Foraging Macrotermes natalensis Fungus-Growing Termites Avoid a Mycopathogen but Not an Entomopathogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites and Termite Nest Collection
2.2. Fungal Pathogens
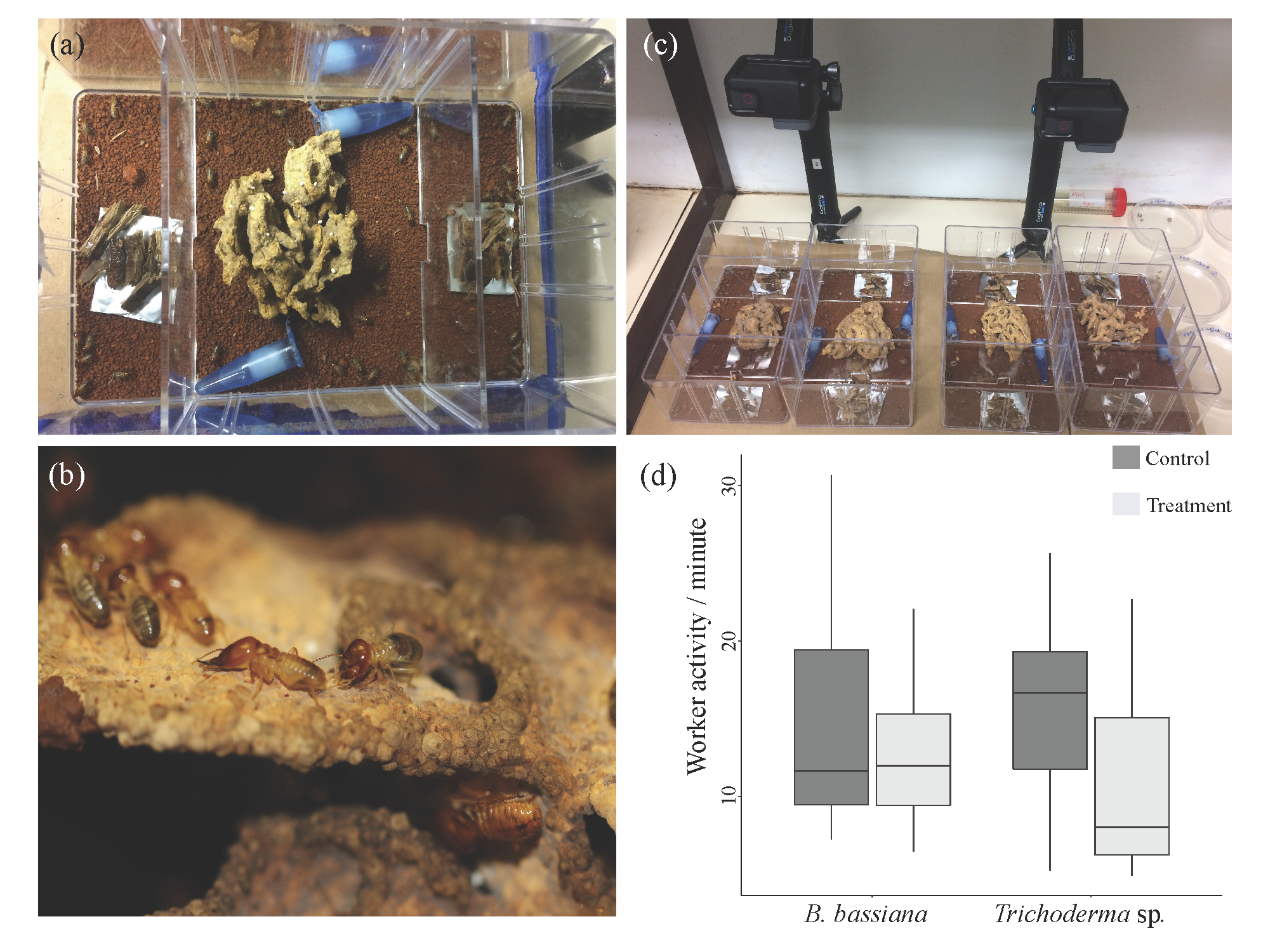
2.3. Behavioural Setup
2.4. Analysis of Behaviours
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugimoto, A.; Bignell, D.E.; MacDonals, J.A. Global impact of termites on the carbon cycle and atmospheric trase gases. In Termites: Evolution, Sociality, Symbiosis, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 409–435. [Google Scholar]
- Brady, S.G.; Larkin, L.; Danforth, B.N. Bees, ants, and stinging wasps (Aculeata). In The Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 264–269. [Google Scholar]
- Rubenstein, D.R.; Abbot, P. The Evolution of social evoliution. In Comparative Social Evolution; Rubenstein, D.R., Abbot, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 1–18. [Google Scholar]
- Correa, M.M.; Silva, P.S.D.; Wirth, R.; Tabarelli, M.; Leal, I.R. How leaf-cutting ants impact forests: Drastic nest effects on light environment and plant assemblages. Oecologia 2010, 162, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Jouquet, P.; Traore, S.; Choosai, C.; Hartmann, C.; Bignell, D. Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur. J. Soil Biol. 2011, 47, 215–222. [Google Scholar] [CrossRef]
- Korb, J.; Thorne, B. Sociality in termites. In Comparative Social Evolution; Rubenstein, D.R., Abbot, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 124–153. [Google Scholar]
- Heinze, J.; Kellner, K.; Seal, J. Sociality in ants. In Comparative Social Evolution; Rubenstein, D.R., Abbot, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 21–49. [Google Scholar]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social immunity. Curr Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, J.J.; Schmid-Hempel, P.; Hughes, W.O.H. Life histories and parasite pressures across the major groups of social insects. In Insect Evolutionary Ecology; Fellowes, M.D.E., Holloway, G.J., Rolff, J., Eds.; CABI Publishing: Oxon, UK, 2005; pp. 139–175. [Google Scholar]
- Liu, L.; Zhao, X.Y.; Tang, Q.B.; Lei, C.L.; Huang, Q.Y. The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Rosengaus, R.B.; Maxmen, A.B.; Coates, L.E.; Traniello, J.F.A. Disease resistance: A benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav. Ecol. Sociobiol. 1998, 44, 125–134. [Google Scholar] [CrossRef]
- Rosengaus, R.B.; Traniello, J.F.; Bulmer, M.S. Ecology, behavior and evolution of disease resistance in termites. In Biology of Termites: A Modern Synthesis; Bignell, D., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 165–191. [Google Scholar]
- Velenovsky, J.F.; Kalisch, J.; Bulmer, M.S. Selective sweeps in Cryptocercus woodroach antifungal proteins. Genetica 2016, 144, 547–552. [Google Scholar] [CrossRef]
- Nalepa, C.A. Origin of termite eusociality: Trophallaxis integrates the social, nutritional, and microbial environments. Ecol. Entomol. 2015, 40, 323–335. [Google Scholar] [CrossRef]
- Hamilton, C.; Bulmer, M.S. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev. Comp. Immunol. 2012, 36, 372–377. [Google Scholar] [CrossRef]
- Meunier, J. Social immunity and the evolution of group living in insects. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140102. [Google Scholar] [CrossRef]
- Walker, T.N.; Hughes, W.O.H. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 2009, 5, 446–448. [Google Scholar] [CrossRef]
- Katariya, L.; Ramesh, P.B.; Gopalappa, T.; Desireddy, S.; Bessiere, J.M.; Borges, R.M. Fungus-farming termites selectively bury weedy fungi that smell different from crop fungi. J. Chem. Ecol. 2017, 43, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Bos, N.; Lefevre, T.; Jensen, A.B.; d’Ettorre, P. Sick ants become unsociable. J. Evol. Biol. 2012, 25, 342–351. [Google Scholar] [CrossRef]
- Prestwich, G.D. Chemical defense by termite soldiers. J. Chem. Ecol. 1979, 5, 459–480. [Google Scholar] [CrossRef]
- He, S.; Johnston, P.R.; Kuropka, B.; Lokatis, S.; Weise, C.; Plarre, R.; Kunte, H.J.; McMahon, D.P. Termite soldiers contribute to social immunity by synthesizing potent oral secretions. Insect. Mol. Biol. 2018, 27, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.; Bot, A.N.M.; Currie, C.R.; Nielsen, M.G.; Boomsma, J.J. Within-colony transmission and the cost of a mutualistic bacterium in the leaf-cutting ant Acromyrmex octospinosus. Funct. Ecol. 2003, 17, 260–269. [Google Scholar] [CrossRef]
- Mburu, D.M.; Ochola, L.; Maniania, N.K.; Njagi, P.G.N.; Gitonga, L.M.; Ndung’u, M.W.; Wanjoya, A.K.; Hassanali, A. Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J. Insect. Physiol. 2009, 55, 774–780. [Google Scholar] [CrossRef]
- Aanen, D.K.; Boomsma, J.J. Social-insect fungus farming. Curr. Biol. 2006, 16, R1014–R1016. [Google Scholar] [CrossRef][Green Version]
- Schultz, T.R.; Brady, S.G. Major evolutionary transitions in ant agriculture. Proc. Natl. Acad. Sci. USA 2008, 105, 5435–5440. [Google Scholar] [CrossRef]
- Aanen, D.K.; Eggleton, P. Fungus-growing termites originated in African rain forest. Curr. Biol. 2005, 15, 851–855. [Google Scholar] [CrossRef]
- Collins, N.M. Consumption of wood by artificially isolated colonies of the fungus-growing termite Macrotermes bellicosus. Entomol. Exp. Appl. 1981, 29, 313–320. [Google Scholar] [CrossRef]
- Weber, N.A. Fungus-growing ants. Science 1966, 153, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, M. Termite symbiotic systems: Efficient bio-recycling of lignocellulose. Appl. Microbiol. Biotechnol. 2003, 61, 1–9. [Google Scholar] [CrossRef]
- da Costa, R.R.; Hu, H.; Li, H.; Poulsen, M. Symbiotic plant biomass decomposition in fungus-growing termites. Insects 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.; Boomsma, J.J. Mutualistic fungi control crop diversity in fungus-growing ants. Science 2005, 307, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Aanen, D.K.; Licht, H.H.D.F.; Debets, A.J.M.; Kerstes, N.A.G.; Hoekstra, R.F.; Boomsma, J.J. High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 2009, 326, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M. Towards an integrated understanding of the consequences of fungus domestication on the fungus-growing termite gut microbiota. Environ. Microbiol. 2015, 17, 2562–2572. [Google Scholar] [CrossRef]
- Currie, C.R. Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 2001, 128, 99–106. [Google Scholar] [CrossRef]
- Visser, A.A.; Kooij, P.W.; Debets, A.J.M.; Kuyper, T.W.; Aanen, D.K. Pseudoxylaria as stowaway of the fungus-growing termite nest: Interaction asymmetry between Pseudoxylaria, Termitomyces and free-living relatives. Fungal Ecol. 2011, 4, 322–332. [Google Scholar] [CrossRef]
- Batra, L.R.; Batra, W.T. Fungus-growing termites of tropical India and associated fungi. J. Kans. Entomol. Soc. 1966, 39, 725–738. [Google Scholar]
- Otani, S.; Challinor, V.; Kildgaard, S.; Kreuzenbeck, N.; Christensen, S.K.; Larsen, L.L.M.; Vreeburg, S.M.E.; Aanen, D.K.; Beemelmanns, C.; Poulsen, M. Disease-free monoculture farming by fungus-growing termites. Sci. Rep. 2019, 9, 8819. [Google Scholar] [CrossRef]
- Um, S.; Fraimout, A.; Sapountzis, P.; Oh, D.-C.; Poulsen, M. The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci. Rep. 2013, 3, 3250. [Google Scholar] [CrossRef] [PubMed]
- Bot, A.N.M.; Ortius-Lechner, D.; Finster, K.; Maile, R.; Boomsma, J.J. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insectes Soc. 2002, 49, 363–370. [Google Scholar] [CrossRef]
- Seipke, R.F.; Barke, J.; Brearley, C.; Hill, L.; Yu, D.W.; Goss, R.J.; Hutchings, M.I. A single Streptomyces symbiont makes multiple antifungals to support the fungus farming ant Acromyrmex octospinosus. PLoS ONE 2011, 6, e22028. [Google Scholar] [CrossRef] [PubMed]
- Morelos-Juarez, C.; Walker, T.N.; Lopes, J.F.S.; Hughes, W.O.H. Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr. Biol. 2010, 20, R553–R554. [Google Scholar] [CrossRef]
- Fernandez-Marin, H.; Zimmerman, J.K.; Nash, D.R.; Boomsma, J.J.; Wcislo, W.T. Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proc. R. Soc. B Biol. Sci. 2009, 276, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marin, H.; Zimmerman, J.K.; Rehner, S.A.; Wcislo, W.T. Active use of the metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B Biol. Sci. 2006, 273, 1689–1695. [Google Scholar] [CrossRef]
- Visser, A.A.; Nobre, T.; Currie, C.R.; Aanen, D.K.; Poulsen, M. Exploring the potential for Actinobacteria as defensive symbionts in fungus-growing termites. Microb. Ecol. 2012, 63, 975–985. [Google Scholar] [CrossRef]
- Heine, D.; Holmes, N.A.; Worsley, S.F.; Santos, A.C.A.; Innocent, T.M.; Scherlach, K.; Patrick, E.H.; Douglas, W.Y.; Murrell, J.C.; Vieria, P.C.; et al. Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen. Nat. Commun. 2018, 9, 2208. [Google Scholar] [CrossRef]
- Poulsen, M.; Cafaro, M.J.; Erhardt, D.P.; Little, A.E.F.; Gerardo, N.M.; Tebbets, B.; Klein, B.S.; Currie, C.R. Variation in Pseudonocardia antibiotic defence helps govern parasite-induced morbidity in Acromyrmex leaf-cutting ants. Environ. Microbiol. Rep. 2010, 2, 534–540. [Google Scholar] [CrossRef]
- Katariya, L.; Ramesh, P.B.; Sharma, A.; Borges, R.M. Local hypoxia generated by live burial is effective in weed control within termite fungus farms. Insectes Soc. 2018, 65, 561–569. [Google Scholar] [CrossRef]
- Xiao, G.H.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.W.; Xie, X.Q.; Shang, Y.; Leger, R.J.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Gunetti, M.; Castiglia, S.; Rustichelli, D.; Mareschi, K.; Sanavio, F.; Muraro, M.; Signorino, E.; Castello, L.; Ferrero, I.; Fagioli, F. Validation of analytical methods in GMP: The disposable Fast Read 102 (R) device, an alternative practical approach for cell counting. J. Transl. Med. 2012, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, C.Y. A laboratory maintenance regime for a fungus-growing termite Macrotermes gilvus (Blattodea: Termitidae). J. Econ. Entomol. 2015, 108, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Bolker, B. GLMM FAQ. 2019. Available online: https://bbolker.github.io/mixedmodels-misc/glmmFAQ.html (accessed on 22 March 2019).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 20 April 2019).
- RStudio Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 20 April 2019).
- de Roode, J.C.; Lefevre, T. Behavioral immunity in insects. Insects 2012, 3, 789–820. [Google Scholar] [CrossRef]
- Mburu, D.M.; Maniania, N.K.; Hassanali, A. Comparison of volatile blends and nucleotide sequences of two Beauveria bassiana isolates of different virulence and repellency toward the termite Macrotermes michealseni. J. Chem. Ecol. 2013, 39, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Bland, J.M.; Gu, W.X. Behavioral and electrophysiological responses of Coptotermes formosanus Shiraki towards entomopathogenic fungal volatiles. Biol. Control 2010, 55, 166–173. [Google Scholar] [CrossRef]
- Yanagawa, A.; Fujiwara-Tsujii, N.; Akino, T.; Yoshimura, T.; Yanagawa, T.; Shimizu, S. Behavioral changes in the termite, Coptotermes formosanus (Isoptera), inoculated with six fungal isolates. J. Invertebr. Pathol. 2011, 107, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Walter, B. Moribund ants leave their nests to die in social isolation. Curr. Biol. 2010, 20, 249–252. [Google Scholar] [CrossRef]
- Rueppell, O.; Hayworth, M.K.; Ross, N.P. Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evolut. Biol. 2010, 23, 1538–1546. [Google Scholar] [CrossRef]
- Currie, C.R.; Stuart, A.E. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. B Biol. Sci. 2001, 268, 1033–1039. [Google Scholar] [CrossRef]
- Katariya, L.; Ramesh, P.B.; Borges, R.M. Dynamic environments of fungus-farming termite mounds exert growth-modulating effects on fungal crop parasites. Environ. Microbiol. 2018, 20, 971–979. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodawatta, K.H.; Poulsen, M.; Bos, N. Foraging Macrotermes natalensis Fungus-Growing Termites Avoid a Mycopathogen but Not an Entomopathogen. Insects 2019, 10, 185. https://doi.org/10.3390/insects10070185
Bodawatta KH, Poulsen M, Bos N. Foraging Macrotermes natalensis Fungus-Growing Termites Avoid a Mycopathogen but Not an Entomopathogen. Insects. 2019; 10(7):185. https://doi.org/10.3390/insects10070185
Chicago/Turabian StyleBodawatta, Kasun H., Michael Poulsen, and Nick Bos. 2019. "Foraging Macrotermes natalensis Fungus-Growing Termites Avoid a Mycopathogen but Not an Entomopathogen" Insects 10, no. 7: 185. https://doi.org/10.3390/insects10070185
APA StyleBodawatta, K. H., Poulsen, M., & Bos, N. (2019). Foraging Macrotermes natalensis Fungus-Growing Termites Avoid a Mycopathogen but Not an Entomopathogen. Insects, 10(7), 185. https://doi.org/10.3390/insects10070185




