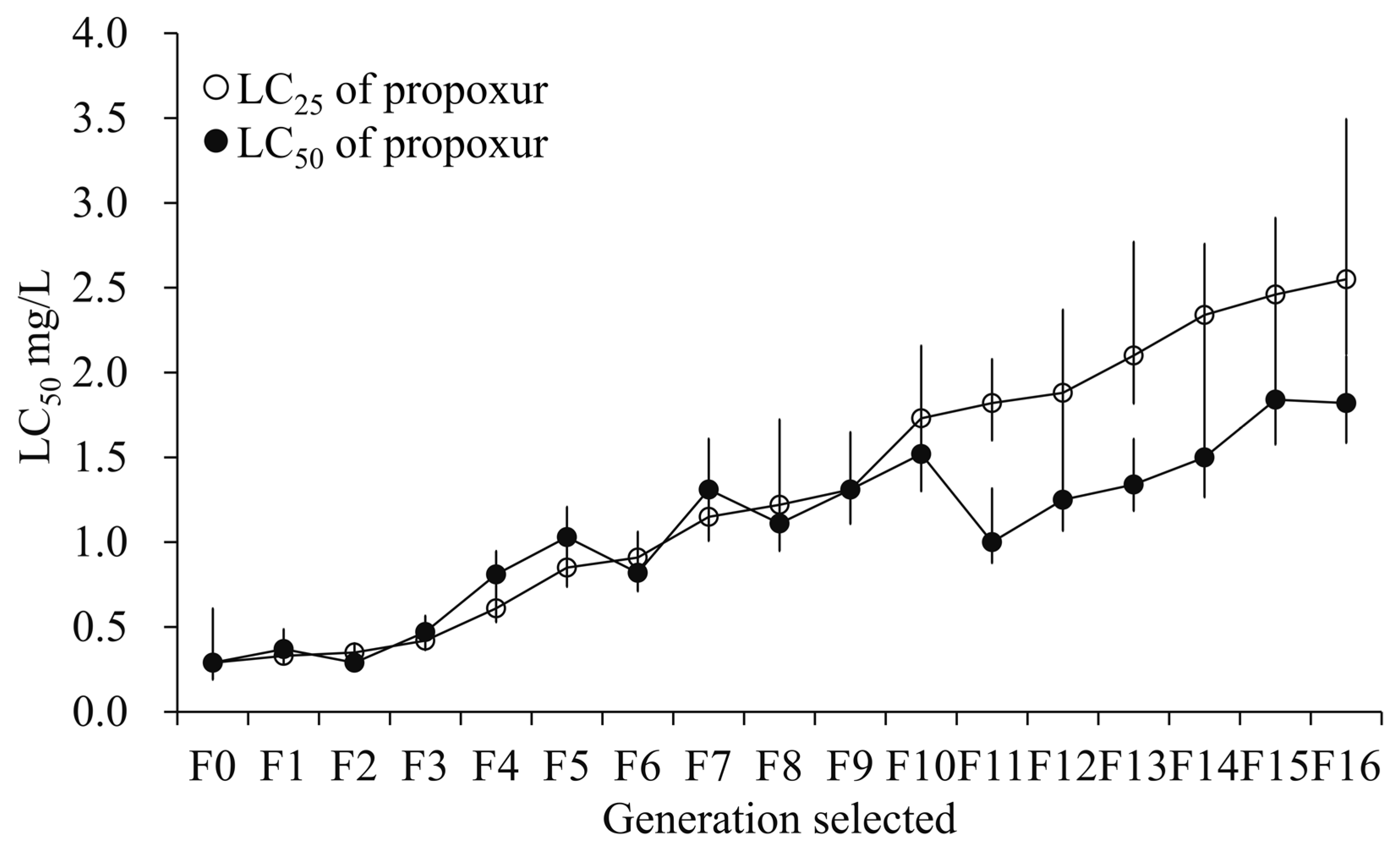
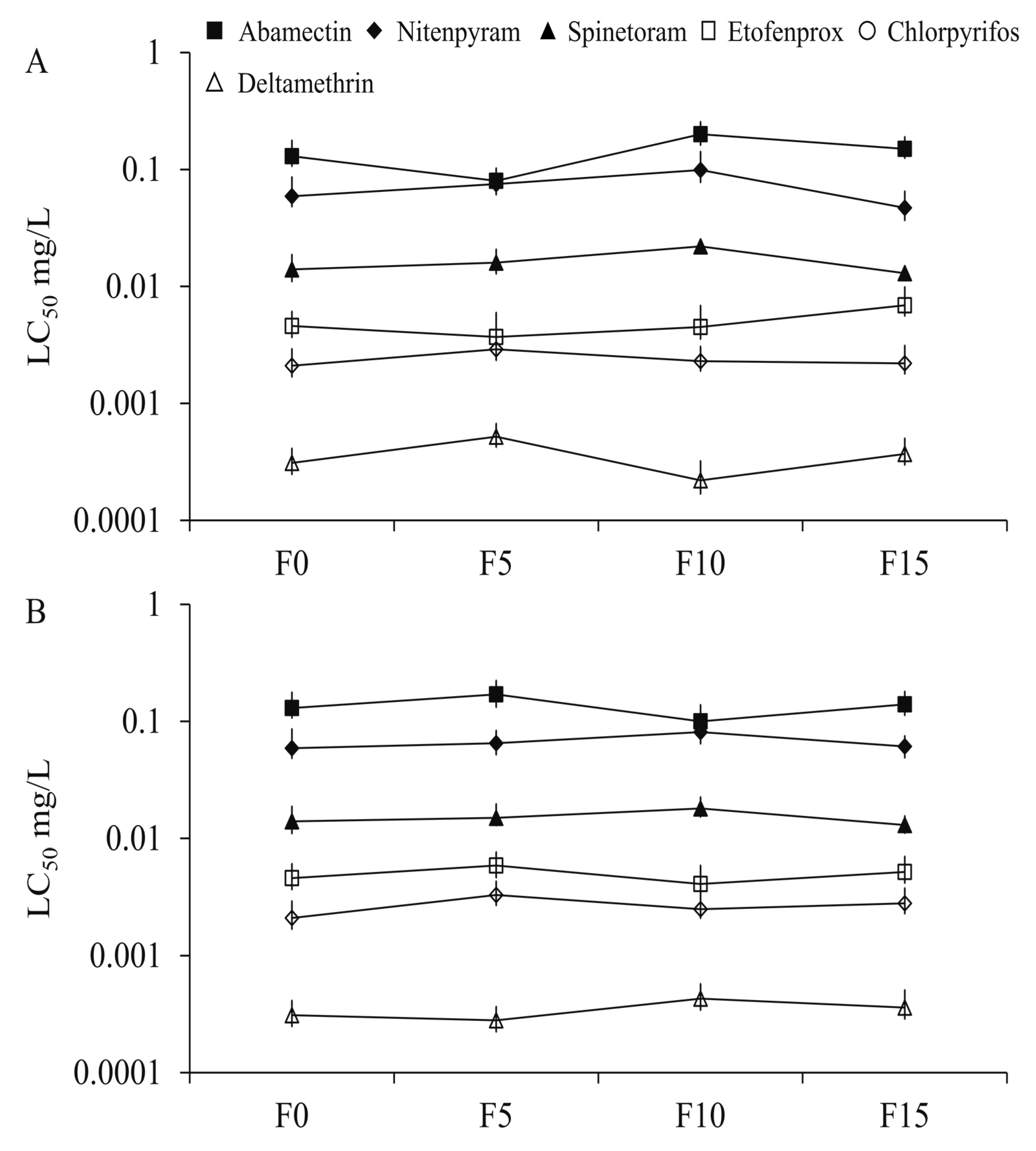
3.1. Resistance Development After Selection with Sublethal Exposure to Propoxur
3.2. Effects on the Developmental Traits of Adults, Pupae, and Eggs of Parental Cx. Quinquefasciatus after Sublethal and Lethal Exposure to Propoxur
3.3. Transgenerational Impact of Propoxur on Demographic Parameters and Developmental Traits
3.4. Analysis of the Possible Resistance Mechanism
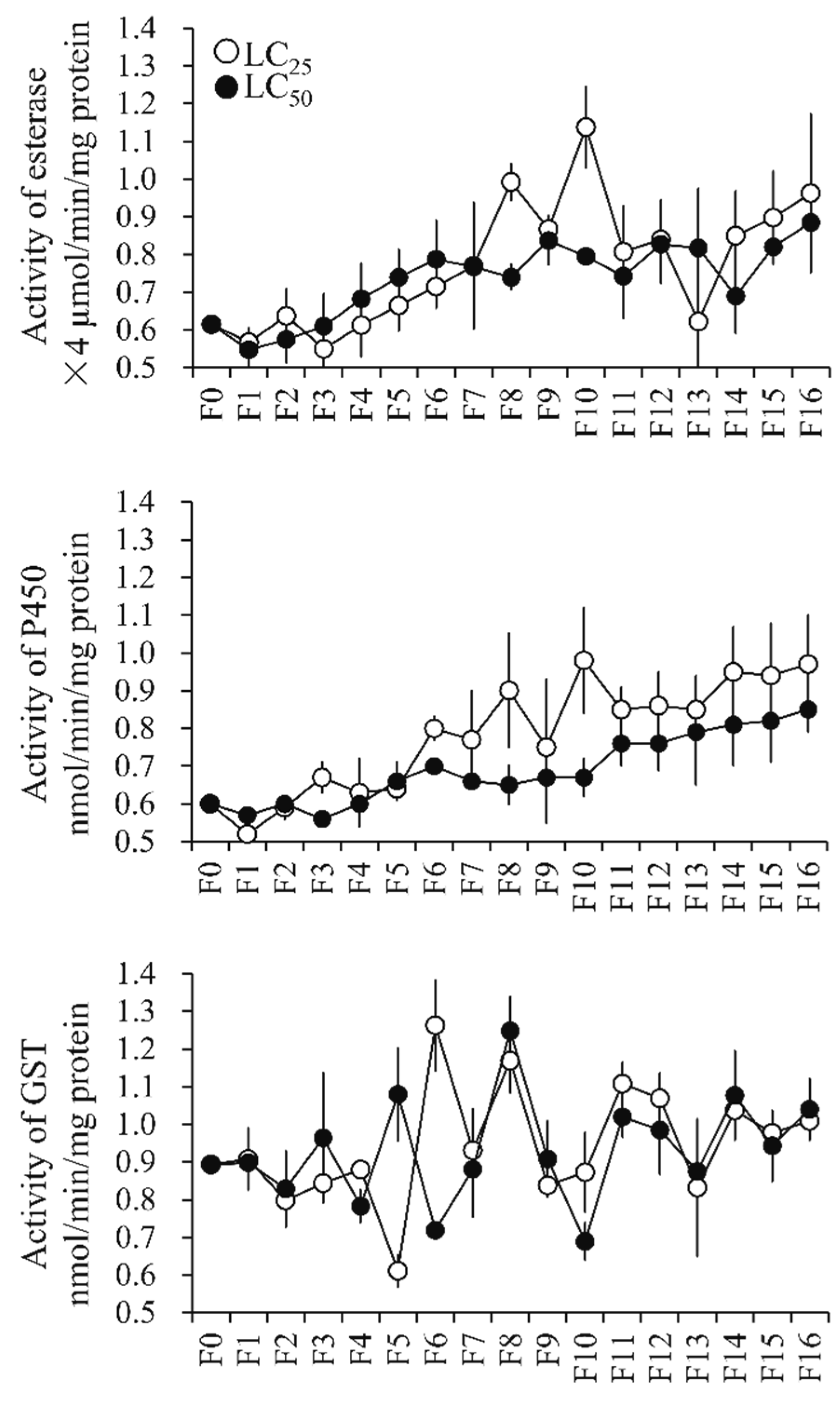
3.4.1. Metabolic Enzyme Activity
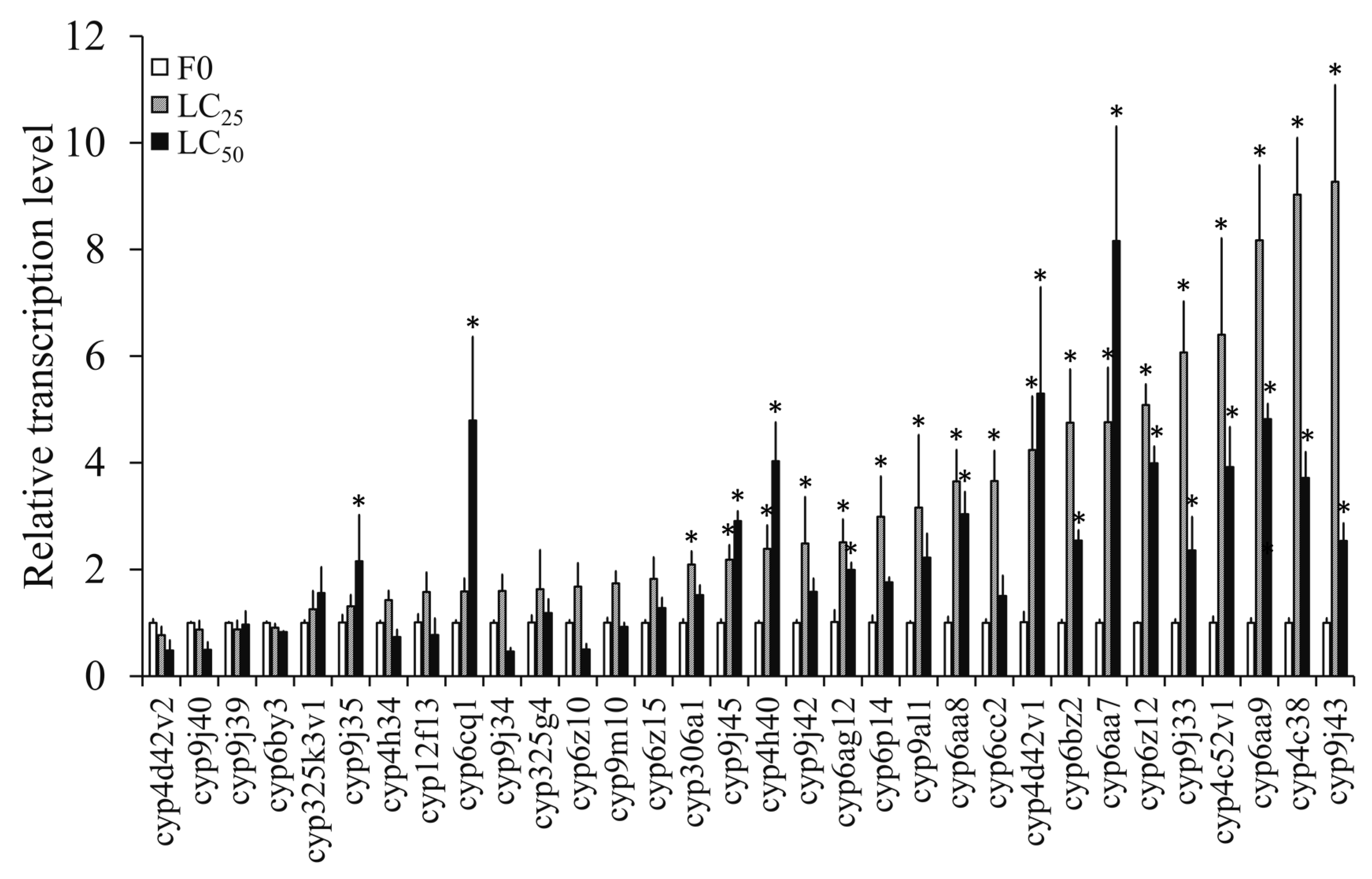
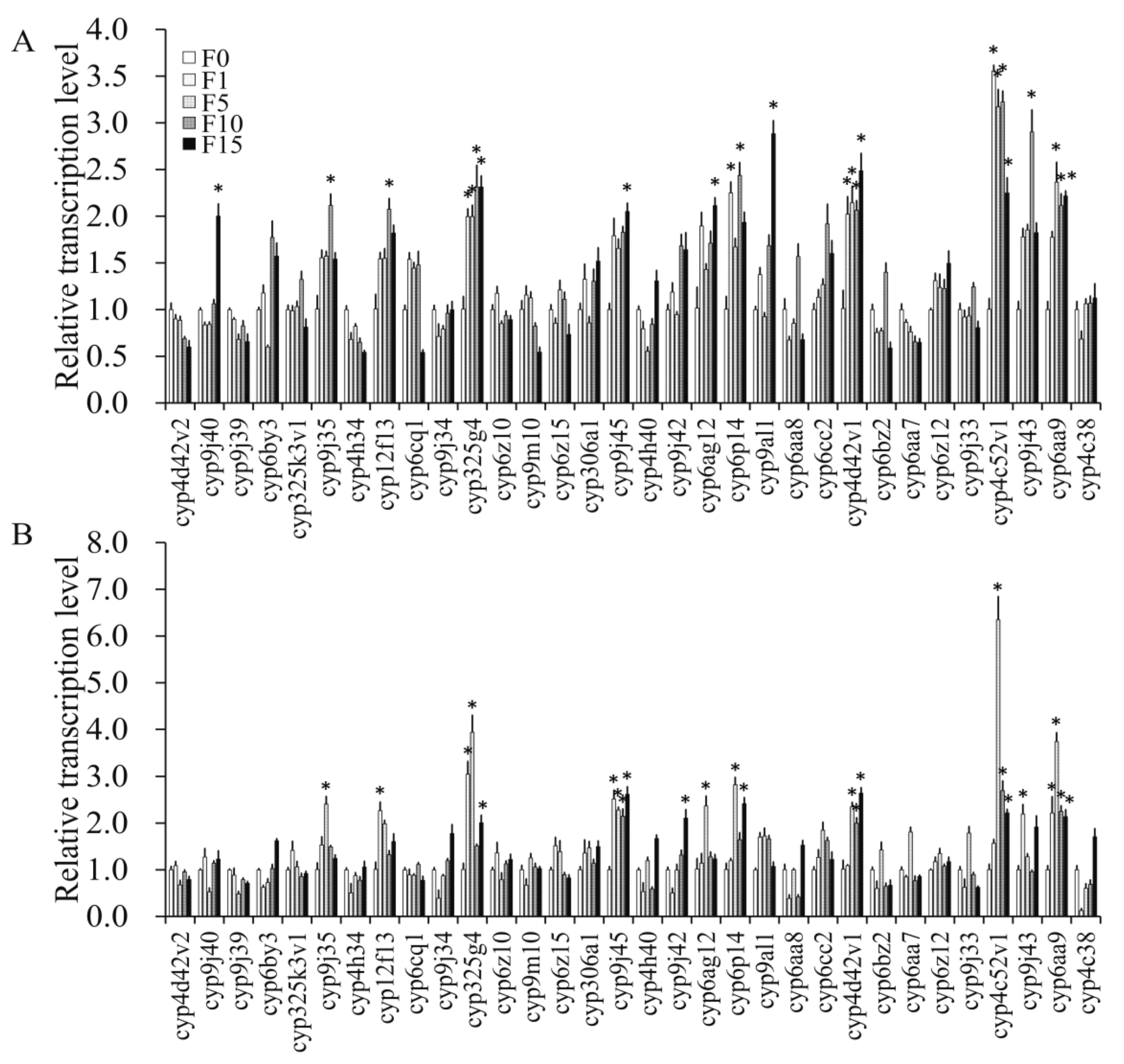
3.4.2. Transcription Levels of P450 Genes in F0 Induced by Propoxur
3.4.3. Transcription Levels of P450 Genes in Propoxur-Sel Strains of F1, F5, F10, and F15
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, X.X.; Li, C.X.X.; Deng, Y.Q.; Xing, D.; Liu, Q.M.; Wu, Q.; Sun, A.J.; Dong, Y.D.; Cao, W.C.; Qin, C.F.; et al. Culex pipiens quinquefasciatus: A potential vector to transmit Zika virus. Emerg. Microbes Infect. 2016, 5, e102. [Google Scholar] [CrossRef] [PubMed]
- Guedes, D.R.D.; Paiva, M.H.S.; Donato, M.M.A.; Barbosa, P.P.; Krokovsky, L.; Rocha, S.W.; Saraiva, K.L.A.; Crespo, M.M.; Barbosa, R.M.R.; Oliveira, C.M.F.; et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes Infect. 2017, 6, e69. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.F.S.; Subramaniam, K.; Steen, K.; Mawejje, H.; Liloglou, T.; Donnelly, M.J.; Wilding, C.S. Detection and quantitation of copy number variation in the voltage-gated sodium channel gene of the mosquito Culex quinquefasciatus. Sci. Rep. 2017, 7, 5821. [Google Scholar] [CrossRef] [PubMed]
- Redekop, W.K.; Lenk, E.J.; Luyendijk, M.; Fitzpatrick, C.; Niessen, L.; Stolk, W.A.; Tediosi, F.; Rijnsburger, A.J.; Bakker, R.; Hontelez, J.A.C.; et al. The socioeconomic benefit to individuals of achieving the 2020 targets for five preventive chemotherapy neglected tropical diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005289. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.R.; Zhang, L.; Gong, Y.H.; Li, T.; Liu, N.N. Gene expression profiles of the southern house mosquito Culex quinquefasciatus during exposure to permethrin. Insect Sci. 2018, 3, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cai, Q.; Yan, J.; Hu, X.; Zheng, D.; Yuan, Z. Single nucleotide deletion of cqm1 gene results in the development of resistance to Bacillus sphaericus in Culex quinquefasciatus. J. Insect Physiol. 2013, 59, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Ji, C.; Zhu, X.B.; Xue, J.W.; Wang, L.H.; Wang, Y. Impact of Bacillus sphaericus exposure on Anopheles dirus’s fecundity and resistance development. Parasitol. Res. 2017, 116, 859–864. [Google Scholar] [CrossRef]
- Cui, F.; Raymond, M.; Qiao, C.L. Insecticide resistance in vector mosquitoes in China. Pest Manag. Sci. 2006, 62, 1013–1022. [Google Scholar] [CrossRef]
- Liang, P.; Tian, Y.A.; Biondi, A.; Desneux, N.; Gao, X.W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 2012, 21, 1889–1898. [Google Scholar] [CrossRef]
- Mattah, P.A.D.; Futagbi, G.; Amekudzi, L.K.; Mattah, M.M.; Souz, D.K.; Kartey-Attipoe, W.D.; Bimi, L.; Wilson, M.D. Diversity in breeding sites and distribution of Anopheles mosquitoes in selected urban areas of southern Ghana. Parasit. Vectors 2017, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Kibuthu, T.W.; Njenga, S.M.; Mbugua, A.K.; Muturi, E.J. Agricultural chemicals: Life changer for mosquito vectors in agricultural landscapes. Parasit. Vectors 2016, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Buhagiar, T.S.; Devine, G.J.; Ritchie, S.A. Effects of sublethal exposure to metofluthrin on the fitness of Aedes aegypti in a domestic setting in Cairns, Queensland. Parasit. Vectors 2017, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Sanil, D.; Shetty, N.J. The effect of sublethal exposure to temephos and propoxur on reproductive fitness and its influence on circadian rhythms of pupation and adult emergence in Anopheles stephensi Liston—A malaria vector. Parasitol. Res. 2012, 111, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Suwansirisilp, K.; Visetson, S.; Prabaripai, A.; Tanasinchayakul, S.; Grieco, J.P.; Bangs, M.J.; Chareonviriyaphap, T. Behavioral responses of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) to four essential oils in Thailand. J. Pest Sci. 2013, 86, 309–320. [Google Scholar] [CrossRef]
- Heckel, D.G. Insecticide resistance after Silent Spring. Science 2012, 337, 1612–1614. [Google Scholar] [CrossRef]
- Seth, V.; Banerjee, B.D.; Bhattacharya, A.; Chakravorty, A.K. Lipid peroxidation, antioxidant enzymes, and glutathione redox system in blood of human poisoning with propoxur. Clin. Biochem. 2000, 33, 683–685. [Google Scholar] [CrossRef]
- Pennington, M.J.; Prager, S.M.; Walton, W.E.; Trumble, T.M. Culex quinquefasciatus larval microbiomes vary with instar and exposure to common wastewater contaminants. Sci. Rep. 2016, 6, 21969. [Google Scholar] [CrossRef]
- Almeida, L.G.; Moraes, L.A.B.; Trigo, J.R.; Omoto, C.; Cônsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef]
- Jiang, Y.; Swale, D.; Carlier, P.R.; Hartsel, J.A.; Ma, M.; Ekström, F.; Bloomquist, J.R. Evaluation of novel carbamate insecticides for neurotoxicity to non-target species. PestiCx. Biochem. Physiol. 2013, 106, 156–161. [Google Scholar] [CrossRef]
- Baron, R.L. Carbamate Insecticides. In Handbook Pesticide Toxicology; Hayes, W.J., Laws, E.R., Eds.; Academic Press: New York, NY, USA, 1991; Volume 3: Classes of Pesticides, pp. 1125–1189. [Google Scholar]
- Eraslan, G.; Kanbur, M.; Silici, S.; Liman, B.C.; Altınordulu, S.; Sarıca, Z. Evaluation of protective effect of bee pollen against propoxur toxicity in rat. Ecotoxicol. Environ. Saf. 2009, 72, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, M.; Priya, S.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Photocatalytic degradation of aqueous propoxur solution using TiO2 and Hβzeolite-supported TiO2. J. Hazard. Mater. 2009, 161, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Dong, Y.; Ran, X.; Wu, Z.; Guo, X.; Zhang, Y.; Xing, D.; Yan, T.; Wang, G.; Zhu, X.; et al. Point mutations associated with organophosphate and carbamate resistance in chinese strains of Culex pipiens quinquefasciatus (Diptera: Culicidae). PLoS ONE 2014, 9, e95260. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Yu, W.T.; Zhang, W.Q.; Yang, Y.H.; Walsh, T.; Oakeshott, J.G.; Wu, Y.D. Variation in P450-mediated fenvalerate resistance levels is not correlated with CYP337B3 genotype in Chinese populations of Helicoverpa armigera. Pestic. Biochem. Physiol. 2015, 121, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Liao, X.; Miao, K.K.; Zhang, K.X.; Wan, H.; Li, J.H. Insecticide resistance monitoring and correlation analysis of insecticides in field populations of the brown planthopper Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2016, 132, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, T.; Reid, W.R.; Yang, T.; Zhang, L. Multiple cytochrome P450 genes: Their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS ONE 2011, 6, e23403. [Google Scholar] [CrossRef]
- Gong, Y.; Li, T.; Zhang, L.; Gao, X.; Liu, N. Permethrin induction of multiple cytochrome P450 genes in insecticide resistant mosquitoes, Culex quinquefasciatus. Int. J. Biol. Sci. 2013, 9, 863–871. [Google Scholar] [CrossRef]
- Li, T.; Liu, N. Regulation of P450-mediated permethrin resistance in Culex quinquefasciatus by the GPCR/Gαs/AC/cAMP/PKA signaling cascade. Biochem. Biophys. Rep. 2017, 12, 12–19. [Google Scholar] [CrossRef]
- Livak, K.L.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rate among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- TWOSEX-MSChart. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 1 July 2019).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: London, UK, 1993; pp. 49–54. [Google Scholar]
- Akköprü, P.E.; Atlihan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.L.; Olson, J.K. Effects of sublethal dosages of insecticides on Culex quinquefasciatus. J. Am. Mosq. Control. Assoc. 1989, 5, 239–246. [Google Scholar] [PubMed]
- Li, C.; Dong, Y.; Zhao, T. Relationship of esterase, acetylcholinesterase and propoxur-resistance to Culex pipiens pallens. Chin. J. Vector Bio. Control 2007, 18, 9–11. [Google Scholar]
- Jiang, H.; Zhen, T.; Wang, H.; Cheng, P.; Sun, C.; Wang, H.; Wang, X.; Zhao, Y. Selection for resistance to propoxur in Culex pipiens pallens and its cross-resistance. Chin. J. Vector Biol. Control 2005, 16, 185–186. [Google Scholar]
- Kou, J.X.; Liu, H.M.; Gong, M.Q. Resistance levels and cross-resistance of Culex pipiens pallens to commonly used chemical pesticides after long-term breeding. Parasitosis Infect. Dis. 2013, 11, 176–179. [Google Scholar]
- Jiang, B.; Li, S.G.; Quan, X.; Xue, Q.J.; Tan, W.B.; Liu, Y.C.X.; Wang, X.G.; Wang, H.W. Experimental study on cross-resistance of Culex pipiens pallens to 3 kinds of chemical pesticides. Chin. J. Schisto Control 2014, 26, 531–533. [Google Scholar]
- Wang, X.; Zhen, T.; Liu, F.; Li, S.; Wang, H.; Zhao, Y.; Sun, C. Cross-resistance of resistant strain of Culex pipiens pallens to chemical insecticides. Chin. J. Vector Biol. Control 1999, 10, 18–20. [Google Scholar]
- Du, Q. Resistance development and cross-resistance of propoxur-resistant Culex Pipiens Pallens and DDVP-resistant Culex Pipiens Pallens. China Trop. Med. 2009, 9, 799–800. [Google Scholar]
- Li, S. The cross -resistance of three resistant strains of Culex pipiens pallens to the five pesticides. China Trop. Med. 2009, 9, 803–804. [Google Scholar]
- Zhang, C.; Li, X.; Dai, W.; Xu, Q.; Zhen, T. Development of resistance of propoxur selected Culex pipiens pallens. China Trop. Med. 2005, 5, 1173–1174. [Google Scholar]
- Valles, S.M.; Yu, S.J. Detection and biochemical characterization of insecticide resistance in the German Cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 1996, 89, 21–26. [Google Scholar] [CrossRef]
- Chai, R.Y.; Lee, C.Y. Insecticide resistance profiles and synergism in field populations of the German Cockroach (Dictyoptera: Blattellidae) from Singapore. J. Econ. Entomol. 2010, 103, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Hardstone, M.C.; Huang, X.; Harrington, L.C.; Scott, J.G. Differences in development, glycogen, and lipid content associated with cytochrome P450-mediated permethrin resistance in Culex pipiens quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Guo, X.X.; Zhang, Y.M.; Dong, Y.D.; Xing, D.; Yan, T.; Wang, G.; Zhang, H.D.; Zhao, T.Y. Identification of genes involved in pyrethroid-, propoxur-, and dichlorvos- insecticides resistance in the mosquitoes, Culex pipiens complex (Diptera: Culicidae). Acta Tropica 2016, 157, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.M.; Wang, K.F.; Zheng, L.; Wu, Z.J.; Miyata, T.; Wu, G. Identification and characterization of a cytochrome P450 CYP6CX1 putatively associated with insecticide resistance in Bemisia tabaci. Insect Sci. 2011, 18, 484–494. [Google Scholar] [CrossRef]
- Wahyuningsih, N.E.; Nurussakinah, F.; Siswoyowati. Ae. aegypti and Ae. albopictus mosquito longevity is longer as impact of exposure to propoxur aerosol insecticide. In Planning for Environmental Sustainability for the Well-Being of Future Humanity, Proceedings of the 1ST UMM International Conference on Pure and Applied Research (UMM-ICOPAR 2015), Malang, East Java, Indonesia, 21–22 August 2015; Admin Conference UMM: Malang, East Java, Indonesia, 2015; pp. 125–131. [Google Scholar]
- Georghiou, G.P. Effects of carbamates on house fly fecundity, longevity and food intake. J. Econ. Entomol. 1965, 58, 58–62. [Google Scholar] [CrossRef]
- Harmon, J.D.; Ross, M.H. Effects of propoxur exposure on females of the German cockroach, Blattela germanica and their oothecae. Entomol. Exp. Appl. 2011, 44, 269–275. [Google Scholar] [CrossRef]
- Lee, C.Y. Sublethal effects of insecticides on longevity, fecundity, and behavior of insect pests: A review. J. Biosci. 2000, 11, 107–112. [Google Scholar]
| G | Cx. quinquefasciatus Treated with LC25 of Propoxur | Cx. quinquefasciatus Treated with LC50 of Propoxur | ||||||
|---|---|---|---|---|---|---|---|---|
| F/M | H% | Sp% | Er% | F/M | H% | Sp% | Er% | |
| F0 | 1:1 a | 92.6 ± 2.6 a | 93.8 ± 1.2 a | 92.2 ± 1.2 a | 1:1 a | 93.6 ± 0.1 a | 98.7 ± 0.4 a | 98.7 ± 0.4 a |
| F1 | 1:5 b | 91.0 ± 0.8 a | 92.6 ± 1.2 a | 89.0 ± 1.3 a | 1:8 b | 87.3 ± 1.2 b | 95.7 ± 2.1 a | 93.8 ± 3.4 a |
| F2 | 1:6 b | 87.3 ± 1.8 b | 93.6 ± 0.9 a | 91.1 ± 1.2 a | 1:9 b | 85.6 ± 0.4 b | 96.3 ± 3.7 a | 92.6 ± 7.4 a |
| F3 | 1:4 b | 85.9 ± 2.4 b | 94.6 ± 0.8 a | 90.9 ± 0.6 a | 1:11 b | 83.8 ± 2.8 b | 90.8 ± 1.4 b | 90.2 ± 1.0 a |
| F4 | 1:4 b | 81.3 ± 0.2 b | 95.0 ± 1.3 a | 92.1 ± 1.1 a | 1:6 b | 82.3 ± 1.0 b | 98.3 ± 0.9 a | 97.5 ± 1.3 a |
| F5 | 1:3 b | 83.8 ± 3.1 b | 94.3 ± 2.1 a | 90.8 ± 1.3 a | 1:6 b | 84.7 ± 1.1 b | 96.9 ± 3.1 a | 92.1 ± 4.2 a |
| F6 | 1:4 b | 84.1 ± 2.3 b | 94.1 ± 0.9 a | 90.5 ± 1.4 a | 1:4 b | 82.4 ± 5.3 b | 96.4 ± 0.2 a | 95.2 ± 1.1 a |
| F7 | 1:5 b | 84.9 ± 0.8 b | 94.8 ± 1.7 a | 92.9 ± 2.1 a | 1:7 b | 81.6 ± 0.8 b | 94.6 ± 2.8 a | 94.6 ± 2.8 a |
| F8 | 1:5 b | 80.6 ± 0.6 b | 91.2 ± 1.8 a | 88.9 ± 2.3 a | 1:8 b | 86.3 ± 2.4 b | 97.8 ± 1.2 a | 95.1 ± 1.3 a |
| F9 | 1:6 b | 81.2 ± 2.0 b | 93.0 ± 0.6 a | 89.8 ± 0.6 a | 1:6 b | 80.1 ± 1.8 b | 97.2 ± 1.8 a | 95.1 ± 3.9 a |
| F10 | 1:6 b | 77.1 ± 1.7 b | 93.1 ± 1.3 a | 88.0 ± 2.4 a | 1:6 b | 82.1 ± 0.2 b | 97.4 ± 1.6 a | 94.2 ± 0.7 a |
| F11 | 1:5 b | 79.6 ± 1.6 b | 93.9 ± 1.7 a | 87.9 ± 1.1 a | 1:9 b | 84.5 ± 2.8 b | 100.0 ± 0.0 a | 87.1 ± 11.4 a |
| F12 | 1:5 b | 81.4 ± 2.3 b | 94.8 ± 1.2 a | 89.7 ± 2.5 a | 1:5 b | 84.9 ± 0.6 b | 94.0 ± 1.1 a | 89.2 ± 2.1 a |
| F13 | 1:4 b | 81.7 ± 1.4 b | 94.3 ± 2.3 a | 90.5 ± 2.0 a | 1:5 b | 79.8 ± 2.8 b | 97.8 ± 2.2 a | 97.8 ± 2.2 a |
| F14 | 1:3 b | 82.8 ± 1.0 b | 93.0 ± 0.6 a | 90.1 ± 0.4 a | 1:10 b | 82.9 ± 1.3 b | 98.2 ± 1.8 a | 96.7 ± 1.7 a |
| F15 | 1:3 b | 82.1 ± 1.2 b | 94.1 ± 1.5 a | 90.8 ± 1.7 a | 1:4 b | 77.4 ± 3.2 b | 94.3 ± 3.2 a | 87.7 ± 2.8 a |
| F16 | 1:5 b | 84.8 ± 1.5 b | 94.3 ± 0.3 a | 90.8 ± 0.8 a | 1:4 b | 82.2 ± 2.2 b | 94.8 ± 2.6 a | 89.3 ± 1.8 a |
| G | Cx. quinquefasciatus Treated with LC25 of Propoxur | Cx. quinquefasciatus Treated with LC50 of Propoxur | ||||||
|---|---|---|---|---|---|---|---|---|
| DT (d) | Fecundity (eggs/♀) | Longevity (d) | F/M | DT (d) | Fecundity (eggs/♀) | Longevity (d) | F/M | |
| F0 | 4.25 ± 0.11 a | 70.22 ± 2.84E-14 a | 39.49 ± 1.31 a | 1:1 a | 4.25 ± 0.11 a | 70.22 ± 2.84E-14 a | 39.49 ± 1.31 a | 1:1 a |
| F1 | 5.49 ± 0.25 b | 70.33 ± 1.42E-14 a | 31.48 ± 1.32 b | 1:2 b | 6.45 ± 0.35 b | 63.44 ± 4.26E-14 b | 31.18 ± 1.36 b | 1:2 b |
| F5 | 6.08 ± 0.32 b | 66.77 ± 2.84E-14 b | 33.83 ± 1.42 b | 1:2 b | 6.78 ± 0.52 b | 64.80 ± 3.89E-14 b | 33.11 ± 1.50 b | 1:3 b |
| F10 | 6.79 ± 0.43 b | 64.35 ± 1.42E-14 b | 31.55 ± 1.31 b | 1:3 b | 7.04 ± 0.56 b | 61.66 ± 1.42E-14 b | 31.79 ± 1.43 b | 1:3 b |
| F15 | 6.46 ± 0.41 b | 67.19 ± 2.84E-14 b | 29.99 ± 1.23 b | 1:3 b | 7.74 ± 0.71 b | 66.96 ± 1.42E-14 b | 29.13 ± 1.54 b | 1:3 b |
| G | Cx. quinquefasciatus Treated with LC25 of Propoxur | Cx. quinquefasciatus Treated with LC50 of Propoxur | ||||||
|---|---|---|---|---|---|---|---|---|
| r (d−1) | R0 | T (d) | λ (d−1) | r (d−1) | R0 | T (d) | λ (d−1) | |
| F0 | 0.16 ± 0.0043 a | 29.52 ± 2.61 a | 20.76 ± 0.00072 a | 1.18 ± 0.0056 a | 0.16 ± 0.043 a | 29.52 ± 2.61 a | 20.76 ± 0.00072 a | 1.18 ± 0.0051 a |
| F1 | 0.13 ± 0.0055 b | 17.40 ± 2.15 b | 22.62 ± 0.00095 b | 1.13 ± 0.0063 b | 0.10 ± 0.0053 b | 14.59 ± 1.95 b | 25.70 ± 0.11 b | 1.11 ± 0.0059 b |
| F5 | 0.11 ± 0.0058 b | 14.19 ± 1.90 b | 23.28 ± 0.00059 b | 1.12 ± 0.0065 b | 0.10 ± 0.0073 b | 10.86 ± 1.81 b | 23.34 ± 0.00081 b | 1.11 ± 0.0081 b |
| F10 | 0.10 ± 0.0061 b | 12.27 ± 1.81 b | 24.57 ± 0.00013 b | 1.11 ± 0.0068 b | 0.10 ± 0.0073 b | 9.99 ± 1.67 b | 23.40 ± 0.0019 b | 1.10 ± 0.0080 b |
| F15 | 0.11 ± 0.0065 b | 12.31 ± 1.83 b | 23.40 ± 0.0011 b | 1.11 ± 0.0072 b | 0.10 ± 0.0074 b | 9.85 ± 1.81 b | 25.55 ± 0.028 b | 1.09 ± 0.0081 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Karungu, S.; Cai, Q.; Yuan, Z.; Hu, X. Effects of Propoxur Exposure on Insecticidal Susceptibility and Developmental Traits in Culex pipiens quinquefasciatus. Insects 2019, 10, 288. https://doi.org/10.3390/insects10090288
Zhang X, Karungu S, Cai Q, Yuan Z, Hu X. Effects of Propoxur Exposure on Insecticidal Susceptibility and Developmental Traits in Culex pipiens quinquefasciatus. Insects. 2019; 10(9):288. https://doi.org/10.3390/insects10090288
Chicago/Turabian StyleZhang, Xiaolei, Samuel Karungu, Quanxin Cai, Zhiming Yuan, and Xiaomin Hu. 2019. "Effects of Propoxur Exposure on Insecticidal Susceptibility and Developmental Traits in Culex pipiens quinquefasciatus" Insects 10, no. 9: 288. https://doi.org/10.3390/insects10090288
APA StyleZhang, X., Karungu, S., Cai, Q., Yuan, Z., & Hu, X. (2019). Effects of Propoxur Exposure on Insecticidal Susceptibility and Developmental Traits in Culex pipiens quinquefasciatus. Insects, 10(9), 288. https://doi.org/10.3390/insects10090288





